The Topical Effect of rhGDF-5 Embedded in a Collagen–Gelatin Scaffold for Accelerated Wound Healing †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Application of rhGDF-5
2.2. Animal and Wound Treatment
- Single application with a dressing of 30 g/m² scaffold with 100 ng rhGDF-5 (n = 6);
- Single application with a dressing of 75 g/m² scaffold with 500 ng rhGDF-5 (n = 6);
- Single application with a dressing of 150 g/m² scaffold with 1000 ng rhGDF-5 (n = 6);
- Single application with a dressing of 90 g/m² scaffold with 5000 ng rhGDF-5 (n = 6);
- Multiple applications with a dressing of 30 g/m² scaffold with 100 ng rhGDF-5 (n = 6);
- Multiple applications with a dressing of 75 g/m² scaffold with 500 ng rhGDF-5 (n = 6);
- Multiple applications with a dressing of 150 g/m² scaffold with 1000 ng rhGDF-5 (n = 6);
- Multiple applications with a dressing of scaffold 90 g/m² with 5000 ng rhGDF-5 (n = 6);
- Single application of the collagen–elastin matrix (n = 6);
- Untreated wounds without a dressing as control (n = 6).
2.3. Experimental Setup
3. Results
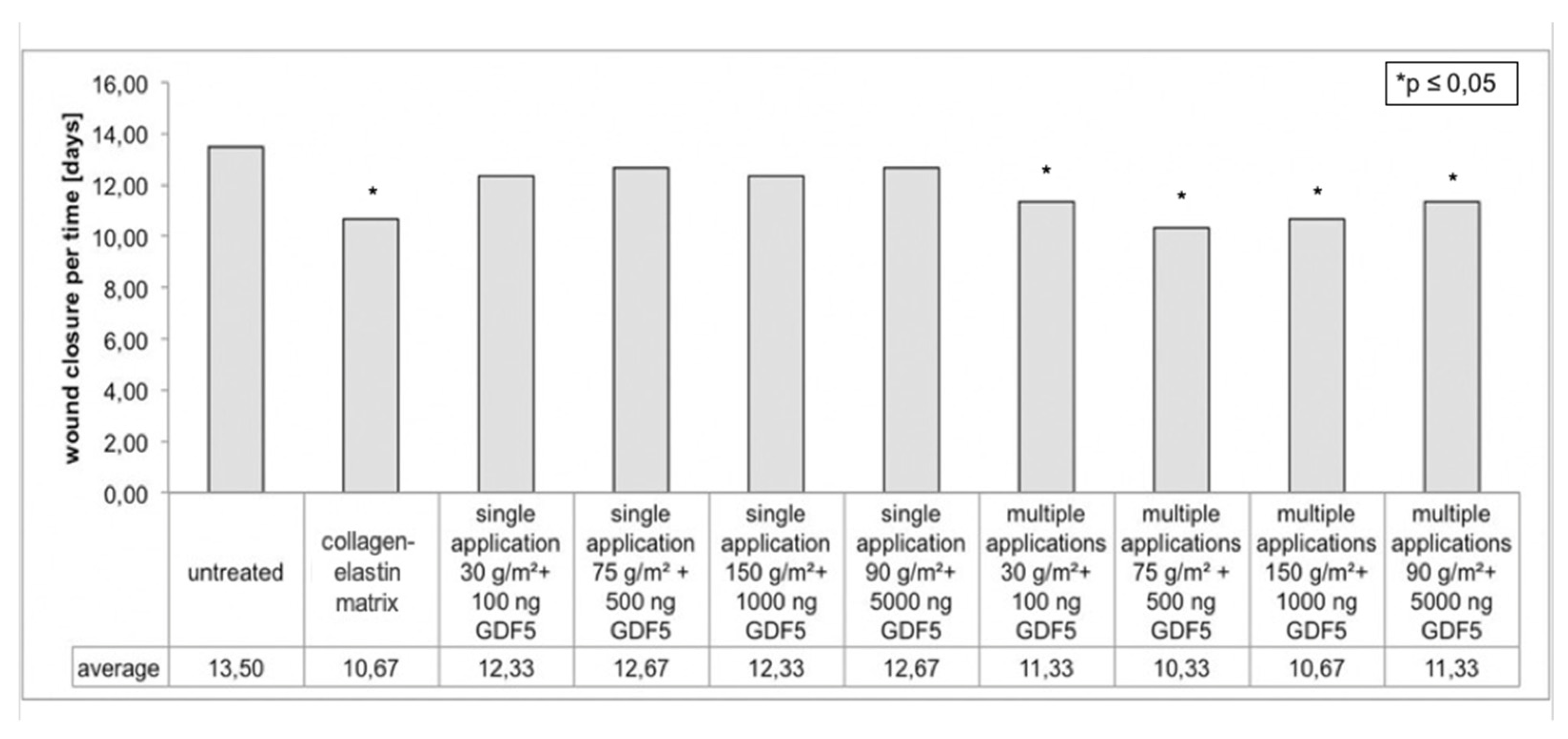
3.1. Planimetric Evaluation
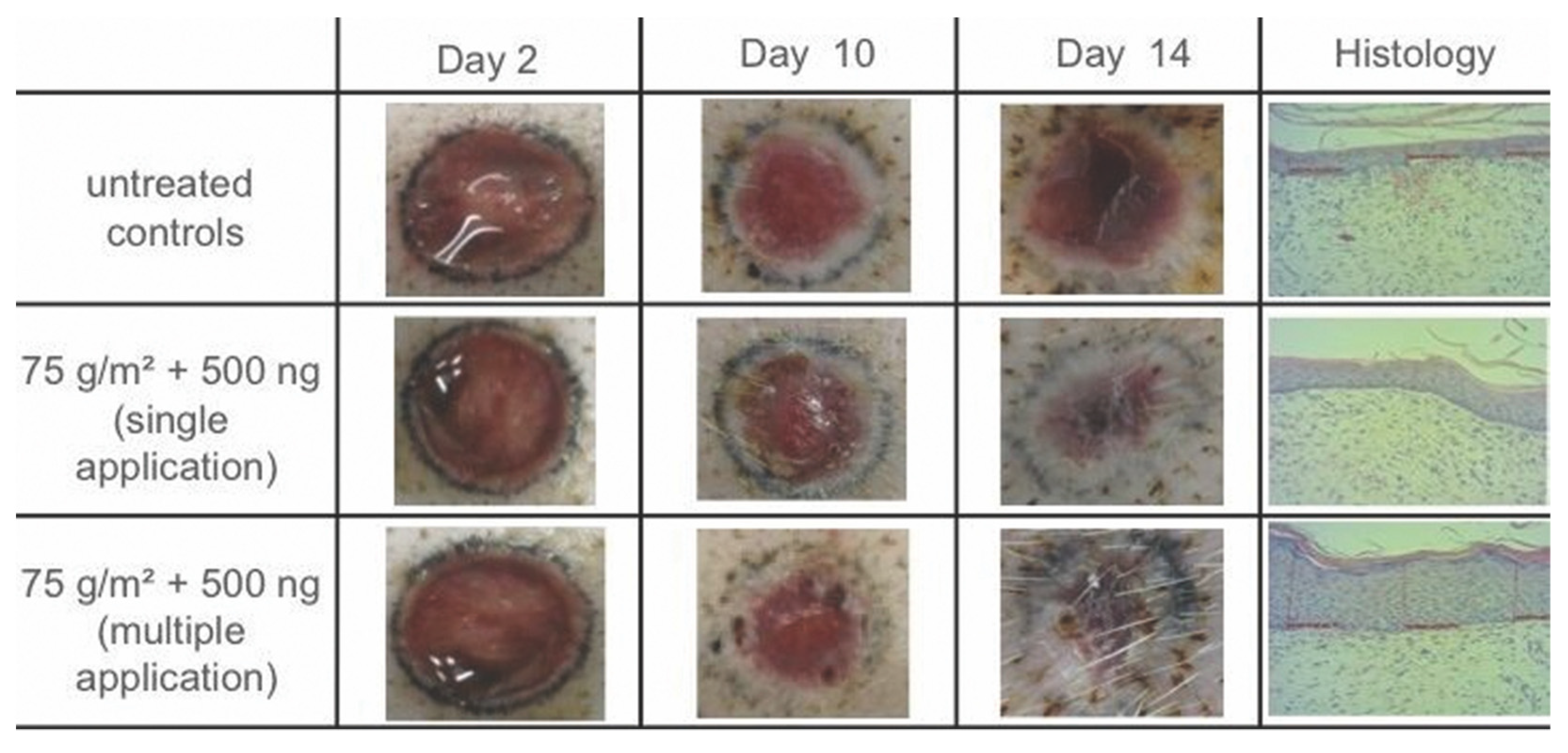
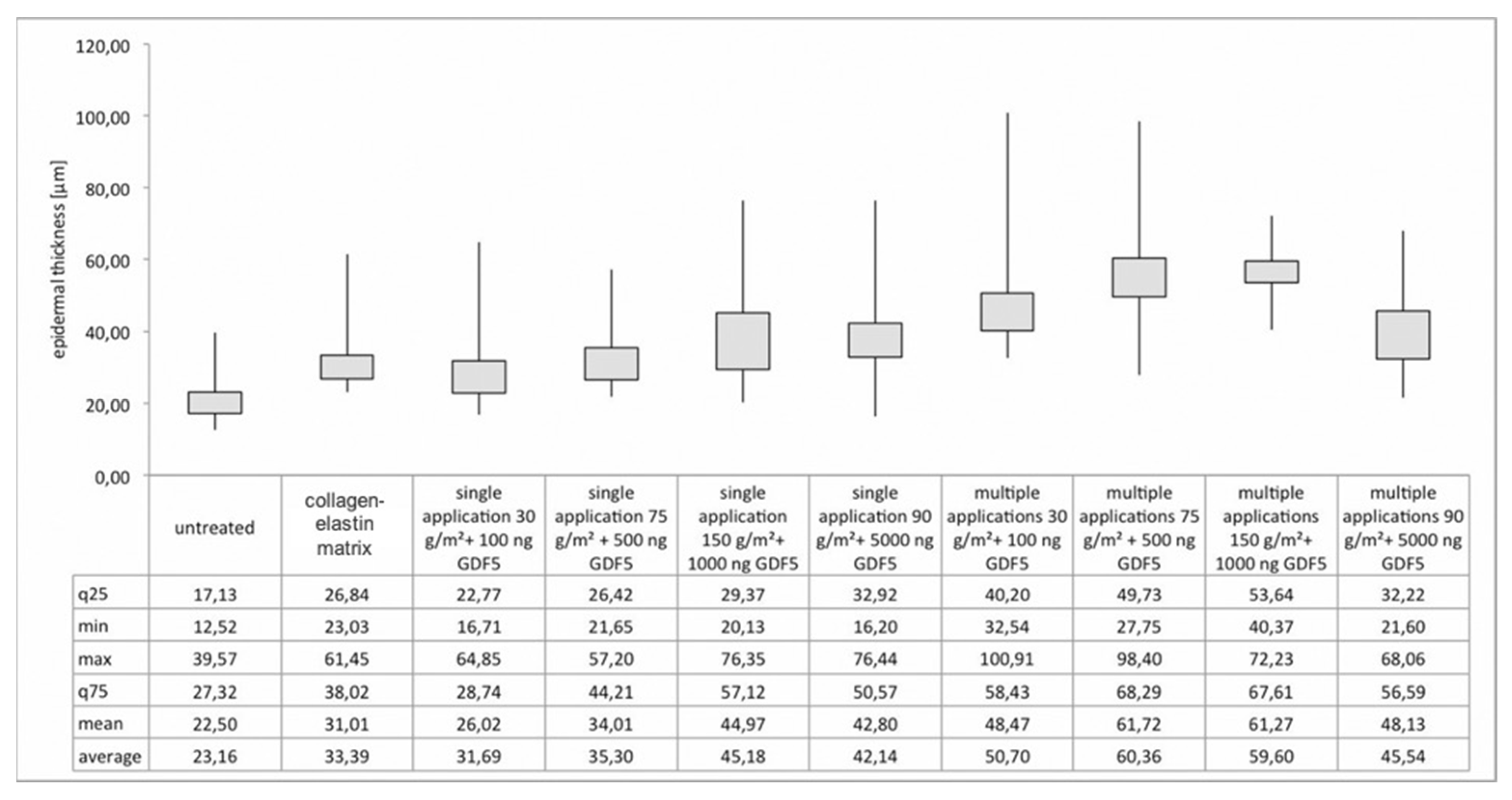
3.2. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Grose, R.; Werner, S. Wound-healing studies in transgenic and knockout mice. Mol. Biotechnol. 2004, 28, 147–166. [Google Scholar] [CrossRef]
- Kiritsy, C.P.; Lynch, A.B.; Lynch, S.E. Role of growth factors in cutaneous wound healing: A review. Crit. Rev. Oral Biol. Med. 1993, 4, 729–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koveker, G.B. Growth factors in clinical practice. Int. J. Clin. Pract. 2000, 54, 590–593. [Google Scholar]
- Lawrence, W.T.; Diegelmann, R.F. Growth factors in wound healing. Clin. Dermatol. 1994, 12, 157–169. [Google Scholar] [CrossRef]
