Abstract
COVID-19 athletes reported persistent and residual symptoms many weeks after initial infection, including cough, fatigue, and neuromuscular disorders. Poor neuromuscular control may cause inefficient movement strategies increasing anterior cruciate ligament load. This is particularly relevant in female athletes, who show a 3-time higher risk than male counterparts. Aim is to evaluate the impairment in thigh muscles activation, body composition, and physical performance after COVID-19 in volleyball athletes. We recruited a cohort of female professional players from the same team. We assessed the pre-activation time of Rectus Femoris (RF), Vastus Medialis (VM), Medial Hamstring (MH), and Lateral Hamstring (LH) before (T0) and after (T1) COVID-19 infection, bioelectrical impedance analysis (BIA), and jump tests. We included 12 athletes with COVID-19 infection diagnosis in January 2021. At T1 we found a significant (p < 0.05) delay (ms) of the activation time of RF (426 ± 188 vs. 152 ± 106); VM (363 ± 192 vs. 140 ± 96); BF (229 ± 60 vs. 150 ± 63); MH (231 ± 88 vs. 203 ± 89), and a significant reduction of body composition at BIA. The neuromotor imbalance of the knee stabilizer muscle in female athletes after COVID-19 infection determines a deficit of knee stabilization. Physicians should consider neuromuscular and metabolic sequelae to identify athletes at higher risk of injury and set up specific neuromuscular rehabilitation protocols.
Keywords:
COVID-19; volley; sports; rehabilitation; electromyography; muscle activity; knee; body composition; ACL 1. Introduction
Anterior cruciate ligament (ACL) injury is one of the most frequent and significant sport related diseases, with a high prevalence in young and active individuals [1]. Female athletes have a 3-time greater incidence of ACL injury than male subjects [2]. This disparity in injury rates is most likely multifactorial and attributable to intrinsic factors, such as the configuration of the intercondylar notch, the loose joint, the differences in anatomic alignment and hormones influence on ligamentous tissue. Furthermore, even extrinsic factors (e.g., muscle imbalances, playing surface, use of orthoses) might play a role in the higher ACL injury risk in female athletes [3,4].
However, the insufficient neuromuscular control during dynamic movements is considered the main risk factor for ACL injury in athletes [5], with a consequent motion asymmetry and inefficient movement strategies [6]. This factor is particularly relevant in female subjects, showing typical muscular recruitment characteristics such as the preferential recruitment of the quadriceps over the hamstrings, the shorter quadriceps latency periods and an unbalanced quadriceps-to-hamstrings strength ratio [7,8,9,10]. In this context, Hewett et al. [11] highlighted the importance of a dynamic neuromuscular control of the knee to prevent ACL injuries. On the other hand, when the system is altered, the preparatory contraction of the thigh muscles might stiffen the knee joint [7,12,13,14].
Therefore, an effective training to reduce these injuries is mandatory in sports involving rapid stopping, cutting and changing of direction (i.e., soccer, basketball, and volleyball) [15]. In fact, about 70–80% of ACL injuries are the consequences of non-contact mechanism associated with landing from a jump, changing of direction or sudden deceleration [1,3,15]. ACL injury is also related to a higher risk of a knee re-injury [12] and long-term disabilities (i.e., early osteoarthritis) that should be adequately managed [16,17].
COVID-19 pandemic has recently been spreading worldwide with a negative impact on all forms of sport, especially in its first wave [18,19]. Indeed, in all the categories, the need to socially distance and stop the spread of disease meant cancellation of routine practices and competitive events [18]. However, in the slow return to normality, National governing bodies and International Federations have published specific protocols focusing on return-to-play, aimed to guarantee the safety of all sports professionals [18].
Despite COVID-19 clinical manifestations are mainly respiratory, major cardiac complications have been also reported, leading to acute myocarditis [20,21,22,23]. Moreover, COVID-19 positive athletes might present persistent and residual symptoms many weeks to months after initial infection, including cough, tachycardia, extreme fatigue and neuromuscular disorders [24,25]. Lastly, COVID-19 could be responsible for a decline of efficiency of neuromuscular system, changes of body mass and composition and a consequent loss in terms of performance and endurance with a higher risk of injury [5].
A neuromuscular involvement in COVID-19 patients has been widely described in literature, due to the presence of impairments of peripheral nerves, neuromuscular junction, muscles and cranial nerves [26]. As highlighted by Huang et colleagues in a cohort of 2469 patients, even 6 months after acute COVID-19 infection, 63% of the subjects showed fatigue or muscle weakness [27]. Recently, a systematic review and metanalysis focused on neurological manifestations in COVID-19 infection showing that myalgia was the most frequent neurological symptom in patients with COVID-19 infection, suggesting a possible deep impact of the disease on the neuromuscular system [28]. Furthermore, an observational study on 214 COVID-19 patients reported peripheral nervous system involvement in up to 9% of patients [29]. Although the association between COVID-19 and some peripheral nerve disorders such as Guillain-Barré syndrome has been debated [30], a link between COVID-19 and peripheral nerve disorders has been widely suggested [31]. At least one third of the COVID-19 patients showed elevation of creatine kinase levels and COVID-19 associated myopathies are reported in literature [32]. Early reports showed considerable musculoskeletal sequelae in COVID-19 patients [33] and electromyography (EMG) studies showed various combinations of neuropathic or myopathic changes [34].
On the other hand, peripheral nerve injury developed after COVID-19 could depend on “a molecular mimicry” mechanism. The transcriptome of SARS-CoV-2 presents molecular similarities with several human protein epitopes, causing a cytokine storm and various autoantibodies, potentially culminating in an autoimmune state leading to an aberrant attack on healthy host tissue. The autoimmune cascade caused by COVID-19 may occur through multiple pathways including molecular mimicry, epitope spreading, bystander activation, autoantibodies production and increase of effector B-cells [35].
