Abstract
Neuromodulators at the periphery, such as neuromuscular electrical stimulation (NMES), have been developed as add-on tools to regain upper extremity (UE) paresis after stroke, but this recovery has often been limited. To overcome these limits, novel strategies to enhance neural reorganization and functional recovery are needed. This review aims to discuss possible strategies for enhancing the benefits of NMES. To date, NMES studies have involved some therapeutic concerns that have been addressed under various conditions, such as the time of post-stroke and stroke severity and/or with heterogeneous stimulation parameters, such as target muscles, doses or durations of treatment and outcome measures. We began by identifying factors sensitive to NMES benefits among heterogeneous conditions and parameters, based on the “progress rate (PR)”, defined as the gains in UE function scores per intervention duration. Our analysis disclosed that the benefits might be affected by the target muscles, stroke severity and time period after stroke. Likewise, repetitive peripheral neuromuscular magnetic stimulation (rPMS) is expected to facilitate motor recovery, as already demonstrated by a successful study. In parallel, our efforts should be devoted to further understanding the precise neural mechanism of how neuromodulators make UE function recovery occur, thereby leading to overcoming the limits. In this study, we discuss the possible neural mechanisms.
1. Introduction
Stroke is a leading cause of disability, with a greater incidence in older age groups. Poststroke disabilities affect upper extremity (UE) function. More severe UE paresis in patients with stroke more profoundly impairs the performance of daily living activities. Full recovery from UE paresis for all survivors is the ultimate goal during rehabilitation intervention. Neuromodulators at the periphery, such as neuromuscular electrical stimulation (NMES), or neuromodulators over the skull, such as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current electrical stimulation (t-DCS), have been proven to be useful tools for treating UE paresis after a stroke [1,2,3,4,5,6]. In this review, we focus on neuromodulators at the periphery.
We begin with some therapeutic concerns regarding NMES. Specifically, NMES studies have provided heterogeneous stimulation parameters, such as target muscles, doses or durations of treatment, as well as outcome measures and/or different conditions, such as time post-stroke, and stroke severity. To the best of our knowledge, currently, there are only a few systematic reviews and meta-analyses due to the substantial heterogeneity among the relevant studies [7,8,9]. A review demonstrated a statistically significant benefit from NMES applied within 2 months of onset [7]. Another review supported the supplementary use of NMES in the first 4 weeks [8]. A third review, by Howlett et al., demonstrated a major effect of NMES on upper-limb activity [9]. On the other hand, the superiority of NMES compared to standard care was still reportedly controversial [10], perhaps because of a lack of optimal treatment parameters for NMES application. To determine whether these factors may influence motor recovery, we focus on the “progress rate (PR)” as an index of gains, defined as the gains in UE function scores (Fugl–Meyer upper extremity scores) divided by treatment duration. These attempts may offer some clues regarding the optimal parameters and/or conditions for maximizing the NMES benefits for UE motor recovery after stroke, thereby, hopefully, overcoming the disability.
This is followed by a description of the expectations from and the possibility of repetitive peripheral neuromuscular magnetic stimulation (rPMS), which is probably comparable to the usefulness of NMES. As might be expected, a recent report indicated the effectiveness of rPMS in UE motor recovery in the acute phase of a stroke [11].
We end this article by discussing the possible mechanism through which neural reorganization occurs during the administration of NMES or rPMS to affected muscles. As another step toward overcoming motor disability after a stroke, our continuous efforts should be devoted to providing further understanding of the mechanism underlying NMES or rPMS benefits.
2. Materials and Methods
Search Strategy
The references used in this review were obtained in January 2021 by a search of MEDLINE, PubMed, Web of Science and Cochrane Library online databases. Articles published between inception and January 2021 were retrieved using the following search terms: FES, neuromuscular electrical stimulation (NMES), upper extremity, stroke (Figure 1).
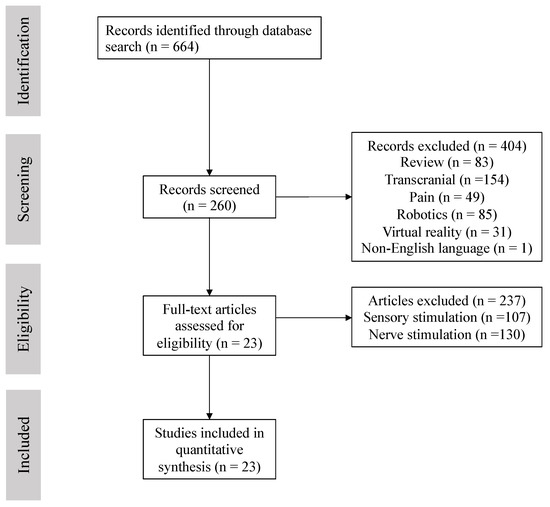
Figure 1.
Flow diagram for included studies.
Inclusion criteria were as follows: (1) patients > 18 years diagnosed with ischemic or hemorrhagic stroke, (2) first stroke onset and single lesion, (3) duration and dose described, (4) target muscles described, (5) available outcome measures of pre- and post-treatment, (6) transcutaneously non-invasive NMES. Exclusion criteria: (1) adolescents/children, (2) multifocal or recurrent stroke, (3) t-DCS or rTMS, (4) robotics, (5) virtual reality, (6) peripheral nerve stimulation (including invasive (implanted or percutaneous) stimulation and PNS at a level above the sensory threshold but below the motor threshold), (7) sensory stimulation (including somatosensory stimulation, priming, mobilization and tactile stimulation (MTS), proprioceptive stimulation, transcutaneous vague nerve stimulation, cervical spine afferent stimulation), (8) review or case studies. As a result, a total of 23 RCT trials was included (n = 833 participants) [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Seven RCT trials explored the effectiveness of NMES on UE function for acute patients (n = 250). Seven RCT trials dealt with subacute patients (n = 248), and nine RCT trails were for chronic patients (n = 335) (Table 1).

Table 1.
Summary of the included NMES studies.