- Coerper, S.; Koveker, G.; Gottwald, T.; Becker, H.D. Cutaneous wound healing: Clinical evaluation of locally active growth factors-an overview. Zentralbl. Chir. 1996, 121, 49–51. [Google Scholar] [PubMed]
- Scheid, A.; Meuli, M.; Gassmann, M.; Wenger, R.H. Genetically modified mouse models in studies on cutaneous wound healing. Exp. Physiol. 2000, 85, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Sena, K.; Sumner, D.R.; Virdi, A.S. Modulation of VEGF expression in rat bone marrow stromal cells by GDF-5. Connect. Tissue Res. 2007, 48, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Plichta, J.K.; Radek, K.A. Sugar-coating wound repair: A review of FGF-10 and dermatan sulfate in wound healing and their potential application in burn wounds. J. Burn Care Res. 2012, 33, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Li, X.; Beck, G.; Balian, G.; Shen, F.H. Growth and differentiation factor-5 (GDF-5) stimulates osteogenic differentiation and increases vascular endothelial growth factor (VEGF) levels in fat-derived stromal cells in vitro. Bone 2007, 40, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Dines, J.S.; Cross, M.B.; Dines, D.; Pantazopoulos, C.; Kim, H.J.; Razzano, P.; Grande, D. In vitro analysis of an rhGDF-5 suture coating process and the effects of rhGDF-5 on rat tendon fibroblasts. Growth Factors 2011, 29, 1–7. [Google Scholar] [CrossRef]
- Forslund, C.; Rueger, D.; Aspenberg, P. A comparative dose-response study of cartilage-derived morphogenetic protein (CDMP)-1, -2 and -3 for tendon healing in rats. J. Orthop. Res. 2003, 21, 617–621. [Google Scholar] [CrossRef]
