Study of Cocoa Pod Husks Thermal Decomposition
Abstract
1. Introduction
- Thermogravimetric analysis, including the proposal of a kinetic model that allows adjusting the experimental data. In this line, a new fitting strategy is proposed. Although this strategy has been applied to the CPH decomposition, it can be extrapolated to any other TG/DTG experimental data.
- Identification of the main volatiles obtained under different pyrolysis conditions, which can guide the selection of the best operating parameters to maximize a compound or a certain type of compounds.
2. Materials and Methods
2.1. Cocoa Pod Husk (CPH)
2.2. Thermogravimetric Analysis (TGA)
2.3. Elemental Analysis
2.4. Analytical Pyrolysis EGA Analysis
- Flash pyrolysis at 400 °C
- Slow pyrolysis at three different temperature ranges: 80–150 °C, 150–350 °C and 350–500 °C. The heating rate was 50 °C min−1.
3. Results and Discussion
3.1. CPH Characterization
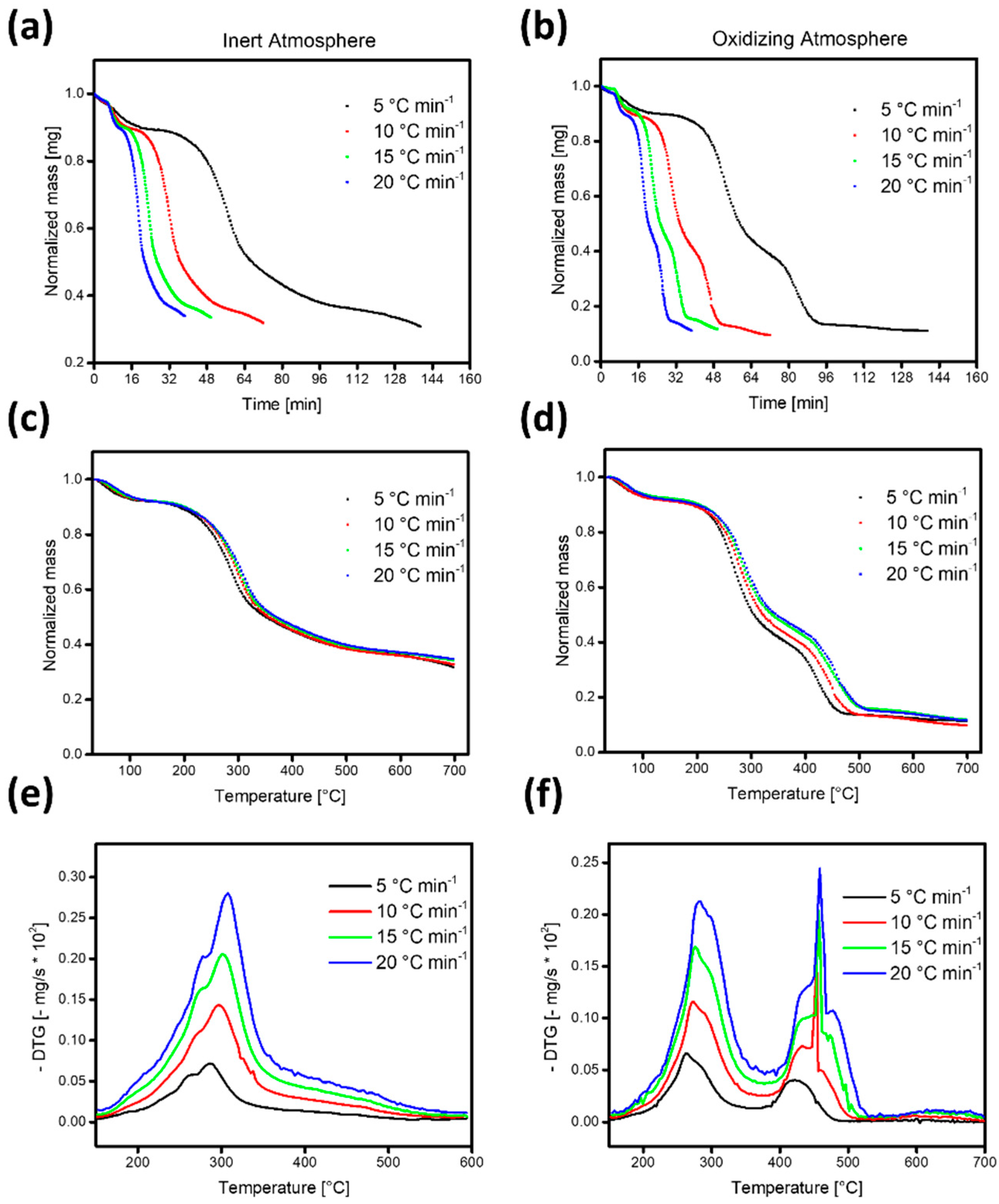
3.2. Thermal Decomposition of CPH