Moreover, Anker et al. [36] reported that patients with COVID-19 are prone to develop significant weight loss that could affect most of all muscle mass. More in detail, weight loss might be caused by a massive inflammatory reaction that disturbs tissue homeostasis and is boosted by concomitant malnutrition and immobilization [36].
Thus, it is reasonable to hypothesize that COVID-19 could have a negative influence on the neuromuscular system, body composition and performance increasing the injury risk and decreasing the performance in team sports [37,38].
In this context, surface EMG (sEMG) integrated with inertial measurement unit (IMU) provides an insight into how the neuromuscular system behaves [39]. More in detail, it is possible to determine the timing of muscles excitation, describing when a muscle “turns on” [39,40] and to record muscle activity of the quadriceps and hamstrings to determine the recruitment pattern and the time of pre-activation as the time of initial contraction in preparation of landing [41,42]. Moreover, bioelectrical impedance analysis (BIA) represents an accurate method in the assessment of athletes body composition when compared with reference techniques (dual X–ray absorptiometry (DXA)) [43].
Therefore, the present observational study aims to characterize the impairment of thigh muscles neuromuscular activation, muscle mass and physical performance in female volley athletes after COVID-19 infection.
2. Materials and Methods
2.1. Participants
We retrospectively assessed medical records of professional Southern Italy team female volleyball players team participating to the 2020–2021 University Sport Screening Program for ACL injury performed by the Physical and Rehabilitative Medicine Unit of the University “Magna Graecia”, Catanzaro, Italy. We included elite athletes aged more than 18 years, with a 5-year experience in the young leagues; d) diagnosis of COVID-19 in the same period (January 2021). Exclusion criteria were previous (two years) surgery at lower extremity; lower limb pathologies or unsolved musculoskeletal syndromes with direct consequences on sports participation; participation in ACL exercise prevention program; d) history of previous neuromuscular disorders. Subjects were a sked to read and sign an informed consent.
All researchers involved were educated in protecting the privacy of the participants. This study was accepted by the local Institutional Review Board (61/10 p. 392), respecting the Declaration of Helsinki and the ethical guidelines of the re-sponsible governmental organization and.
2.2. Outcome Measures
The primary outcome was the pre-activation time of the knee stabilizer muscles of the dominant leg: rectus femoris (RF), vastus medialis (VM), biceps femoris (BF), and medial hamstrings (MH) were evaluated through sEMG. We placed the electrodes on the dominant leg [41,42], according to the recommendations of the sEMG recommendations for Not-Invasive Assessment of Muscles (SENIAM) [44]. The knee stabilizer muscles’ time of pre-activation is computed as the interval of time between the beginning of muscle activation and the contact with the floor, evaluated by sEMG (FREE EMG 1000; BTS Bioengineering Spa, Garbagnate M.se-Milano, ITA), through 80 mm bipolar surface electrodes (Ambu, Neuroline, Ballerup, Denmark) [45]. Raw sEMG sample recording frequency was 1000 Hz. An IMU (G-sensor, BTS Bioengineering Spa, Garbagnate M.se-Milano, ITA) provided kinematic data. We positioned the elastic band containing the IMU on lumbar vertebrae (L5). IMU signal provided the exact time of floor contact that we used as reference to calculate muscle pre-activation. These signals were elaborated using sEMG Analyzer software (BTS Bioengineering Spa, Garbagnate M.se-Milano, ITA) through the “drop fall” protocol [46]. Each athlete was asked to move vertically, without controlling the land, from a 32 cm platform. All these evaluations were performed by a physician with a 5-year expertise in sEMG measurement (Figure 1).
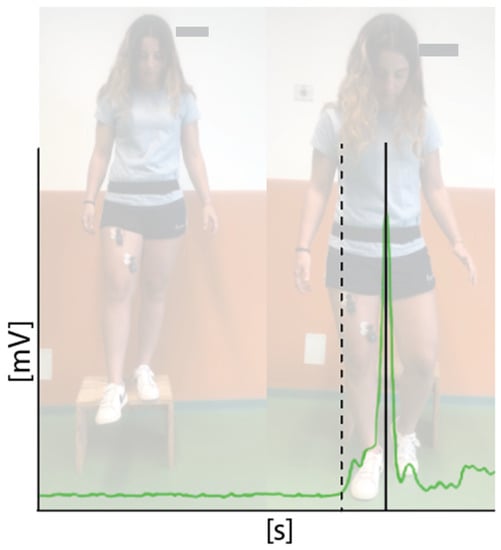
Figure 1.
sEMG signal of vastus medialis (green line) of one athlete. Pre-activation time is measured as the interval time between the activation of the muscle (dashed line) and the floor contact.
Body composition was assessed as secondary outcome, through the BIA (Akern s.r.l, Montacchiello, Pisa, Italy) evaluating: body cellular mass-BCM, fat mass (%), fat mass weight (kg), free fat mass weight (kg), muscle mass (kg), extra-cellular mass-ECM (kg), ECM/BCM ratio, phase angle (°), total body water (kg), intracellular water (kg), extra-cellular water (kg), O2 consumption (mL/min), and basal metabolic rate (kcal). Two low-impedance electrodes were positioned on the right hand and on the corresponding foot.
Lastly, we assessed the power of the lower limb muscles, through counter movement jump (CMJ), and squat jump (SJ) tests. CMJ assessed the ability to rapidly generate power in stretch-shortening cycle activities, while the SJ assessed the ability to quickly produce power in concentric movements [47].