Statistical analyses were performed by Kruskal–Wallis Test and Mann–Whitney U test for nonparametric data using SPSS ver. 26. Results were accepted as statistically significant at p < 0.05. Finally, the effect size (r value) of outcome measures was calculated to measure the magnitude of the treatment effect: r = Z/√N, where N is the total number of samples. r values of 0.1, 0.3 and 0.5 represent small, moderate and large effect sizes, respectively.
3. Results
3.1. Heterogeneous NMES Conditions
The NMES conditions among the included studies were generally heterogeneous, such as treatment intensity, dose of intervention (30 min to 9 h per day), duration of intervention (10 days to 5 months), time since stroke (7 days to 3.49 years from onset), severity of paresis and stimulated target muscles.
3.2. Effects of Time Post-Stroke on NMES Benefits
The participants received NMES at different times following stroke, categorized by three phases of stroke: acute (<1 month from onset), subacute (>1 month – <6 months) and chronic (>6 months). The average PR was 0.951 ± 0.293 (mean ± SE) for acute patients (n = 7 studies), 0.547 ± 0.142 for subacute patients (n = 7) and 0.382 ± 0.113 for chronic patients (n = 11) (Figure 2). There was only a trend toward the usefulness of early intervention. Statistically, however, there was no significant difference among the three phases (p = 0.16 by Kruskal–Wallis Test). Furthermore, we found no correlation between time since stroke (within 1 month: 5 trials) and PR.
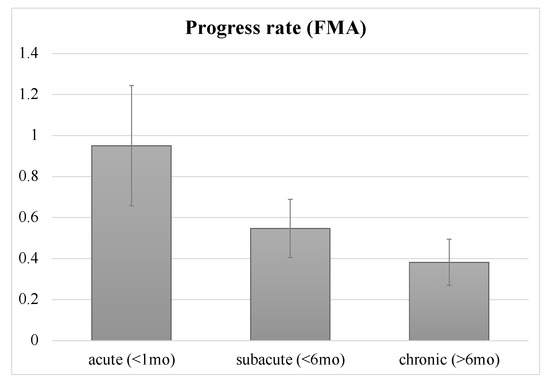
Figure 2.
Progress rate of patients at acute, subacute and chronic phases. Error bars indicate standard errors. There were no significant differences, only a trend, among the three phases. FMA: Fugl-Meyer motor assessment scale for upper extremity; mo: month.
3.3. Influence of Stroke Severity
The prognosis of motor recovery may depend on the post-stroke severity of UE paresis. Likewise, the severity of UE paresis may influence the efficacy of NMES in UE function. We investigated whether patients with relatively severe paresis would experience greater motor recovery through the application of NMES. The mean FMA pre-treatment score was 21.06. When two groups were classified based on the mean, the PR of the under-mean group (mean FMA score: 11.3 ± 1.98) was 0.94 ± 0.20 and that of the over-mean group (29.93 ± 2.39) was 0.334 ± 0.08 (Figure 3). The PR differed significantly between the groups (z = 2.614, p = 0.009). The under-mean group demonstrated a large effect size (r = 0.60) on FMA-UE, suggesting the high clinical significance of FMA-UE. This suggests that NMES might be more effective for patients with severe UE paresis than for those with moderate paresis. However, we could not find a significant correlation between FMA-UE scores at pre-treatment and PR.
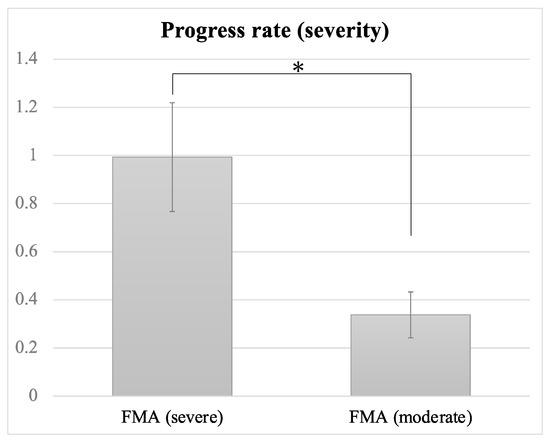
Figure 3.
Progress rates of patients with severe and moderate paresis. Error bars indicate standard errors. Significant difference is denoted by *; FMA: Fugl-Meyer motor assessment scale for upper extremity.
3.4. Target Muscles: Whole UE Versus Wrist/Finger Extensors
Most of the included studies preferred to only apply NMES to the wrist and finger extensors as the target muscles. When the preferred target muscles were the wrist and finger extensors/flexors, the PR was 0.398 ± 0.08 (mean ± SE), whereas in the case of the whole UE, including the shoulder flexors, elbow extensors/flexors, wrist extensors/flexors and finger extensors, the PR was 1.01 ± 0.27 (Figure 4). The PR of the FMA-UE scores differed significantly between the finger/wrist group and the whole-UE group (z = 2.393, p = 0.017). The whole-UE group demonstrated a large effect size (r = 0.54) on FMA-UE, suggesting the high clinical significance in FMA-UE. This finding suggests that the application of NMES to the whole UE might be advantageous compared to its application to the wrist/fingers to regain post-stroke UE function.
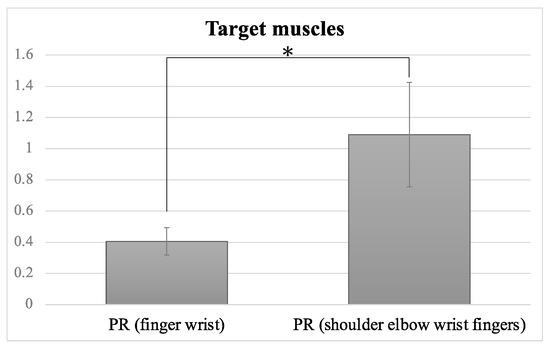
Figure 4.
Progress rates (PR) of different target muscles. Error bars indicate standard errors. There was a significant difference between the finger and wrist flexors/extensor group and the whole-UE (shoulder, elbow, wrist, fingers) group. Significant difference is denoted by *.