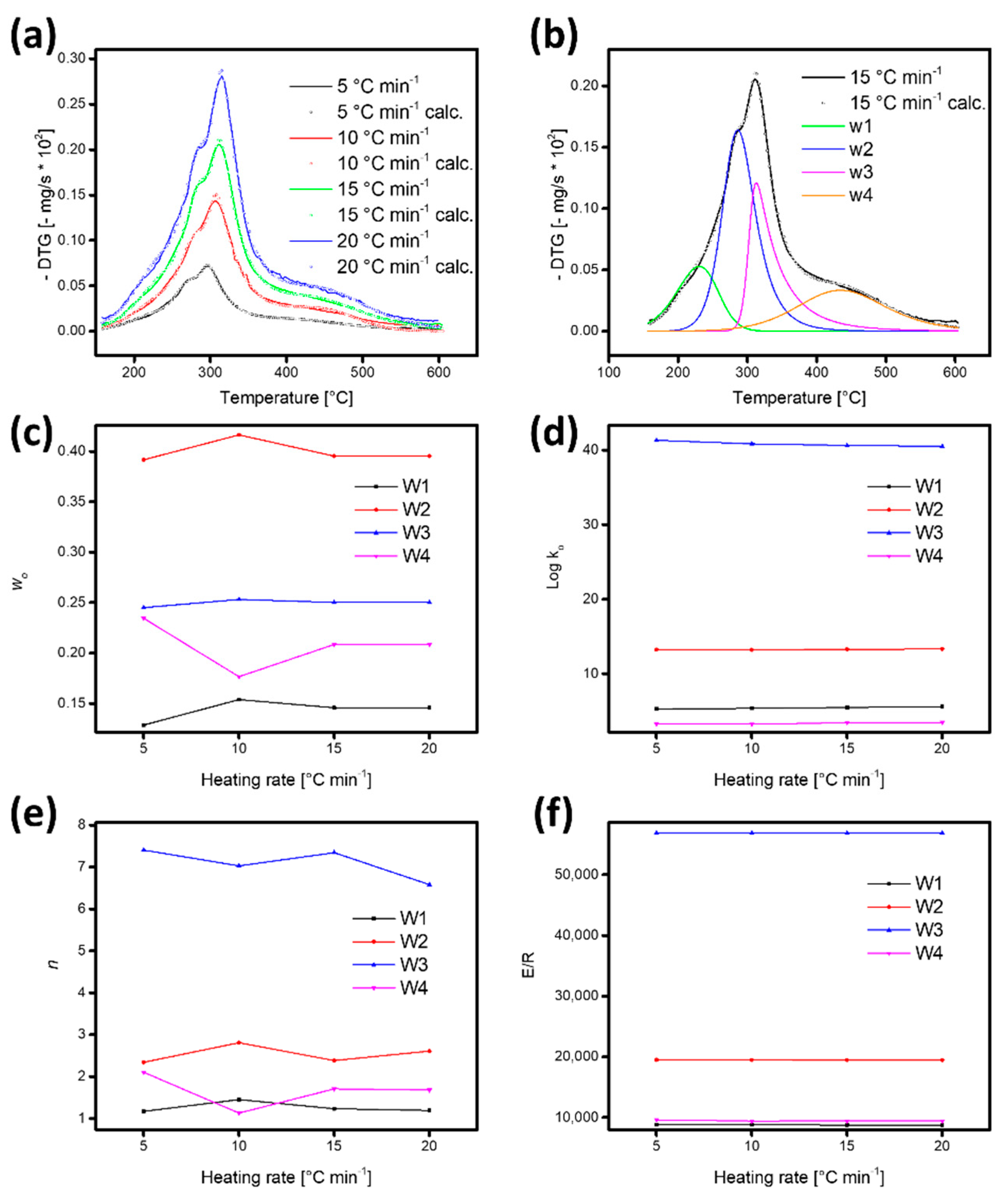
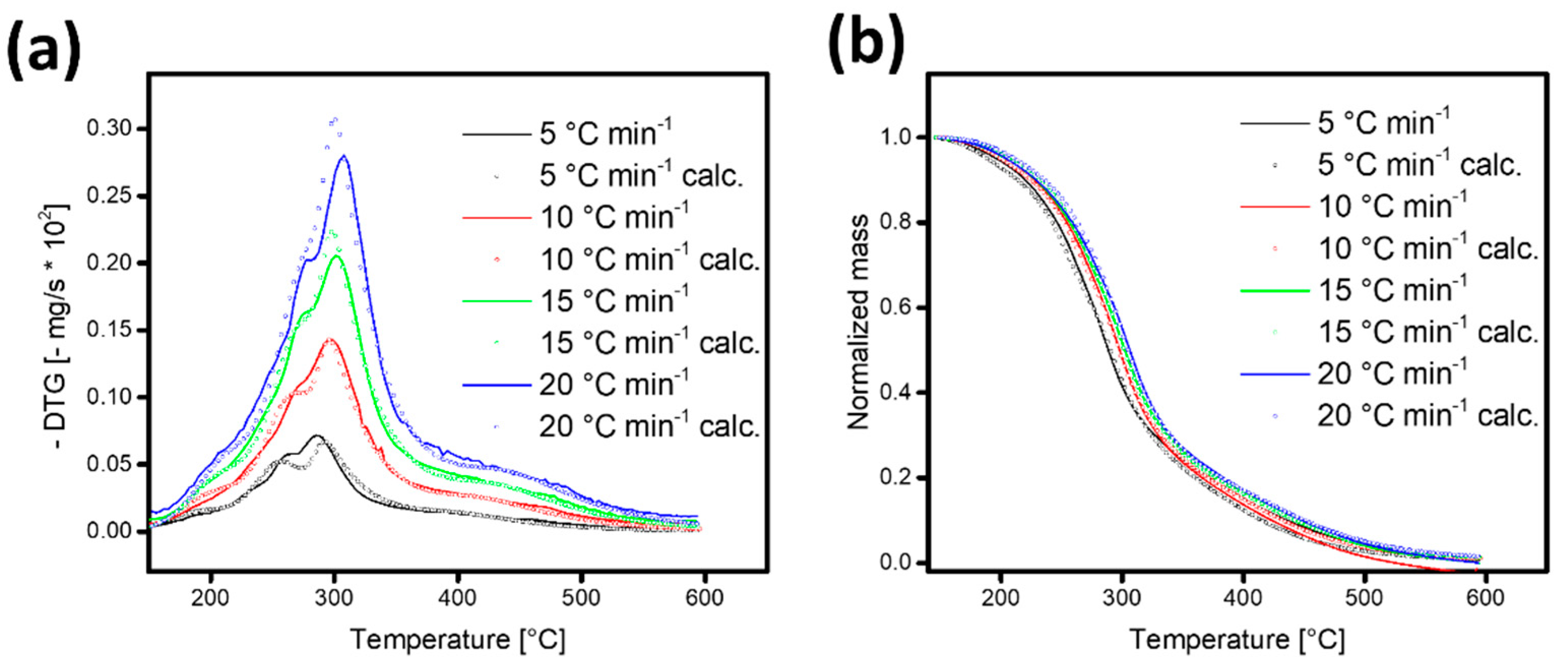
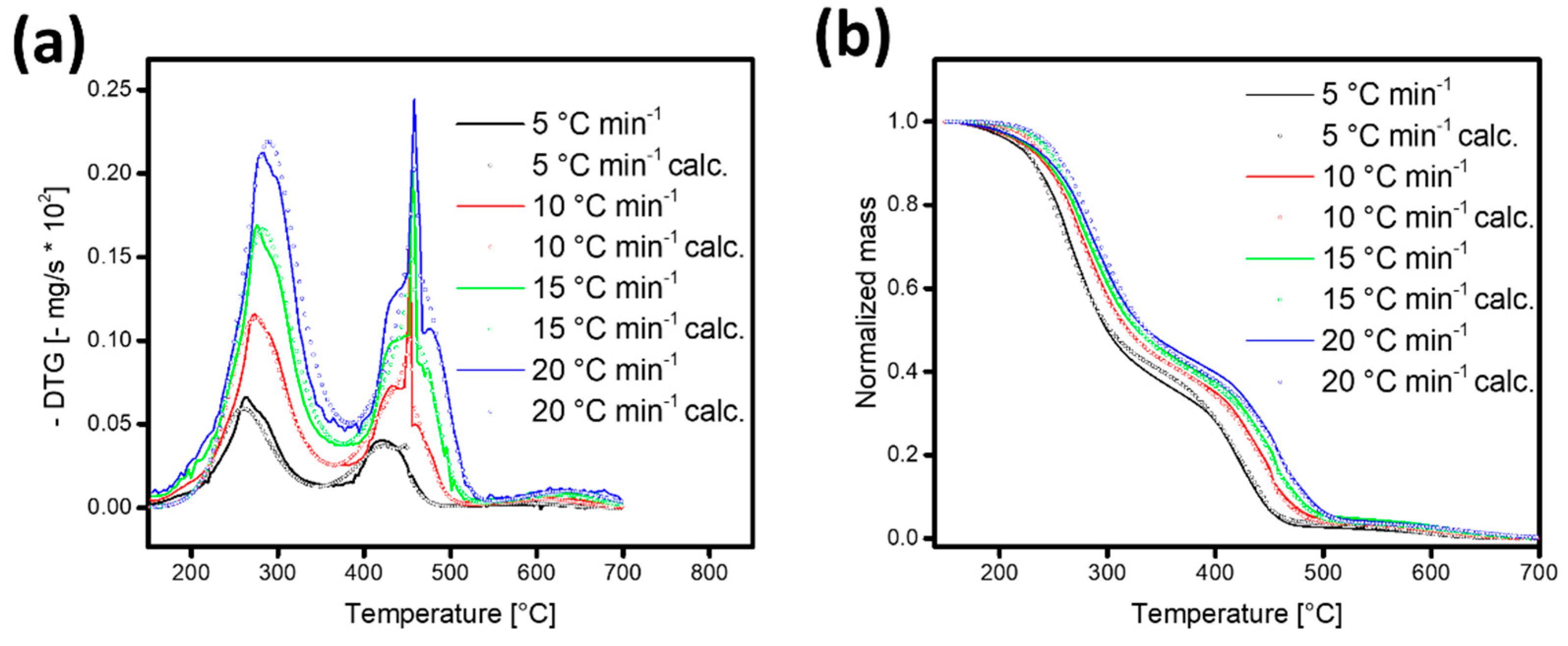
3.3. Kinetic Model and Fitting Strategy
3.4. Kinetic Parameters
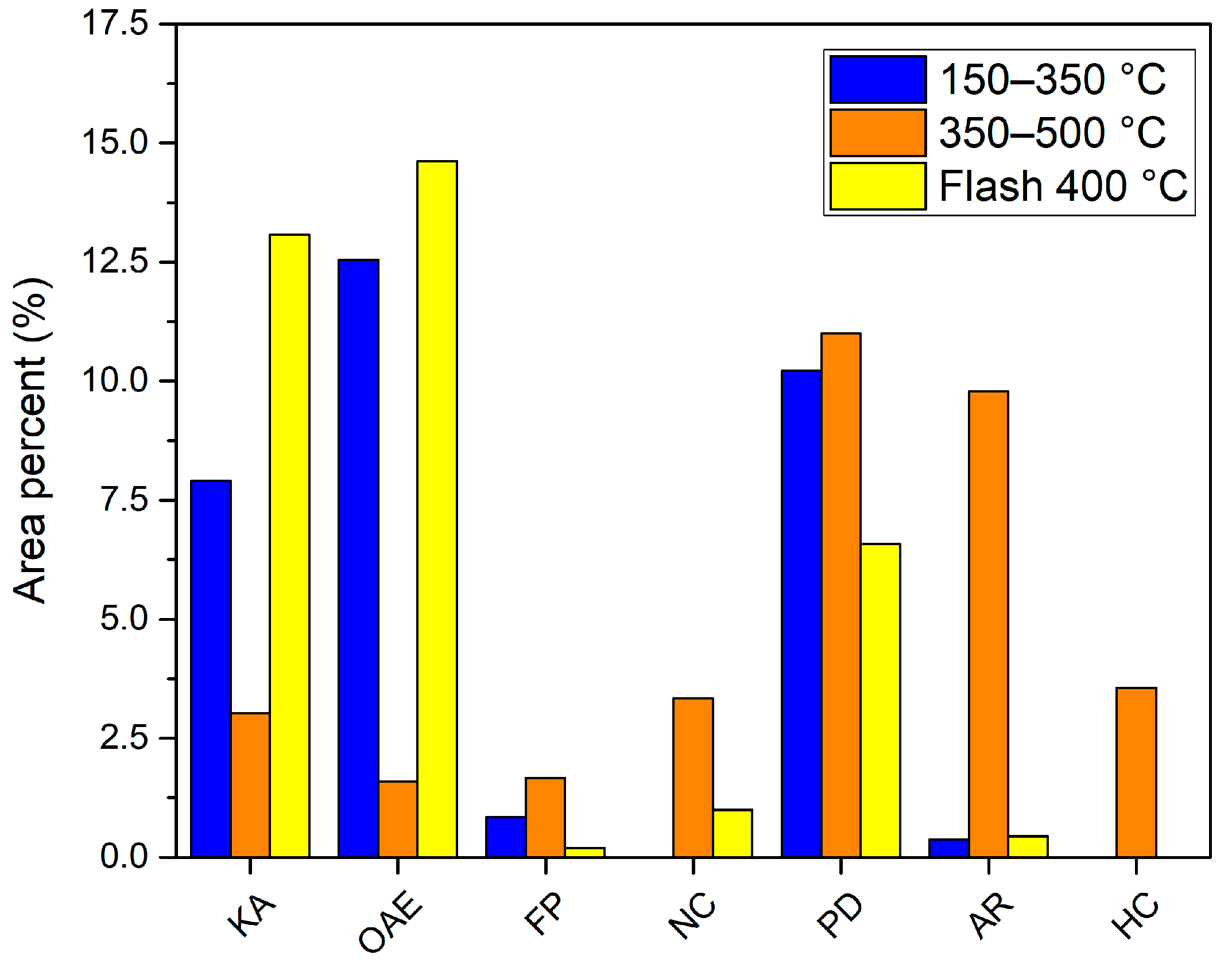
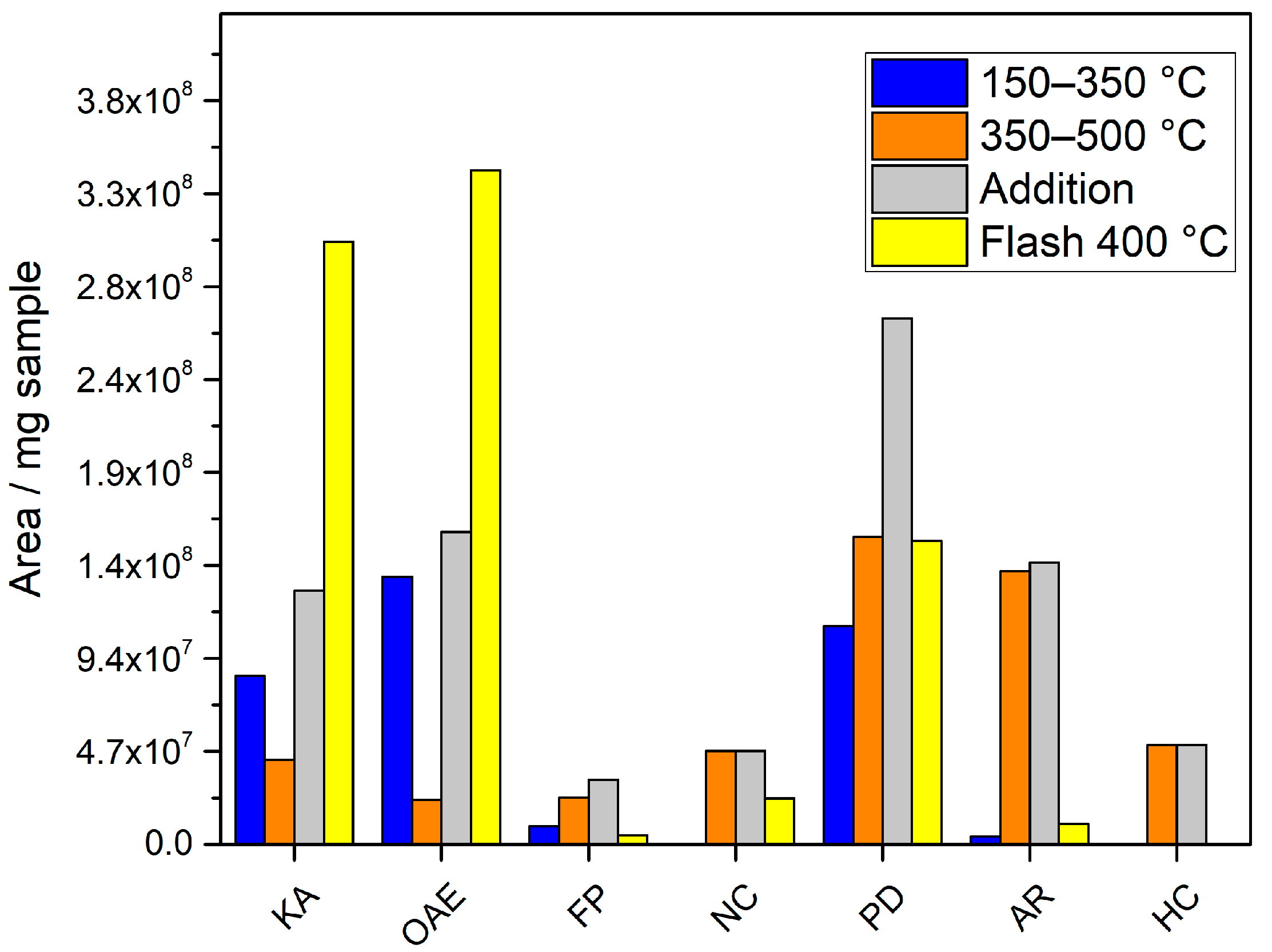
3.5. Analytical Pyrolysis EGA Analysis
- 80–150 °C. Only water is detected in this range. The temperature is not high enough to release any other volatiles.
- 150–350 °C. Besides water and CO2, which are the main compounds detected, 