- Hayashi, M.; Zhao, C.; An, K.N.; Amadio, P.C. The effects of growth and differentiation factor 5 on bone marrow stromal cell transplants in an in vitro tendon healing model. J. Hand Surg. Eur. Vol. 2011, 36, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, R.; Kumbar, S.G.; Laurencin, C.T.; Balian, G.; Chhabra, A.B. Tendon tissue engineering: Adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomed. Mater. 2011, 6, 025011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Fang, L.; Li, H.; Li, X.; Cheng, B.; Sheng, Z. Adipose tissue extract enhances skin wound healing. Wound Repair Regen. 2007, 15, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, J.L.; Rath, R.; Held, M.; Petersen, W.; Werner, J.O.; Schaller, H.E.; Rahmanian-Schwarz, A. Frequent Application of the New Gelatin-Collagen Nonwoven Accelerates Wound Healing. Adv. Skin Wound Care 2016, 29, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Papini, R. Management of burn injuries of various depths. BMJ 2004, 329, 158–160. [Google Scholar] [CrossRef] [Green Version]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Kemp, P.D. Tissue engineering and cell-populated collagen matrices. Methods Mol. Biol. 2009, 522, 363–370. [Google Scholar]
- Yamashita, H.; Shimizu, A.; Kato, M.; Nishitoh, H.; Ichijo, H.; Hanyu, A.; Morita, I.; Kimura, M.; Makishima, F.; Miyazono, K. Growth/differentiation factor-5 induces angiogenesis in vivo. Exp. Cell Res. 1997, 235, 218–226. [Google Scholar] [CrossRef]
- Koria, P. Delivery of growth factors for tissue regeneration and wound healing. BioDrugs 2012, 26, 163–175. [Google Scholar] [CrossRef]
- Held, M.; Rahmanian-Schwarz, A.; Schiefer, J.; Rath, R.; Werner, J.O.; Rahmanian, S.; Schaller, H.E.; Petersen, W. A Novel Collagen-Gelatin Scaffold for the Treatment of Deep Dermal Wounds-An Evaluation in a Minipig Model. Dermatol. Surg. 2016, 42, 751–756. [Google Scholar] [CrossRef]