3.5. Influence of Dose and Duration on NMES Benefit
The length of the dose (20 min to 9 h per day) and duration (10 days to 5 months) of NMES treatment varied widely among the included studies. The mean dose was 80.68 ± 22.73 min (SE) and the mean duration was 29.15 ± 4.23 days. Unexpectedly, neither the correlation between treatment dose and PR nor between duration and PR was significant.
3.6. Sensitivity of Measure Outcomes to Motor Function
The most frequently used measure was FMA (20/23 trials) among the included studies. The Action Research Arm Test (ARAT) followed (seven trials) (Figure 5). Four studies used the box and block test (BBT) and two studies selected the Wolf motor function test (WMFT). According to data from Obayashi et al. [11,32], the FMA scores were closely associated with those of WMFT (R2 = 0.723) (Figure 6a). By contrast, both the FMA and WMFT scores showed no association with the BBT scores (Figure 6b,c).
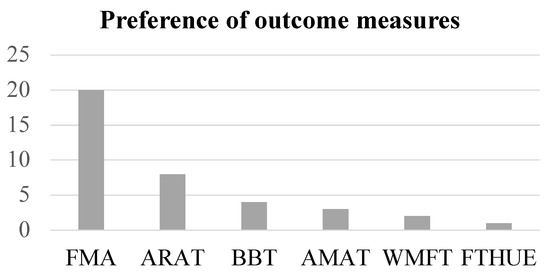
Figure 5.
The preference of outcome measures used is shown. FMA: Fugl-Meyer motor assessment scale for upper extremity; ARAT: action research arm test; BBT: box and block test; AMAT: arm motor ability test; WMFT: Wolf motor function test; FTHUE: functional test for hemiparetic upper extremity.
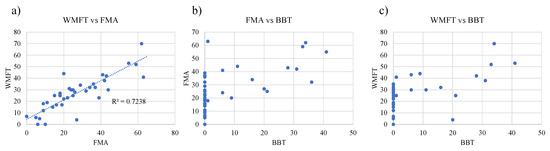
Figure 6.
The sensitivity of outcome measures to UE motor function. Comparisons of sensitivity were made among FMA, WMFT and BBT as (a) WMFT vs. FMA, (b) FAM vs. BBT, (c) WMFT vs. BBT. Note that FMA has almost the same sensitivity to severe UE motor function as WMFT, and that of FMA has a close association with WMFT, while BBT is less sensitive than FMA and WMFT.
4. Discussion
In this review, we confirmed that NMES was effective for the functional recovery of UE paresis in all phases of stroke. We identified crucial factors that influence NMES benefits, such as target muscles, severity and outcome measures. However, these factors are only the tip of the iceberg, and their identification is followed by descriptions of possible considerations likely to contribute to maximizing the effectiveness of neuromodulators in motor recovery or minimizing recovery limits.
4.1. Time to NMES following Stroke: Is Earlier Intervention More Effective?
In general, the greatest gain in recovery tends to occur immediately after stroke, with slower gains over time [35]. A previous study suggested that critical time windows exist during which the brain is more responsive to training-dependent plasticity [36]. In particular, the first month after stroke onset offers the highest rate of recovery. Another study suggested that intervention training in the early phase of stroke can promote motor improvement rapidly, with a delayed enlargement of the motor map relative to behavioral changes [37]. With regard to the effectiveness of rTMS, there may be optimal time windows for motor recovery as well. A recent study suggested the timing-dependent effectiveness of rTMS applied pos-stroke in the following descending order: acute phase > subacute phase and >chronic phase [38]. The same may be true for other neuromodulators. Therefore, we anticipated the superiority of earlier intervention with NMES over later intervention in terms of functional recovery. However, there was only a trend toward the usefulness of early intervention of NMES, but no significance (Figure 2). Furthermore, there was no correlation between the time since stroke and PR. These results may, however, be mainly due to a lack of statistical power because of the small sample size.
Interestingly, a recent report discovered the surprising role of reactive astrocytes as phagocytes in the clearance or synaptic remodeling of the penumbra area in the acute phase (from 7 days of onset) of cerebral infarct [39]. This supports the concept that early intervention may provide the advantage of motor recovery after a stroke. Accordingly, it is plausible that NMES is most effective when it is applied during the optimum therapeutic window.
4.2. Stroke Severity
Although neural reorganization occurs soon after stroke, functional recovery by conventional intervention has often been limited. The probability of motor recovery after a stroke depends on its severity, and a prognosis of functional recovery may be formed within 12 weeks of stroke onset [40]. Neurological impairments recover most dramatically within 30 days of stroke onset, but patients with moderate and severe disability recover within up to 3 and 6 months, respectively [41]. So far, the relationship between NMES benefits and severity has remained unclear. The present findings suggest that survivors of severe stroke may be more sensitive to NMES than survivors of moderate stroke, regardless of the length of time since stroke (Figure 3). On the other hand, our results suggest that there is no correlation between severity and PR; this implies the existence of a therapeutic window for NMES in terms of severity. Further study is required.
4.3. Target Muscles
Our results suggest that the application of NMES to the whole UE was more effective for the UE function of stroke survivors than its application to the wrist and finger extensors (Figure 4). These findings indicate that NMES benefits may depend upon the muscles targeted by NMES. In the included studies, the majority of NMES studies applied NMES to the wrist/ finger extensors. Some authors claimed that NMES was applied to the wrist/finger extensors because of the time required to apply the multiple electrodes to cover the whole UE. Nevertheless, our present analysis suggests that the application to the whole UE is strategically advantageous in the enlargement of ADL as well as motor recovery compared to the wrist/fingers. A recent report also supports this view [32].