3 other compounds clearly stand out among all: acetic acid, 2,6-dimethoxy-4-(2-propenyl)-phenol and 1-hydroxy-2-propanone. The presence of other ketones, phenol derivatives and organic acids is detected in lower percentages.
- 350–500 °C. By increasing temperature, the number of volatiles formed increases. In fact, the total chromatographic area obtained in this range is 20% higher than the area obtained in the previous one. Water and CO2 continue to be the main compounds, but a greater number of volatiles, more than 200, is detected. The yields of all of them are more similar and, unlike the previous temperature range, the compounds with higher percentages do not stand out too much from the rest. The increase in temperature has increased the generation of compounds but has decreased the selectivity of the process, i.e., many more compounds with lower yield. The 3 compounds with the highest area percentage are 2-methoxy-phenol, toluene and 2-methyl-furan. Many other phenol derivatives (4-methyl-phenol, 2,3-dimethyl-phenol, 2-methoxy-4-propyl-phenol, 2,6-dimethoxy-4-(2-propenyl)-phenol), aromatics (benzene, ethylbenzene, xylene, naphthalene derivatives), organic acids (C16, C18) and other functional groups are also identified.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahbandeh, M. Cocoa Bean Production Worldwide 2018/19 & 2020/21, by Country. Available online: https://www.statista.com/statistics/263855/cocoa-bean-production-worldwide-by-region/ (accessed on 28 August 2022).