More in detail, we considered the functional tests at the baseline (T0), 8 days before the COVID-19 positive nasopharyngeal swabs, and at return-to-training (T1) 3 days after the COVID-19 negative conversion at the RT-PCR test (see Figure 2 for further details).
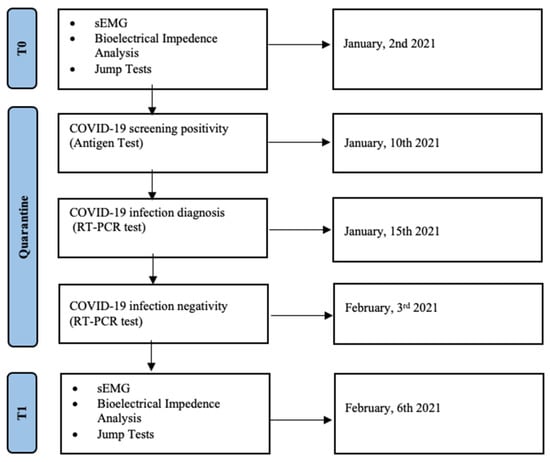
Figure 2.
Timeline of this retrospective study on volleyball athletes affected by COVID-19.
2.3. Statistical Analysis
Data were analyzed with R software (version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria). We used Shapiro–Wilk for testing normality of data. Considering the pre-activation time (assessed in ms) as primary outcome, the minimum sample size (n = 10) was calculated based on a previous study performed by our group [41], taking into account a potential 10% drop-out rate. Mean and standard deviation were used to describe the data, t-Student test was used for significance testing, as appropriate for paired samples, and Pearson’s r for parametric correlations. Plots were outlined using Graph PRISM (version 4.0 for Windows; GraphPad Software, San Diego, CA, USA). We calculated a post-hoc power analysis for the main outcomes considered.
3. Results
We included 12 athletes, mean aged 20.5 ± 6.2 years, with a mean height of 180.8 ± 7.9 cm, of the same team competing in the Second Division of the Italian National Volley Championship. All the athletes had COVID-19 mild-infection in the same frame time (January 2021) and they were isolated for 25 days. Paracetamol was administered for symptoms control. The most prevalent symptom was fever reported by 7 athletes, followed by headache (6 athletes), loss of taste or smell (5 athletes), muscle aches (4 athletes), and cough (2 athletes). The mean duration of symptoms was 4.0 ± 2.1 days.
The electromyographic analysis of the knee stabilizer muscles showed at T1 a significant delay of the RF activation time (426 ± 188 vs. 152 ± 106 ms; p = 0.001); VM (363 ± 192 vs. 140 ± 96 ms; p = 0.019); BF (229 ± 60 vs. 150 ± 63; p = 0.006); MH (T0: 231 ± 88 vs. 203 ± 89 ms; p = 0.011). Further details are shown in Table 1.

Table 1.
Differences in pre-activation time (ms) of knee stabilizer muscles before COVID-19 infection (T0) and at the return-to-training day (T1) comparing the same study population (n = 12).
At T1 the BIA showed a statistically significant reduction of muscle mass (41.24 ± 3.06 vs. 39.38 ± 2.63 kg; p = 0.003); fat mass weight (19.82 ± 4.45 vs. 18.4 ± 4.04 kg; p = 0.009), ECM (22.36 ± 1.98 vs. 23.43 ± 2.38 kg; p = 0.035), BCM (32.92 ± 4.53 vs. 31.27 ± 3.81 kg; p = 0.007), and phase angle (7.81 ± 0.72 vs. 7.02 ± 0.44 degrees; p = 0.003). Further details on the BIA evaluation are depicted by Table 2.

Table 2.
Bioelectrical impedance analysis of female volleyball athletes (n = 10) before COVID-19 infection (T0) and at the return-to-training day (T1).
At T1 we found a negative trend, even though not significant, in terms of CMJ flight time (0.56 ± 0.04 vs. 0.55 ± 0.03 s; p = 0.113) and height (35.7 ± 12.3 vs. 35.2 ± 11.9 cm; p = 0.103); and SJ (flight time 0.467 ± 0.15 vs. 0.461 ± 0.14 s; p = 0.144) and height (32.04 ± 5.13 vs. 31.25 ± 4.67 cm; p = 0.140) after COVID-19.
4. Discussion
This retrospective study investigated the effects of COVID-19 infection on neuromuscular activation pattern of the knee stabilizer muscle in professional volleyball female players. The athletes enrolled had only mild COVID-19 symptoms, thus, our study mainly focused on the consequent athletic condition impairment. To the best of our knowledge, this is the first study investigating the effects of COVID-19 on neuromuscular system in a cluster of athletes at high risk of ACL injury.
We found a significant delay in the muscular activation time of VM, RF, MH, and LH at the return-to-training compared with their own values before COVID-19 infection. Moreover, in the athletes who reported fever there was a significant reduction of the phase angle at the BIA. Neuromuscular activation patterns and the rate of recruitment of thigh muscle fibers, particularly quadriceps and hamstrings, play a key role in providing dynamic stability and reducing the risk of injury [7,48]. MH and LH are a dynamic ACL agonist, with various tendon insertions that can be used selectively to control the limb during functional task [49,50,51]. Thus, a delay in hamstring activation does not contrast the anterior displacement of the tibia, increasing anterior tibial shear force and magnify ACL loading during sport specific task [16]. The VM and RF through their indirect connection to the patellar ligament, which runs in the center of the two femoral condyles, have a stabilizing moment in both varus and valgus conditions [17]. Therefore, a delay in quadriceps muscle activation represents a dynamic instability of the knee during the initial phase of the cutting movements, from the pre-contact to the acceptance of weight phase [17]. Moreover, the late activation of VM and an unbalanced mediolateral quadriceps musculature recruitment pattern increases the apparent valgus dynamic position of the knee, increasing ACL load at landing after a jump [46,52].