4.4. Is a Higher Dose of NMES More Beneficial?
In general, higher doses and longer duration of standard care intervention may produce better functional outcomes. Typically, the efficacy of constrained-induced (CI) therapy may support this view. In fact, few studies seem to have paid attention to this consideration. Intuitively, it was expected that a higher dose of NMES is more effective for motor recovery. However, the present findings suggest that higher dose and/or longer duration may not always produce better outcomes. The dose–response relationship between NMES and UE function for stroke patients remains uncertain [19,22]. A trial by Hsu et al. compared the NMES benefits between low doses (30 min/session) and high doses (60 min/session) of NMES and reported no significant difference [19]. In a study by Page et al., three different doses (30 min, 60 min, 120 min) of NMES were applied for the chronic phase; the authors suggested that a dose of 120 min is most effective for UE function [22]. They also suggested that future studies should investigate various combinations of treatment dose administration for designing intervention programs suitable for clinical practice. In addition, a recent study demonstrated that 20 min of NMES could facilitate UE motor recovery in the acute phase of stroke [32]. It implied that, by comparison, lower doses and shorter durations of intervention ameliorate UE disability during the early acute phase of stroke. In support of this, the effective threshold of minimal dose and duration needs to be identified.
4.5. Which of the NMES Modes Is More Effective?
NMES features two modes, the EMG-triggered mode and the cyclic mode. As well as EMG-triggered NMES, the post-stroke application of cyclic NMES to UE paresis after stroke was considered effective for UE improvement [16]. Most of the included studies (21/23 trials), however, used EMG-triggered NMES; therefore, we could not compare the NMES benefits between the two modes, leaving us unable to address the issue of whether cyclic or EMG-triggered NMES is more effective.
EMG-triggered NMES can provide weaker contraction of target muscles in severe stroke patients than cyclic NMES, whereas survivors with moderate or mild paresis experience stronger contractions with EMG-triggered NMES than with cyclic NMES. Actually, EMG-triggered NMES for severe paretic patients cannot enhance the fully volitional contraction of target muscles. It is possible that insufficient muscle contraction by electrical stimulation may be nonproductive, resulting in unsatisfactory benefits from NMES. NMES might be more effective for motor recovery when it is appropriately decided which mode of NMES is applied to each of the paretic muscles, including the shoulder, elbow, wrist and fingers during the early acute phase of stroke. The selection of mode may depend upon the volitional contraction of each of the paretic muscles. Accordingly, a recent study examined the effect of coupled cyclic and EMG-triggered NMES with whole UE on motor function [32]. In other words, EMG-triggered NMES was applied to each of the relatively moderate paretic muscles (stroke impairment assessment set (SIAS) level 3), while cyclic NMES was applied to severe paretic muscles (SIAS 1–2). The results suggested that the PR of the FMA-UE scores differed significantly between the coupled NMES group and the standard care group (2.54 for the coupled NMES group and 1.10 for the standard care group) (p = 0.036). The NMES group demonstrated a large effect size (r = 0.50) on FMA-UE, suggesting the high clinical significance of FMA-UE. These findings suggest that a new strategy of coupled NMES, depending on the severity of targeted paretic muscles, might be more effective than the application of the alternative mode.
4.6. Sensitivity of Measure Outcomes to Motor Function
The sensitivity of outcome measures for motor function is a seemingly important factor in determining whether NMES is effective for UE function. Which of the outcome measures are the most sensitive to changes in UE function? It is likely that FMA-UE features almost the same sensitivity to severe UE paresis as WMFT-FAS (Figure 6a). BBT seemed less sensitive to UE function than FMA and WMlFT (Figure 6b,c). More specifically, BBT marked zero even when the FMA scores and WMFT were estimated as 40 and 35, respectively. Accordingly, it is plausible that the included studies used FMA most frequently as outcome measures for motor recovery when applying NMES. It is notable, however, that full FMA-UE scores do not mean full recovery from paresis. For patients with mild paresis, FMA is saturated. Instead, BBT is available. This means that the sensitivity and suitability of outcome measures may depend on stroke severity. In the near future, a new universal outcome measure, independent of the severity of UE paresis, is expected to be established.
4.7. Possibility of rPMS for Recovery from UE Paresis
The use of rPMS can be characterized by penetration into deeper regions of muscles without pain. Given that peripheral stimulation for affected muscles, such as NMES, can enhance motor recovery, rPMS could improve the motor function of UE paresis. So far, however, there have been no studies to support the effectiveness of rPMS on upper extremity paresis despite some efforts using various parameters [42,43,44,45,46,47]. Recently, a study reported the effectiveness of rPMS in UE motor function recovery when applied to affected UE muscles (shoulder, elbow, wrist, fingers) in the early acute phase of stroke (mean stroke duration of 9.2 days) [11]. The PR of the FMA-UE scores after 7.8 session-rPMS treatment (n = 10; mean scores 14.6/66 before intervention) significantly differed from the standard care (SC) group (n = 9; mean scores 19.0/66 before treatment), i.e., 2.65 for the rPMS group and 1.10 for the SC group, respectively (p = 0.003). The rPMS group demonstrated a large effect size (r = 0.68) on FMA-UE, suggesting high clinical significance in FMA-UE.
The advantages and disadvantages of rPMS have been summarized [48]. The authors of this summary pointed out that the advantages of rPMS over NMES were the absence of pain, deeper penetration, the generation of higher muscle torque and its applicability to children, while the disadvantages were the overheating of the coil and the exposure of a larger area stimulated with increased intensity. So far, however, no recommendations have been provided regarding the parameters of rPMS application, such as coil design, duty cycle, duration, frequency and intensity.
4.8. Effects of Neuromodulators: Long-Term and Long-Lasting?
When applied over motor cortical areas to treat UE paresis, rTMS and t-DCS are both expected to be potential tools for improving motor function after stroke by modulating cortical excitability [1,2,3,4]. Both modulators not only feature similar concerns as NMES, but also create two other issues that must be raised. One is that rTMS and/or t-DCS have been proven to produce a short-term effect on UE function after stroke in the acute, subacute or chronic phases, but it still remains unknown whether the beneficial effect would be long-lasting. The other is that it is unclear whether rTMS and/or t-DCS would lead to greater motor recovery following more treatment sessions. A recent review addressed this issue by comparing the short-term effects of rTMS on motor recovery after stroke with the long-term effects [38]. The review suggested the session-number-dependent effect of rTMS on UE paresis recovery after stroke but that increasing the session number to five produced the most benefits before a plateau is reached. Subsequently, the therapeutic effect was rapidly lost after the use of more than 15 sessions. Similarly, after the initial five sessions of t-DCS were administrated to chronic stroke patients, the more sessions of t-DCS were performed, the less effective they became over time [49]. It remains unclear how or why this phenomenon occurs. In spite of our efforts, we have not found any studies that address the above issues concerning NMES and rPMS. It is likely that ceiling effects of NMES and rPMS may also exist. Further study is required.