- Agencia de Regulación y Control Fito y Zoosanitario Cocoa Export during 2021. Available online: https://www.agrocalidad.gob.ec/wp-content/uploads/2022/02/Informe-cacao.pdf (accessed on 28 August 2022).
- Acebo-Guerrero, Y.; Hernández-Rodríguez, A.; Heydrich-Pérez, M.; El Jaziri, M.; Hernández-Lauzardo, A.N. Management of Black Pod Rot in Cacao (Theobroma Cacao L.): A Review. Fruits 2012, 67, 41–48. [Google Scholar] [CrossRef]
- Acosta, N.; De Vrieze, J.; Sandoval, V.; Sinche, D.; Wierinck, I.; Rabaey, K. Cocoa Residues as Viable Biomass for Renewable Energy Production through Anaerobic Digestion. Bioresour. Technol. 2018, 265, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Golveia, J.C.S.; Bara, M.T.F.; Santiago, M.F.; Campos, L.C.; Schimidt, F. Cocoa Agro-Industrial Residue (Theobroma cacao) as Inducer of the Production of Fungal Laccase and Kojic Acid for Application in the Biodegradation of 17-α-Ethinylestradiol. J. Braz. Chem. Soc. 2020, 31, 2023–2029. [Google Scholar] [CrossRef]
- Yusof, F.; Khanahmadi, S.; Amid, A.; Mahmod, S.S. Cocoa Pod Husk, a New Source of Hydrolase Enzymes for Preparation of Cross-Linked Enzyme Aggregate. Springerplus 2016, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomah, B.D. Cocoa (Theobroma cacao L.) Pod Husk: Renewable Source of Bioactive Compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; de Mello Castanho Amboni, R.D.; de Oliveira Petkowicz, C.L. Cacao Pod Husks (Theobroma cacao L.): Composition and Hot-Water-Soluble Pectins. Ind. Crops Prod. 2011, 34, 1173–1181. [Google Scholar] [CrossRef]
- Ayeni, L.S. Integrated Application of Cocoa Pod Ash and NPK Fertilizer on Soil Chemical Properties and Yield of Tomato. Am. J. Sustain. Agric. 2008, 2, 333–337. [Google Scholar]
- Ayeni, L.S.; Adetunji, M.T.; Ojeniyi, S.O.; Ewulo, B.S.; Adeyemo, A.J. Comparative and Cumulative Effect of Cocoa Pod Husk Ash and Poultry Manure on Soil and Maize Nutrient Contents and Yield. Am. J. Sustain. Agric. 2008, 2, 92–97. [Google Scholar]
- Putri, R.E.; Kasim, A.; Emriadi; Asben, A. Pyrolysis and Characterization of Liquid Smoke from Cacao Pod Husks. IOP Conf. Ser. Earth Environ. Sci. 2019, 327, 012011. [Google Scholar] [CrossRef]
- Mansur, D.; Tago, T.; Masuda, T.; Abimanyu, H. Conversion of Cacao Pod Husks by Pyrolysis and Catalytic Reaction to Produce Useful Chemicals. Biomass Bioenergy 2014, 66, 275–285. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Tsai, W.-T.; Liu, S.-C.; Lin, Y.-Q. Thermochemical Characterization of Biochar from Cocoa Pod Husk Prepared at Low Pyrolysis Temperature. Biomass Convers. Biorefinery 2018, 8, 237–243. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Hsu, C.-H.; Lin, Y.-Q.; Tsai, C.-H.; Chen, W.-S.; Chang, Y.-T. Enhancing the Pore Properties and Adsorption Performance of Cocoa Pod Husk (CPH)-Derived Biochars via Post-Acid Treatment. Process 2020, 8, 144. [Google Scholar] [CrossRef]
- Bahrun, A.; Yunus Fahimuddin, M.; Safuan, L.O.; Harjoni Kilowasid, L.M.; Singh, R. Effects of Cocoa Pod Husk Biochar on Growth of Cocoa Seedlings in Southeast Sulawesi-Indonesia. Asian J. Crop Sci. 2018, 10, 22–30. [Google Scholar] [CrossRef]