The electrophysiological changes documented in COVID-19 athletes could increase the ACL injury risk. Moreover, according to Nepal et al. [53] electromyography plays a key role in COVID-19 to evidence myopathic and neuropathic changes, although performing complete electrophysiological examination, especially in patients with worst conditions [26], was extremely challenging during the pandemic. In this context, Agergaard and colleagues [54], used quantitative EMG studies and found myopathic changes in 11 (55%) of 20 patients examined, complaining long term fatigue after COVID-19 infection, The authors hypothesized that myopathy rather than neuropathy could be a possible explanation of physical fatigue in long-term COVID-19 patients even in non-hospitalized ones.
There are several reports about the specific tropism of COVID-19 for neural and muscular tissue, including peripheral nerve, neuromuscular junction and muscle tissue [21,26]. Potential nervous system infections could be caused by the direct entrance of the virus via the lamina cribrosa or, through systemic circulatory dissemination subsequent lungs infection, leading to a higher risk of developing transient or persistent neuromuscular and/or neurological sequelae [55]. COVID-19 might lead to neurological sequelae by attacking the central and peripheric nervous system in several ways, including vascular, inflammatory and/or direct neuronal injury, damaging dopaminergic system, basal ganglia and limbic system [55]. Indeed, neuromuscular complications of COVID-19 might be directly or indirectly related to coronavirus infection [25]; thus, it is possible that the virus may be neurotropic and could directly infect and damage motor neurons and peripheral nerves, with symptoms developed after an interval of 12–20 days after COVID-19 infection onset [26]. Likewise, as many as a third of patients infected with other coronavirus infections showed myalgia, elevated CKs and rhabdomyolysis suggesting that coronavirus infections may cause a viral myositis [25,26]. Moreover, levels of LDH above the normative were found in subject with myalgia and fatigue [56], and elevated levels of myoglobin are reported in patients with severe COVID-19 infection [37]. Whether the creatine kinase levels elevation and myopathic damage are supposed to be caused by toxic effects of cytokines, viral infection of muscle, or other mechanisms is still unclear, but data from muscle-biopsy advise an important pathophysiologic responsibility of severe immune activation commonly featured in COVID-19 patients [57]. The affinity of COVID-19 for neural tissue could in part explain the alteration of neuromuscular recruitment pattern of the knee stabilizer muscle caused by an immune-mediated disease of peripheral nerve myelin sheath or Schwann cells due to the glycoproteins on the surface of the virus that resemble glycoconjugates in human nervous tissue [26,58]. The antibodies formed against the viral surface glycoproteins act against the glycoconjugates on the neural tissue and cause a chronic inflammatory demyelinating polyneuropathy [26,59] that results in an alteration of propagation of action potentials by saltatory conduction [60], leading to an alteration of timing of muscular activation.
Moreover, the COVID-19 influenced the athletes’ body composition with a weight loss, an increase of ECM/BCM ratio and a reduction of phase angle that are useful markers for sarcopenia or malnutrition [61,62]. Anker et al. [36] reported that body wasting in patients with COVID-19 is determined by several factors, including loss of taste and appetite, immobilization, inflammation and fever, catabolic–anabolic imbalance, and COVID-19 organ-specific complications [36]. In particular, Di Filippo et al. [63] reported that patients with mild COVID-19 managed at home might suffer from malnutrition and alterations of smell and taste, as well as fatigue and lack of appetite, which are common symptoms that could affect food intake. In this context, we found an interesting association between the loss of taste and poor nutritional outcomes: this symptom, reported by more than 40% of the athletes enrolled in this study, is significantly correlated with weight loss, reduction of muscle mass and ICW.
It is essential to ensure an adequate protein intake for athletes to provide a net anabolic effect thanks to the synergistic interaction between exercise and macronutrient consumption [64]. More in detail, an acute amino acids feeding significantly increases the rate of muscle protein synthesis [64,65], most of all in the hours right before and during exercise, maximizing muscle repair and enhancing strength- and hypertrophy-related adaptations [66,67]. This could be achieved through a higher activation (phosphorylation) of mTOR pathway (a key signaling protein present in myocytes that is linked to the synthesis of muscle proteins) and its downstream mRNA translational signaling proteins (i.e., p70s6 kinase and eIF4BP) that are strongly related to muscle hypertrophy [68,69]. Moreover, phase angle has recently emerged as a sensitive indicator of cellular health, with higher values reflecting cell membrane integrity of living tissue [70], and is correlated positively with maximum muscle power output, handgrip strength and peak expiratory flow [71]. This worsening of metabolic outcomes could explain the negative trend of explosive power of the lower limbs showed in our results that could have a further effect in increasing the risk of injury in these athletes [72]. The retraining procedures should consider all the neuromuscular and nutritional impairments before return-to-competition, addressing these systemic changes for enhancing sports performance and reducing the risk of injury in athletes affected by COVID-19.
Taking into account these findings, early rehabilitative interventions are crucial to reduce the possible neurological sequelae in these subjects [55]. Muscle deconditioning occurs very early with bed rest, involving a decline in muscle mass, strength and aerobic efficiency and a prompt and effective management of neuromuscular weakness could improve the patient’s status [55]. Thus, we recommended a neuromuscular training protocol to enhance unconscious motor responses through the simultaneous stimulation of afferent signals and central mechanisms responsible for dynamic joint control. We proposed exercises specifically designed to induce compensatory changes in muscle activation patterns and the main treatment goals were improving the ability to produce a fast and adequate muscle activation pattern, increasing dynamic joint stability and restoring movement patterns and abilities essential during sports activities [73,74]. This could be obtained introducing structured neuromuscular rehabilitative programs including perturbation, balance training and strength exercises, focusing on a higher consciousness and control of knees and ankles during all the sport related-activities in order to improve muscle reactivity via the muscle spindle that quickly identifies unexpected perturbations [41].