4.9. Other Possible Factors: Gender Difference
Some reports have pointed out the gender difference in functional outcomes after a stroke [50], as well as the influence of gender on functional reorganization after a stroke [51]. The former suggested that females experienced worse functional recovery than males. Furthermore, gender difference in functional outcome was significantly modified by stroke severity, with the differences being prominent for mild and moderate but not for severe stroke. The latter study estimated gender differences in brain excitability in the acute phase of stroke, demonstrating gender differences in the functional asymmetry of inter-hemispheric excitability in an opposite manner. Therefore, it is plausible that the beneficial effect of NMES or other neuromodulations, such as rTMS and t-DCS, on motor recovery might be influenced by gender difference. However, to the best of our knowledge, there no study has focused on gender difference in terms of the benefits of neuromodulation. Further studies are required.
4.10. Neural Basis for the Benefits from Neuromodulators
There is increasing evidence that motor recovery after stroke is associated with reorganization of the damaged brain [52,53]. Specifically, Nudo et al. demonstrated that, during intensive rehabilitative training, monkeys recovered from UE paresis after infarction in association with enlargement of the motor map representing the disabled forearm [52].
How do neuromodulators at the periphery regain motor recovery? It is speculated that the administration of NMES or rPMS to affected UE muscles could induce cortical reorganization of the affected hemisphere [23,45,54,55]. Some studies have proposed the possible neural mechanism underlying UE function recovery mediated by NMES. A near-infrared spectroscopy study suggested the association of the ipsilateral sensorimotor cortex with the improvement in UE function by EMG-triggered NMES in chronic-phase stroke patients [23]. Seventeen patients in the chronic phase of stroke underwent NMES applied to affected UE once or twice a week for 5 months. After this period, UE function improved and hemodynamic responses in the sensory-motor cortex (SMC), measured by NIRS, were altered to be bilaterally activated in a hemisphere-dominant way during affected wrist/finger extension with EMG-triggered NMES relative to voluntary of wrist/finger extension. This activation shift to the affected SMC may be related to cortical plasticity induced by NMES, in conjunction with motor recovery. This view is supported by a previous study [53] (Figure 7). Another neuroimaging study demonstrated that the contralateral SMC was activated when administering NMES to the wrist extensor and flexor muscles [54]. In addition, a previous animal study addressed the effect of forepaw electrical stimulation during a 90 min period of right middle cerebral artery (MCA) occlusion on neurological and tissue outcomes in a rat model of reversible focal forebrain ischemia [56]. The cortical and striatal infarct volume were both significantly reduced in the group with stimulated forepaw contralateral to the occlusion relative to the ipsilateral stimulated group (48% total reduction). This suggested the neuroprotective effect of peripheral electrical stimulation contralateral to the ischemic hemisphere in the rat ischemic model. It remains unknown how this protection takes place, but it is most likely related to the contribution of NMES to neuronal reorganization. Altogether, NMES could produce cortical reorganization associated with motor recovery.
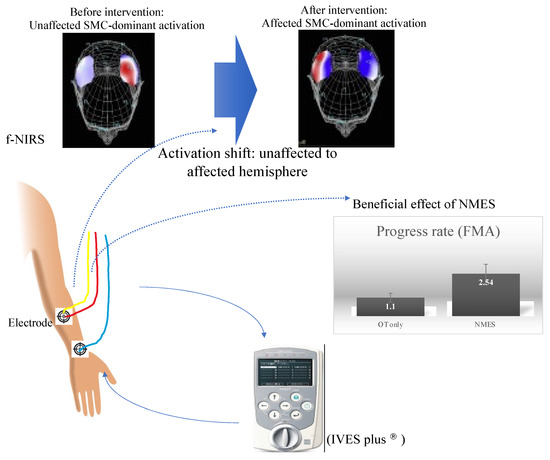
Figure 7.
NMES setting (placement of electrodes applied to FDS) and neural basis underlying the beneficial effect of NMES on motor recovery. The scheme illustrates that NMES, when applied to affected forearm muscles, can facilitate motor recovery as shown by augmented PR and subsequently generate cortical reorganization of affected SMC activation, revealed by f-NIRS. FDS: flexor digitorum superficialis; SMC: sensory-motor cortex.
A previous PET activation study [45] delineated the reorganization of the motor map induced by rPMS. The authors demonstrated that regional cerebral blood flow (rCBF) increases in the superior posterior parietal cortex and that the premotor cortex is associated with an improvement in spasticity in the paretic arm following rPMS treatment. The use of rPMS might produce cortical neuroplastic changes in conjunction with motor recovery.
Another study of corticospinal excitability tested by means of transcranial magnetic stimulation demonstrated that rPMS generated an increase in motor-evoked potential (MEP) amplitude and a decrease in MEP latency [55]. In other words, higher MEP amplitudes represent the recruitment of a larger volume of residual pyramidal neurons in the M1 area after stroke [57]. Shorter MEP latencies can be interpreted as two potential changes: (1) better synchronicity of multiple descending volleys (I-wave) arising from rPMS, thus leading to a more efficient depolarization of spinal motor neurons [58] and (2) better recruitment of short-latency corticocortical projections from premotor areas to primary motor cortices (area 4) [59]. In other words, rPMS-induced proprioceptive inflows can be conveyed to the affected hemisphere mediated by thalamocortical and corticocortical fibers, resulting in the potentially enhanced synaptic connectivity of premotor and residual M1 cells [44].