- Petersen, W.; Rahmanian-Schwarz, A.; Werner, J.O.; Schiefer, J.; Rothenberger, J.; Hubner, G.; Schaller, H.E.; Held, M. The use of collagen-based matrices in the treatment of full-thickness wounds. Burns 2016, 42, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Shores, J.T.; Gabriel, A.; Gupta, S. Skin substitutes and alternatives: A review. Adv. Skin Wound Care. 2007, 20, 493–508; quiz 509–510. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, J.L.; Held, M.; Fuchs, P.C.; Demir, E.; Ploger, F.; Schaller, H.E.; Rahmanian-Schwarz, A. Growth Differentiation Factor 5 Accelerates Wound Closure and Improves Skin Quality During Repair of Full-Thickness Skin Defects. Adv. Skin Wound Care. 2017, 30, 223–229. [Google Scholar] [CrossRef]
- Paul, S.P.; Blaikley, S.; Chinthapalli, R. Clinical update: Febrile convulsion in childhood. Community Pract. 2012, 85, 36–38. [Google Scholar] [PubMed]
- Graves, R.C.; Oehler, K.; Tingle, L.E. Febrile seizures: Risks, evaluation, and prognosis. Am. Fam. Phys. 2012, 85, 149–153. [Google Scholar]
- Auvin, S.; Desnous, B.; Bellavoine, V.; Gressens, P.; Boespflug-Tanguy, O. Febrile seizure: Underlying mechanisms, consequences and management. Arch Pediatr. 2010, 17, 686–687. [Google Scholar] [CrossRef]
- Fetveit, A. Assessment of febrile seizures in children. Eur. J. Pediatr. 2008, 167, 17–27. [Google Scholar] [CrossRef]
- Millar, J.S. Evaluation and treatment of the child with febrile seizure. Am. Fam. Phys. 2006, 73, 1761–1764. [Google Scholar]
- Kolokythas, P.; Aust, M.C.; Vogt, P.M.; Paulsen, F. Dermal subsitute with the collagen-elastin matrix Matriderm in burn injuries: A comprehensive review. Handchir. Mikrochir. Plast Chir. 2008, 40, 367–371. [Google Scholar] [CrossRef]
- Harvey, E.J.; Berger, R.A.; Osterman, A.L.; Fernandez, D.L.; Weiss, A.P. Bone-tissue-bone repairs for scapholunate dissociation. J. Hand Surg. Am. 2007, 32, 256–264. [Google Scholar] [CrossRef]