This study presents some limitations: first, the limited sample size that could not allow an adequate external generalizability; second, sEMG measurement could be influenced by muscle crosstalk, albeit the most appropriate electrode size and electrode placement were carefully selected; third, there are various factors that can affect BIA results, such as temperature, and sensitivity to conductive surface of electrodes. Further study limitations that should be noticed are the post-hoc nature of our analysis and the use of patient-reported symptoms.
5. Conclusions
Taken together, our findings suggested that the deficit of quadriceps varus/valgus stabilization during ACL stressful movements could lead to an increased risk of injury in female professional volleyball players after COVID-19. The early detection of this neuromuscular imbalance at knee stabilizer muscles might be useful to define the best training strategy for these athletes. Physician should pay attention on neuromuscular and metabolic issues after COVID-19 infection to identify athletes with a higher risk of injury and set up tailored and effective neuromuscular rehabilitation protocols.
Author Contributions
Conceptualization, A.d.S., A.D., M.I. and A.A.; methodology, A.d.S. and M.I.; software, A.D. and N.M.; formal analysis, N.M.; investigation, A.D., N.M., R.S. and T.I.; resources, A.A.; data curation, A.d.S., A.D., C.C. and M.I.; writing—original draft preparation, A.d.S. and A.D., writing—review and editing, M.I. and A.A.; visualization, N.M., R.S., C.C., G.F., F.F., T.I., L.L. and T.P.; supervision, A.d.S., M.I. and A.A.; submission, M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Dataset is available on request.
Acknowledgments
We would like to thank Vincenzo Capilupi and Andrea Rotella for their support in this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sepúlveda, F.; Sánchez, L.; Amy, E.; Micheo, W. Anterior Cruciate Ligament Injury: Return to Play, Function and Long-Term Considerations. Curr. Sports Med. Rep. 2017, 16, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Prodromos, C.; Han, Y.; Rogowski, J.; Joyce, B.; Shi, K. A Meta-analysis of the Incidence of Anterior Cruciate Ligament Tears as a Function of Gender, Sport, and a Knee Injury–Reduction Regimen. Arthrosc. J. Arthrosc. Relat. Surg. 2007, 23, 1320–1325.e6. [Google Scholar] [CrossRef]
- Moeller, J.L.; Lamb, M.M. Anterior Cruciate Ligament Injuries in Female Athletes: Why Are Women More Susceptible? Physician Sportsmed. 1997, 25, 31–54. [Google Scholar] [CrossRef]
- Palmieri-Smith, R.M.; McLean, S.G.; Ashton-Miller, J.A.; Wojtys, E.M. Association of Quadriceps and Hamstrings Cocontraction Patterns with Knee Joint Loading. J. Athl. Train. 2009, 44, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Bisciotti, G.N.; Eirale, C.; Corsini, A.; Baudot, C.; Saillant, G.; Chalabi, H. Return to football training and competition after lockdown caused by the COVID-19 pandemic: Medical recommendations. Biol. Sport 2020, 37, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Zazulak, B.T.; Hewett, T.E.; Reeves, N.P.; Goldberg, B.; Cholewicki, J. Deficits in Neuromuscular Control of the Trunk Predict Knee Injury Risk: A Prospective Biomechanical-Epidemiologic Study. Am. J. Sports Med. 2007, 35, 1123–1130. [Google Scholar] [CrossRef]
- Medina, J.M.; McLeod, T.C.V.; Howell, S.K.; Kingma, J.J. Timing of neuromuscular activation of the quadriceps and hamstrings prior to landing in high school male athletes, female athletes, and female non-athletes. J. Electromyogr. Kinesiol. 2008, 18, 591–597. [Google Scholar] [CrossRef]
- Hewett, T.E.; Stroupe, A.L.; Nance, T.A.; Noyes, F.R. Plyometric Training in Female Athletes. Decreased Impact Forces and Increased Hamstring Torques. Am. J. Sports Med. 1996, 24, 765–773. [Google Scholar] [CrossRef]
- Huston, L.J.; Wojtys, E.M. Neuromuscular Performance Characteristics in Elite Female Athletes. Am. J. Sports Med. 1996, 24, 427–436. [Google Scholar] [CrossRef]
- Shultz, S.J.; Perrin, D.H. Using surface electromyography to assess sex differences in neuromuscular response characteristics. J. Athl. Train. 1999, 34, 165–176. [Google Scholar]
- Hewett, T.E.; Zazulak, B.T.; Myer, G.D.; Ford, K. A review of electromyographic activation levels, timing differences, and increased anterior cruciate ligament injury incidence in female athletes. Br. J. Sports Med. 2005, 39, 347–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldén, M.; Hägglund, M.; Magnusson, H.; Ekstrand, J. ACL Injuries in Men’s Professional Football: A 15-Year Prospective Study on Time Trends and Return-to-Play Rates Reveals Only 65% of Players Still Play at the Top Level 3 Years after ACL Rupture. Br. J. Sports Med. 2016, 50, 744–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemann, B.L.; Lephart, S.M. The sensorimotor system, part I: The physiologic basis of functional joint stability. J. Athl. Train. 2002, 37, 71–79. [Google Scholar] [PubMed]