4.11. Limitations
To date, NMES studies have included several considerations. Because of the small sample size, the results should be interpreted with caution. We need to accumulate a larger sample size to provide stronger evidence. Further study exploring the causal relationship between motor recovery and factors such as time to intervention, intensity, dose, duration, stroke severity, or gender are required. Furthermore, we need to investigate whether the long-term effects of NMES or rPMS differ from short-term effects, or how long NMES or rPMS benefits endure. At least, both responders and non-responders to NMES or rPMS application seemed to exist in our study. Some participants showed a prominently accelerated PR not only in the NMES intervention group but also in the standard care group. Understanding fully what causes patients to exhibit different responses to NMES would be valuable; this knowledge might provide a crucial clue as to how to overcome motor recovery limits.
5. Conclusions
This review identified factors influencing the benefits of NMES, such as target muscles, stroke severity and time post-stroke. In addition, other factors, such as dose, duration and NMES modes need to be considered to optimize the NMES effects. Further exploration of specific treatment parameters is required to establish a definitive approach to the improvement of severe UE paresis immediately after stroke onset. Simultaneously, we need to further understand the precise neural mechanisms underlying how neuromodulators act beneficially in motor recovery. Such a comprehensive approach should be integrated to produce new strategies, resulting in the overcoming of recovery limits.
Author Contributions
The review was conceptualized by S.O. The literature search and data collection were performed by S.O. and H.S. The manuscript was written by S.O. Funding acquisition by S.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Grants-in-Aid for Scientific Research, Japan Society for the Promotion of Science (JSPS), grant number 19K11355.
Institutional Review Board Statement
This study was approved by our institutional ethics committee (Registered number: 1831).
Informed Consent Statement
All participants provided written informed consent, and all procedures performed in this study complied with the National Ethical Guidelines for Medical and Health Research involving Human Participants and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank the co-medical staff, Rina Takahashi, Mitsugu Onuki, Suzuka Homma, Sumika Hirono, Misa Kumagai, Yosuke Hamaya, in our acute rehabilitation care unit for their cooperation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hsu, W.Y.; Cheng, C.H.; Liao, K.K.; Lee, I.H.; Lin, Y.Y. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: A meta-analysis. Stroke 2012, 43, 1849–1857. [Google Scholar] [CrossRef] [Green Version]
- Le, Q.; Qu, Y.; Tao, Y.; Zhu, S. Effects of repetitive transcranial magnetic stimulation on hand function recovery and excitability of the motor cortex after stroke: A meta-analysis. Am. J. Phys. Med. Rehabil. 2014, 93, 422–430. [Google Scholar] [CrossRef]
- Hummel, F.; Celnik, P.; Giraux, P.; Floel, A.; Wu, W.H.; Gerloff, C.; Cohen, L.G. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 2005, 128, 490–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlaug, G.; Renga, V.; Nair, D. Transcranial direct current stimulation in stroke recovery. Arch. Neurol. 2008, 65, 1571–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daly, J.J.; Hogan, N.; Perepezko, E.M.; Krebs, H.I.; Rogers, J.M.; Goyal, K.S.; Dohring, M.E.; Fredrickson, E.; Nethery, J.; Ruff, R.L. Response to upper-limb robotics and functional neuromuscular stimulation following stroke. J. Rehabil. Res. Dev. 2005, 42, 723–736. [Google Scholar] [CrossRef]
- Popovic, D.B.; Popovic, M.B.; Sinkjaer, T. Neurorehabilitation of upper extremities in humans with sensory-motor impairment. Neuromodulation 2002, 5, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Eraifej, J.; Clark, W.; France, B.; Desando, S.; Moore, D. Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: A systematic review and meta-analysis. Syst. Rev. 2017, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Wattchow, K.A.; McDonnell, M.N.; Hillier, S.L. Rehabilitation Interventions for Upper Limb Function in the First Four Weeks Following Stroke: A Systematic Review and Meta-Analysis of the Evidence. Arch. Phys. Med. Rehabil. 2018, 99, 367–382. [Google Scholar] [CrossRef]
- Howlett, O.A.; Lannin, N.A.; Ada, L.; McKinstry, C. Functional electrical stimulation improves activity after stroke: A systematic review with meta-analysis. Arch. Phys. Med. Rehabil. 2015, 96, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Whitall, J. Stroke rehabilitation research: Time to answer more specific questions? NeuroRehabilit. Neural Repair 2004, 18, 3–8. [Google Scholar] [CrossRef]
- Obayashi, S.; Takahashi, R. Repetitive peripheral magnetic stimulation improves severe upper limb paresis in early acute phase stroke survivors. NeuroRehabilitation 2020, 46, 569–575. [Google Scholar] [CrossRef]
- Francisco, G.; Chae, J.; Chawla, H.; Kirshblum, S.; Zorowitz, R.; Lewis, G.; Pang, S. Electromyogram-triggered neuromuscular stimulation for improving the arm function of acute stroke survivors: A randomized pilot study. Arch. Phys. Med. Rehabil. 1998, 79, 570–575. [Google Scholar] [CrossRef]
- Chae, J.; Bethoux, F.; Bohine, T.; Dobos, L.; Davis, T.; Friedl, A. Neuromuscular stimulation for upper extremity motor and functional recovery in acute hemiplegia. Stroke 1998, 29, 975–979. [Google Scholar] [CrossRef] [Green Version]