- Muller, M.; Reik, M.; Sauerbier, M.; Germann, G. A new bone-ligament-bone autograft from the plantar plates of the toes and its potential use in scapholunate reconstruction: An anatomical study. Ann. Plast. Surg. 2008, 61, 463–467. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, S.H.; Oh, G.J.; Roh, S.G.; Lee, N.H.; Yang, K.M. Management of defects on lower extremities with the use of matriderm and skin graft. Arch. Plast. Surg. 2014, 41, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Min, J.H.; Yun, I.S.; Lew, D.H.; Roh, T.S.; Lee, W.J. The use of matriderm and autologous skin graft in the treatment of full thickness skin defects. Arch. Plast. Surg. 2014, 41, 330–336. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisler, W.; Held, M.; Rahmanian-Schwarz, A.; Schiefer, J.; Rahmanian, S.; Daigeler, A.; Baur, J.-O. The Topical Effect of rhGDF-5 Embedded in a Collagen–Gelatin Scaffold for Accelerated Wound Healing. Appl. Sci. 2022, 12, 867. https://doi.org/10.3390/app12020867
Eisler W, Held M, Rahmanian-Schwarz A, Schiefer J, Rahmanian S, Daigeler A, Baur J-O. The Topical Effect of rhGDF-5 Embedded in a Collagen–Gelatin Scaffold for Accelerated Wound Healing. Applied Sciences. 2022; 12(2):867. https://doi.org/10.3390/app12020867
Chicago/Turabian StyleEisler, Wiebke, Manuel Held, Afshin Rahmanian-Schwarz, Jennifer Schiefer, Shahab Rahmanian, Adrien Daigeler, and Jan-Ole Baur. 2022. "The Topical Effect of rhGDF-5 Embedded in a Collagen–Gelatin Scaffold for Accelerated Wound Healing" Applied Sciences 12, no. 2: 867. https://doi.org/10.3390/app12020867
APA StyleEisler, W., Held, M., Rahmanian-Schwarz, A., Schiefer, J., Rahmanian, S., Daigeler, A., & Baur, J.-O. (2022). The Topical Effect of rhGDF-5 Embedded in a Collagen–Gelatin Scaffold for Accelerated Wound Healing. Applied Sciences, 12(2), 867. https://doi.org/10.3390/app12020867