- Riemann, B.L.; Lephart, S.M. The sensorimotor system, part II: The role of proprioception in motor control and functional joint stability. J. Athl. Train. 2002, 37, 80–84. [Google Scholar]
- Begalle, R.L.; Distefano, L.J.; Blackburn, T.; Padua, D.A. Quadriceps and Hamstrings Coactivation During Common Therapeutic Exercises. J. Athl. Train. 2012, 47, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Tognolo, L.; Maccarone, M.C.; De Trane, S.; Scanu, A.; Masiero, S.; Fiore, P. Therapeutic Exercise and Conservative Injection Treatment for Early Knee Osteoarthritis in Athletes: A Scoping Review. Medicina. 2022, 58, 69. [Google Scholar] [CrossRef]
- de Sire, A.; Stagno, D.; Minetto, M.A.; Cisari, C.; Baricich, A.; Invernizzi, M. Long-term effects of intra-articular oxygen-ozone therapy versus hyaluronic acid in older people affected by knee osteoarthritis: A randomized single-blind extension study. J. Back Musculoskelet Rehabil. 2020, 33, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, B.; James, M.; Koller, D.; Lindholm, J.; Mavromati, D.; Parrish, R.; Rodenberg, R. The impact of COVID-19 on sports: A mid-way assessment. Int. Sports Law J. 2020, 20, 115–119. [Google Scholar] [CrossRef]
- Marotta, N.; DE Sire, A.; Gimigliano, A.; Demeco, A.; Moggio, L.; Vescio, A.; Iona, T.; Ammendolia, A. Impact of COVID-19 lockdown on the epidemiology of soccer muscle injuries in Italian Serie A professional football players. J. Sports Med. Phys. Fit. 2021. [Google Scholar] [CrossRef]
- Andrenelli, E.; Negrini, F.; de Sire, A.; Arienti, C.; Patrini, M.; Negrini, S.; Ceravolo, M.G.; International Multiprofessional Steering Committee of Cochrane Rehabilitation REH-COVER action. Systematic rapid living review on rehabilitation needs due to COVID-19: Update to 31 May 2020. Eur. J. Phys. Rehabil. Med. 2020, 56, 508–514. [Google Scholar] [CrossRef]
- Negrini, F.; de Sire, A.; Andrenelli, E.; Lazzarini, S.G.; Patrini, M.; Ceravolo, M.G.; International Multiprofessional Steering Committee of Cochrane Rehabilitation REH-COVER action. Rehabilitation and COVID-19: The Cochrane Rehabilitation 2020 rapid living systematic review. Update as of July 31st, 2020. Eur. J. Phys. Rehabil. Med. 2020, 56, 652–657. [Google Scholar] [CrossRef]
- de Sire, A.; Andrenelli, E.; Negrini, F.; Patrini, M.; Lazzarini, S.G.; Ceravolo, M.G.; International Multiprofessional Steering Committee of Cochrane Rehabilitation REH-COVER Action. Rehabilitation and COVID-19: A rapid living systematic review by Cochrane Rehabilitation Field updated as of December 31st, 2020 and synthesis of the scientific literature of 2020. Eur. J. Phys. Rehabil. Med. 2021, 57, 161–188. [Google Scholar] [CrossRef]
- Demeco, A.; Marotta, N.; Barletta, M.; Pino, I.; Marinaro, C.; Petraroli, A.; Moggio, L.; Ammendolia, A. Rehabilitation of patients post-COVID-19 infection: A literature review. J. Int. Med Res. 2020, 48, 0300060520948382. [Google Scholar] [CrossRef]
- Wilson, M.G.; Hull, J.H.; Rogers, J.; Pollock, N.; Dodd, M.; Haines, J.; Harris, S.; Loosemore, M.; Malhotra, A.; Pieles, G.; et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: A practical guide for sport and exercise medicine physicians. Br. J. Sports Med. 2020, 54, 1157–1161. [Google Scholar] [CrossRef]
- Guidon, A.C.; Amato, A.A. COVID-19 and neuromuscular disorders. Neurology 2020, 94, 959–969. [Google Scholar] [CrossRef] [Green Version]
- Paliwal, V.K.; Garg, R.K.; Gupta, A.; Tejan, N. Neuromuscular presentations in patients with COVID-19. Neurol. Sci. 2020, 41, 3039–3056. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Collantes, M.E.V.; Espiritu, A.I.; Sy, M.C.C.; Anlacan, V.M.M.; Jamora, R.D.G. Neurological Manifestations in COVID-19 Infection: A Systematic Review and Meta-Analysis. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2021, 48, 66–76. [Google Scholar] [CrossRef]
- Xu, X.-W.; Wu, X.; Jiang, X.-G.; Xu, K.-J.; Ying, L.-J.; Ma, C.-L.; Li, S.-B.; Wang, H.-Y.; Zhang, S.; Gao, H.-N.; et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-CoV-2) outside of Wuhan, China: Retrospective case series. BMJ 2020, 368, m606. [Google Scholar] [CrossRef] [Green Version]
- Keddie, S.; Pakpoor, J.; Mousele, C.; Pipis, M.; Machado, P.M.; Foster, M.; Record, C.J.; Keh, R.Y.S.; Fehmi, J.; Paterson, R.W.; et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain 2020, 144, 682–693. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Abdelhak, A.; Foschi, M.; Tumani, H.; Otto, M. Guillain–Barré syndrome spectrum associated with COVID-19: An up-to-date systematic review of 73 cases. J. Neurol. 2020, 268, 1133–1170. [Google Scholar] [CrossRef]
- Manzano, G.S.; Woods, J.K.; Amato, A.A. COVID-19–Associated Myopathy Caused by Type I Interferonopathy. N. Engl. J. Med. 2020, 383, 2389–2390. [Google Scholar] [CrossRef]