- Powell, J.; Pandyan, A.D.; Granat, M.; Cameron, M.; Stott, D.J. Electrical stimulation of wrist extensors in poststroke hemiplegia. Stroke 1999, 30, 1384–1389. [Google Scholar] [CrossRef] [Green Version]
- Cauraugh, J.; Light, K.; Kim, S.; Thigpen, M.; Behrman, A. Chronic motor dysfunction after stroke: Recovering wrist and finger extension by electromyography-triggered neuromuscular stimulation. Stroke 2000, 31, 1360–1364. [Google Scholar] [CrossRef]
- Mann, G.E.; Burridge, J.H.; Malone, L.J.; Strike, P.W. A pilot study to investigate the effects of electrical stimulation on recovery of hand function and sensation in subacute stroke patients. Neuromodulation 2005, 8, 193–202. [Google Scholar] [CrossRef]
- Thrasher, T.A.; Zivanovic, V.; McIlroy, W.; Popovic, M.R. Rehabilitation of reaching and grasping function in severe hemiplegic patients using functional electrical stimulation therapy. NeuroRehabilit. Neural Repair 2008, 22, 706–714. [Google Scholar] [CrossRef]
- Chan, M.K.; Tong, R.K.; Chung, K.Y. Bilateral upper limb training with functional electric stimulation in patients with chronic stroke. NeuroRehabilit. Neural Repair 2009, 23, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.S.; Hu, M.H.; Wang, Y.H.; Yip, P.K.; Chiu, J.W.; Hsieh, C.L. Dose-response relation between neuromuscular electrical stimulation and upper-extremity function in patients with stroke. Stroke 2010, 41, 821–824. [Google Scholar] [CrossRef] [Green Version]
- Yun, G.J.; Chun, M.H.; Park, J.Y.; Kim, B.R. The synergic effects of mirror therapy and neuromuscular electrical stimulation for hand function in stroke patients. Ann. Rehabil. Med. 2011, 35, 316–321. [Google Scholar] [CrossRef]
- Shindo, K.; Fujiwara, T.; Hara, J.; Oba, H.; Hotta, F.; Tsuji, T.; Hase, K.; Liu, M. Effectiveness of hybrid assistive neuromuscular dynamic stimulation therapy in patients with subacute stroke: A randomized controlled pilot trial. NeuroRehabilit. Neural Repair 2011, 25, 830–837. [Google Scholar] [CrossRef]
- Page, S.J.; Levin, L.; Hermann, V.; Dunning, K.; Levine, P. Longer versus shorter daily durations of electrical stimulation during task-specific practice in moderately impaired stroke. Arch. Phys. Med. Rehabil. 2012, 93, 200–206. [Google Scholar] [CrossRef]
- Hara, Y.; Obayashi, S.; Tsujiuchi, K.; Muraoka, Y. The effects of electromyography-controlled functional electrical stimulation on upper extremity function and cortical perfusion in stroke patients. Clin. Neurophysiol. 2013, 124, 2008–2015. [Google Scholar] [CrossRef]
- Boyaci, A.; Topuz, O.; Alkan, H.; Ozgen, M.; Sarsan, A.; Yildiz, N.; Ardic, F. Comparison of the effectiveness of active and passive neuromuscular electrical stimulation of hemiplegic upper extremities: A randomized, controlled trial. Int. J. Rehabil. Res. 2013, 36, 315–322. [Google Scholar] [CrossRef]
- McCabe, J.; Monkiewicz, M.; Holcomb, J.; Pundik, S.; Daly, J.J. Comparison of robotics, functional electrical stimulation, and motor learning methods for treatment of persistent upper extremity dysfunction after stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2015, 96, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Amasyali, S.Y.; Yaliman, A. Comparison of the effects of mirror therapy and electromyography-triggered neuromuscular stimulation on hand functions in stroke patients: A pilot study. Int. J. Rehabil. Res. 2016, 39, 302–307. [Google Scholar] [CrossRef]
- Kwakkel, G.; Winters, C.; van Wegen, E.E.; Nijland, R.H.; van Kuijk, A.A.; Visser-Meily, A.; de Groot, J.; de Vlugt, E.; Arendzen, J.H.; Geurts, A.C.; et al. Effects of Unilateral Upper Limb Training in Two Distinct Prognostic Groups Early After Stroke: The EXPLICIT-Stroke Randomized Clinical Trial. NeuroRehabilit. Neural Repair 2016, 30, 804–816. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.D.; Page, S.J.; Delahanty, M.; Knutson, J.S.; Gunzler, D.D.; Sheffler, L.R.; Chae, J. Upper-Limb Recovery After Stroke: A Randomized Controlled Trial Comparing EMG-Triggered, Cyclic, and Sensory Electrical Stimulation. NeuroRehabilit. Neural Repair 2016, 30, 978–987. [Google Scholar] [CrossRef] [Green Version]
- Carda, S.; Biasiucci, A.; Maesani, A.; Ionta, S.; Moncharmont, J.; Clarke, S.; Murray, M.M.; Millan, J.D.R. Electrically Assisted Movement Therapy in Chronic Stroke Patients With Severe Upper Limb Paresis: A Pilot, Single-Blind, Randomized Crossover Study. Arch. Phys. Med. Rehabil. 2017, 98, 1628–1635.e2. [Google Scholar] [CrossRef] [Green Version]
- Jonsdottir, J.; Thorsen, R.; Aprile, I.; Galeri, S.; Spannocchi, G.; Beghi, E.; Bianchi, E.; Montesano, A.; Ferrarin, M. Arm rehabilitation in post stroke subjects: A randomized controlled trial on the efficacy of myoelectrically driven FES applied in a task-oriented approach. PLoS ONE 2017, 12, e0188642. [Google Scholar] [CrossRef]
- Qian, Q.; Hu, X.; Lai, Q.; Ng, S.C.; Zheng, Y.; Poon, W. Early Stroke Rehabilitation of the Upper Limb Assisted with an Electromyography-Driven Neuromuscular Electrical Stimulation-Robotic Arm. Front. Neurol. 2017, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, S.; Takahashi, R.; Onuki, M. Upper limb recovery in early acute phase stroke survivors by coupled EMG-triggered and cyclic neuromuscular electrical stimulation. NeuroRehabilitation 2020, 46, 417–422. [Google Scholar] [CrossRef]
- Lin, Z.; Yan, T. Long-term effectiveness of neuromuscular electrical stimulation for promoting motor recovery of the upper extremity after stroke. J. Rehabil. Med. 2011, 43, 506–510. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Park, J.H.; Jung, M.Y.; Yoo, E.Y. Effects of Task-Oriented Training as an Added Treatment to Electromyogram-Triggered Neuromuscular Stimulation on Upper Extremity Function in Chronic Stroke Patients. Occup. Ther. Int. 2016, 23, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Kwakkel, G.; Kollen, B.; Twisk, J. Impact of time on improvement of outcome after stroke. Stroke 2006, 37, 2348–2353. [Google Scholar] [CrossRef] [Green Version]