- Disser, N.P.; De Micheli, A.J.; Schonk, M.M.; Konnaris, M.A.; Piacentini, A.N.; Edon, D.L.; Toresdahl, B.G.; Rodeo, S.A.; Casey, E.K.; Mendias, C.L. Musculoskeletal Consequences of COVID-19. J. Bone Jt. Surg. Am. 2020, 102, 1197–1204. [Google Scholar] [CrossRef]
- Abdel-Mannan, O.; Eyre, M.; Löbel, U.; Bamford, A.; Eltze, C.; Hameed, B.; Hemingway, C.; Hacohen, Y. Neurologic and Radiographic Findings Associated With COVID-19 Infection in Children. JAMA Neurol. 2020, 77, 1440–1445. [Google Scholar] [CrossRef]
- Gupta, M.; Weaver, D.F. COVID-19 as a Trigger of Brain Autoimmunity. ACS Chem. Neurosci. 2021, 12, 2558–2561. [Google Scholar] [CrossRef] [PubMed]
- Anker, M.S.; Landmesser, U.; von Haehling, S.; Butler, J.; Coats, A.J.S.; Anker, S.D. Weight loss, malnutrition, and cachexia in COVID-19: Facts and numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 9–13. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Skeletal Muscle Damage in COVID-19: A Call for Action. Medicina 2021, 57, 372. [Google Scholar] [CrossRef]
- Sarto, F.; Impellizzeri, F.M.; Spörri, J.; Porcelli, S.; Olmo, J.; Requena, B.; Suarez-Arrones, L.; Arundale, A.; Bilsborough, J.; Buchheit, M.; et al. Impact of Potential Physiological Changes due to COVID-19 Home Confinement on Athlete Health Protection in Elite Sports: A Call for Awareness in Sports Programming. Sports Med. 2020, 50, 1417–1419. [Google Scholar] [CrossRef]
- Vigotsky, A.D.; Halperin, I.; Lehman, G.J.; Trajano, G.; Vieira, T.M.; Vigotsky, A.D.; Halperin, I.; Lehman, G.J.; Trajano, G.; Vieira, T.M. Interpreting Signal Amplitudes in Surface Electromyography Studies in Sport and Rehabilitation Sciences. Front. Physiol. 2017, 8, 985. [Google Scholar] [CrossRef] [Green Version]
- Demeco, A.; Marotta, N.; Moggio, L.; Pino, I.; Marinaro, C.; Barletta, M.; Petraroli, A.; Palumbo, A.; Ammendolia, A. Quantitative analysis of movements in facial nerve palsy with surface electromyography and kinematic analysis. J. Electromyogr. Kinesiol. 2021, 56, 102485. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Demeco, A.; Marotta, N.; Moggio, L.; Palumbo, A.; Iona, T.; Ammendolia, A. Anterior Cruciate Ligament Injury Prevention Exercises: Could a Neuromuscular Warm-Up Improve Muscle Pre-Activation before a Soccer Game? A Proof-of-Principle Study on Professional Football Players. Appl. Sci. 2021, 11, 4958. [Google Scholar] [CrossRef]
- de Sire, A.; Marotta, N.; Demeco, A.; Moggio, L.; Paola, P.; Marotta, M.; Iona, T.; Invernizzi, M.; Leigheb, M.; Ammendolia, A. Electromyographic Assessment of Anterior Cruciate Ligament Injury Risk in Male Tennis Players: Which Role for Visual Input? A Proof-of-Concept Study. Diagnostics 2021, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Toselli, S.; Marini, E.; Latessa, P.M.; Benedetti, L.; Campa, F. Maturity Related Differences in Body Composition Assessed by Classic and Specific Bioimpedance Vector Analysis among Male Elite Youth Soccer Players. Int. J. Environ. Res. Public Health 2020, 17, 729. [Google Scholar] [CrossRef] [Green Version]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Sacco, I.C.N.; Gomes, A.A.; Otuzi, M.E.; Pripas, D.; Onodera, A.N. A method for better positioning bipolar electrodes for lower limb EMG recordings during dynamic contractions. J. Neurosci. Methods 2009, 180, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Marotta, N.; Demeco, A.; De Scorpio, G.; Indino, A.; Iona, T.; Ammendolia, A. Late Activation of the Vastus Medialis in Determining the Risk of Anterior Cruciate Ligament Injury in Soccer Players. J. Sport Rehabil. 2020, 29, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Van Hooren, B.; Zolotarjova, J. The Difference between Countermovement and Squat Jump Performances: A Review of Underlying Mechanisms With Practical Applications. J. Strength Cond. Res. 2017, 31, 2011–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaxman, T.E.; Smith, A.J.J.; Benoit, D.L. Sex-related differences in neuromuscular control: Implications for injury mechanisms or healthy stabilisation strategies? J. Orthop. Res. 2014, 32, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Letafatkar, A.; Rajabi, R.; Tekamejani, E.E.; Minoonejad, H. Effects of perturbation training on knee flexion angle and quadriceps to hamstring cocontraction of female athletes with quadriceps dominance deficit: Pre–post intervention study. Knee 2015, 22, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.J.A.; Simic, M.; Myer, G.D.; Ford, K.; Hewett, T.E.; Pappas, E. The Effects of Injury Prevention Programs on the Biomechanics of Landing Tasks: A Systematic Review with Meta-analysis. Am. J. Sports Med. 2017, 46, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Hamstrings Cocontraction Reduces Internal Rotation, Anterior Translation, and Anterior Cruciate Ligament Load in Weight-bearing Flexion-MacWilliams-1999-Journal of Orthopaedic Research-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/jor.1100170605 (accessed on 5 June 2020).
- Marotta, N.; Demeco, A.; Moggio, L.; Isabello, L.; Iona, T.; Ammendolia, A. Correlation between Dynamic Knee Valgus and Quadriceps Activation Time in Female Athletes. J. Phys. Educ. Sport 2020, 20, 2508–2512. [Google Scholar] [CrossRef]
- Nepal, G.; Shrestha, G.S.; Rehrig, J.H.; Gajurel, B.P.; Ojha, R.; Agrawal, A.; Panthi, S.; Khatri, B.; Adhikari, I. Neurological Manifestations of COVID-19 Associated Multi-system Inflammatory Syndrome in Children: A Systematic Review and Meta-analysis. J. Nepal Health Res. Counc. 2021, 19, 10–18. [Google Scholar] [CrossRef]
- Agergaard, J.; Leth, S.; Pedersen, T.H.; Harbo, T.; Blicher, J.U.; Karlsson, P.; Østergaard, L.; Andersen, H.; Tankisi, H. Myopathic changes in patients with long-term fatigue after COVID-19. Clin. Neurophysiol. 2021, 132, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Pincherle, A.; Jöhr, J.; Pancini, L.; Leocani, L.; Vecchia, L.D.; Ryvlin, P.; Schiff, N.D.; Diserens, K. Intensive Care Admission and Early Neuro-Rehabilitation. Lessons for COVID-19? Front. Neurol. 2020, 11, 880. [Google Scholar] [CrossRef]
- Tuzun, S.; Keles, A.; Okutan, D.; Yildiran, T.; Palamar, D. Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with COVID-19. Eur. J. Phys. Rehabil. Med. 2021, 57, 653–662. [Google Scholar] [CrossRef]
- Zhang, H.; Charmchi, Z.; Seidman, R.J.; Anziska, Y.; Do, V.V.; Perk, J. COVID -19–associated myositis with severe proximal and bulbar weakness. Muscle Nerve 2020, 62, E57–E60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lopez, E.J.; Soneji, D.J.; Azevedo, C.J.; Patel, V.R. Myelin Oligodendrocyte Glycoprotein Antibody–Associated Optic Neuritis and Myelitis in COVID-19. J. Neuro-Ophthalmol. 2020, 40, 398–402. [Google Scholar] [CrossRef]
- Lucchese, G.; Flöel, A. SARS-CoV-2 and Guillain-Barré syndrome: Molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress Chaperon- 2020, 25, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Kane, N.M.; Oware, A. Nerve conduction and electromyography studies. J. Neurol. 2012, 259, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Avram, M.M.; Fein, P.A.; Borawski, C.; Chattopadhyay, J.; Matza, B. Extracellular mass/body cell mass ratio is an independent predictor of survival in peritoneal dialysis patients. Kidney Int. 2010, 78, S37–S40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirose, S.; Nakajima, T.; Nozawa, N.; Katayanagi, S.; Ishizaka, H.; Mizushima, Y.; Matsumoto, K.; Nishikawa, K.; Toyama, Y.; Takahashi, R.; et al. Phase Angle as an Indicator of Sarcopenia, Malnutrition, and Cachexia in Inpatients with Cardiovascular Diseases. J. Clin. Med. 2020, 9, 2554. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.; De Lorenzo, R.; D’Amico, M.; Sofia, V.; Roveri, L.; Mele, R.; Saibene, A.; Rovere-Querini, P.; Conte, C. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: A post-hoc analysis of a prospective cohort study. Clin. Nutr. 2020, 40, 2420–2426. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [Green Version]
- Tipton, K.D.; Ferrando, A.A.; Phillips, S.M.; Doyle, D.; Wolfe, R.R. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am. J. Physiol. Metab. 1999, 276, E628–E634. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S. Influence of Nutrition on Responses to Resistance Training. Med. Sci. Sports Exerc. 2004, 36, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Kerksick, C.; Harvey, T.; Stout, J.; Campbell, B.; Wilborn, C.; Kreider, R.; Kalman, D.; Ziegenfuss, T.; Lopez, H.; Landis, J.; et al. International Society of Sports Nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2008, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farnfield, M.M.; Breen, L.; Carey, K.A.; Garnham, A.; Cameron-Smith, D. Activation of mTOR signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion. Appl. Physiol. Nutr. Metab. 2012, 37, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Hulmi, J.J.; Kovanen, V.; Selänne, H.; Kraemer, W.J.; Häkkinen, K.; Mero, A.A. Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids 2009, 37, 297–308. [Google Scholar] [CrossRef]
- Uemura, K.; Yamada, M.; Okamoto, H. Association of bioimpedance phase angle and prospective falls in older adults. Geriatr. Gerontol. Int. 2019, 19, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Buehring, B.; Krueger, D.; Anderson, R.M.; Schoeller, D.A.; Binkley, N. Electrical Properties Assessed by Bioelectrical Impedance Spectroscopy as Biomarkers of Age-related Loss of Skeletal Muscle Quantity and Quality. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2016, 72, 1180–1186. [Google Scholar] [CrossRef] [Green Version]
- Tillin, N.A.; Jimenez-Reyes, P.; Pain, M.T.; Folland, J.P. Neuromuscular performance of explosive power athletes versus untrained individuals. Med. Sci. Sports Exerc. 2010, 42, 781–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risberg, M.A.; Mørk, M.; Jenssen, H.K.; Holm, I. Design and Implementation of a Neuromuscular Training Program Following Anterior Cruciate Ligament Reconstruction. J. Orthop. Sports Phys. Ther. 2001, 31, 620–631. [Google Scholar] [CrossRef] [Green Version]
- Van Melick, N.; Cingel, R.E.H.V.; Brooijmans, F.; Neeter, C.; Van Tienen, T.; Hullegie, W.; Der Sanden, M.W.G.N.-V. Evidence-based clinical practice update: Practice guidelines for anterior cruciate ligament rehabilitation based on a systematic review and multidisciplinary consensus. Br. J. Sports Med. 2016, 50, 1506–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).