- Wahl, A.S.; Omlor, W.; Rubio, J.C.; Chen, J.L.; Zheng, H.; Schroter, A.; Gullo, M.; Weinmann, O.; Kobayashi, K.; Helmchen, F.; et al. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science 2014, 344, 1250–1255. [Google Scholar] [CrossRef]
- Nishibe, M.; Urban, E.T., 3rd; Barbay, S.; Nudo, R.J. Rehabilitative training promotes rapid motor recovery but delayed motor map reorganization in a rat cortical ischemic infarct model. NeuroRehabilit. Neural Repair 2015, 29, 472–482. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, G.; Fan, Y.; Guo, Z.; Chen, H.; Mu, Q. Short- and Long-term Effects of Repetitive Transcranial Magnetic Stimulation on Upper Limb Motor Function after Stroke: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2017, 31, 1137–1153. [Google Scholar] [CrossRef]
- Morizawa, Y.M.; Hirayama, Y.; Ohno, N.; Shibata, S.; Shigetomi, E.; Sui, Y.; Nabekura, J.; Sato, K.; Okajima, F.; Takebayashi, H.; et al. Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat. Commun. 2017, 8, 28. [Google Scholar] [CrossRef]
- Jorgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Vive-Larsen, J.; Stoier, M.; Olsen, T.S. Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1995, 76, 406–412. [Google Scholar] [CrossRef]
- Duncan, P.W.; Lai, S.M.; Keighley, J. Defining post-stroke recovery: Implications for design and interpretation of drug trials. Neuropharmacology 2000, 39, 835–841. [Google Scholar] [CrossRef]
- Barker, A.T. An introduction to the basic principles of magnetic nerve stimulation. J. Clin. Neurophysiol. 1991, 8, 26–37. [Google Scholar] [CrossRef]
- Krewer, C.; Hartl, S.; Muller, F.; Koenig, E. Effects of repetitive peripheral magnetic stimulation on upper-limb spasticity and impairment in patients with spastic hemiparesis: A randomized, double-blind, sham-controlled study. Arch. Phys. Med. Rehabil. 2014, 95, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.D.; Schneider, C. Effects of repetitive peripheral magnetic stimulation on normal or impaired motor control. A review. Neurophysiol. Clin. 2013, 43, 251–260. [Google Scholar] [CrossRef]
- Struppler, A.; Binkofski, F.; Angerer, B.; Bernhardt, M.; Spiegel, S.; Drzezga, A.; Bartenstein, P. A fronto-parietal network is mediating improvement of motor function related to repetitive peripheral magnetic stimulation: A PET-H2O15 study. Neuroimage 2007, 36 (Suppl. 2), T174–T186. [Google Scholar] [CrossRef] [PubMed]
- Struppler, A.; Angerer, B.; Gundisch, C.; Havel, P. Modulatory effect of repetitive peripheral magnetic stimulation on skeletal muscle tone in healthy subjects: Stabilization of the elbow joint. Exp. Brain Res. 2004, 157, 59–66. [Google Scholar] [CrossRef]
- Struppler, A.; Havel, P.; Muller-Barna, P. Facilitation of skilled finger movements by repetitive peripheral magnetic stimulation (RPMS)—A new approach in central paresis. NeuroRehabilitation 2003, 18, 69–82. [Google Scholar] [CrossRef]
- Beaulieu, L.D.; Schneider, C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: A literature review on parameters of application and afferents recruitment. Neurophysiol. Clin. 2015, 45, 223–237. [Google Scholar] [CrossRef]
- Lindenberg, R.; Zhu, L.L.; Schlaug, G. Combined central and peripheral stimulation to facilitate motor recovery after stroke: The effect of number of sessions on outcome. NeuroRehabilit. Neural Repair 2012, 26, 479–483. [Google Scholar] [CrossRef] [Green Version]
- Lisabeth, L.D.; Reeves, M.J.; Baek, J.; Skolarus, L.E.; Brown, D.L.; Zahuranec, D.B.; Smith, M.A.; Morgenstern, L.B. Factors influencing sex differences in poststroke functional outcome. Stroke 2015, 46, 860–863. [Google Scholar] [CrossRef] [Green Version]
- Di Lazzaro, V.; Pellegrino, G.; Di Pino, G.; Ranieri, F.; Lotti, F.; Florio, L.; Capone, F. Human Motor Cortex Functional Changes in Acute Stroke: Gender Effects. Front. Neurosci. 2016, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Nudo, R.J.; Wise, B.M.; SiFuentes, F.; Milliken, G.W. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 1996, 272, 1791–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grefkes, C.; Fink, G.R. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 2014, 13, 206–216. [Google Scholar] [CrossRef]
- Blickenstorfer, A.; Kleiser, R.; Keller, T.; Keisker, B.; Meyer, M.; Riener, R.; Kollias, S. Cortical and subcortical correlates of functional electrical stimulation of wrist extensor and flexor muscles revealed by fMRI. Hum. Brain Mapp. 2009, 30, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Flamand, V.H.; Schneider, C. Noninvasive and painless magnetic stimulation of nerves improved brain motor function and mobility in a cerebral palsy case. Arch. Phys. Med. Rehabil. 2014, 95, 1984–1990. [Google Scholar] [CrossRef]
- Burnett, M.G.; Shimazu, T.; Szabados, T.; Muramatsu, H.; Detre, J.A.; Greenberg, J.H. Electrical forepaw stimulation during reversible forebrain ischemia decreases infarct volume. Stroke 2006, 37, 1327–1331. [Google Scholar] [CrossRef]
- Rothwell, J.C. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J. Neurosci. Methods 1997, 74, 113–122. [Google Scholar] [CrossRef]
- Amassian, V.E.; Stewart, M.; Quirk, G.J.; Rosenthal, J.L. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery 1987, 20, 74–93. [Google Scholar] [CrossRef]
- Volz, L.J.; Hamada, M.; Rothwell, J.C.; Grefkes, C. What Makes the Muscle Twitch: Motor System Connectivity and TMS-Induced Activity. Cereb. Cortex 2015, 25, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).