- Ghysels, S.; Acosta, N.; Estrada, A.; Pala, M.; De Vrieze, J.; Ronsse, F.; Rabaey, K. Integrating Anaerobic Digestion and Slow Pyrolysis Improves the Product Portfolio of a Cocoa Waste Biorefinery. Sustain. Energy Fuels 2020, 4, 3712–3725. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Contreras-Ramos, S.M.; Orozco-Avila, I.; Jaramillo-Flores, E.; Lugo-Cervantes, E. Effect of Fermentation Time and Drying Temperature on Volatile Compounds in Cocoa. Food Chem. 2012, 132, 277–288. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Biomass Proximate Analysis Using Thermogravimetry. Bioresour. Technol. 2013, 139, 1–4. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric Analysis as a New Method to Determine the Lignocellulosic Composition of Biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Grams, J. Chromatographic Analysis of Bio-Oil Formed in Fast Pyrolysis of Lignocellulosic Biomass. Rev. Anal. Chem. 2020, 39, 65–77. [Google Scholar] [CrossRef]
- Lu, F.; Rodriguez-Garcia, J.; Van Damme, I.; Westwood, N.J.; Shaw, L.; Robinson, J.S.; Warren, G.; Chatzifragkou, A.; McQueen Mason, S.; Gomez, L.; et al. Valorisation Strategies for Cocoa Pod Husk and Its Fractions. Curr. Opin. Green Sustain. Chem. 2018, 14, 80–88. [Google Scholar] [CrossRef]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn Growth and Nitrogen Nutrition after Additions of Biochars with Varying Properties to a Temperate Soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Gaur, S.; Reed, T. Thermal Data for Natural and Synthetic Fuels; Taylor & Francis: New York, NY, USA, 1998; ISBN 9780824700706. [Google Scholar]
- Manyà, J.J.; Velo, E.; Puigjaner, L. Kinetics of Biomass Pyrolysis: A Reformulated Three-Parallel-Reactions Model. Ind. Eng. Chem. Res. 2003, 42, 434–441. [Google Scholar] [CrossRef]
- Domene, A.; Conesa, J. Biomasses Pyrolysis and Combustion Kinetics through N-Th Order Parallel Reactions. Thermochim. Acta 2011, 523, 176–181. [Google Scholar] [CrossRef][Green Version]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass Pyrolysis Kinetics: A Comparative Critical Review with Relevant Agricultural Residue Case Studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Chen, D.; Gao, X.; Dollimore, D. A Generalized Form of the Kissinger Equation. Thermochim. Acta 1993, 215, 109–117. [Google Scholar] [CrossRef]
- García, A.N.; Marcilla, A.; Font, R. Thermogravimetric Kinetic Study of the Pyrolysis of Municipal Solid Waste. Thermochim. Acta 1995, 254, 277–304. [Google Scholar] [CrossRef]
- Wang, L.; Lei, H.; Liu, J.; Bu, Q. Thermal Decomposition Behavior and Kinetics for Pyrolysis and Catalytic Pyrolysis of Douglas Fir. RSC Adv. 2018, 8, 2196–2202. [Google Scholar] [CrossRef]
- Hameed, S.; Sharma, A.; Pareek, V.; Wu, H.; Yu, Y. A Review on Biomass Pyrolysis Models: Kinetic, Network and Mechanistic Models. Biomass Bioenergy 2019, 123, 104–122. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Mabon, R.; Broadbelt, L.J. A Critical Review on Hemicellulose Pyrolysis. Energy Technol. 2017, 5, 52–79. [Google Scholar] [CrossRef]
- Kleinert, M.; Barth, T. Phenols from Lignin. Chem. Eng. Technol. 2008, 31, 736–745. [Google Scholar] [CrossRef]
- Chen, L.; Liao, Y.; Guo, Z.; Cao, Y.; Ma, X. Products Distribution and Generation Pathway of Cellulose Pyrolysis. J. Clean. Prod. 2019, 232, 1309–1320. [Google Scholar] [CrossRef]

| Variable | Unit | Value |
|---|---|---|
| Initial moisture | % | 29.9 ± 6.3 |
| Final moisture | % | 10.5 ± 0.2 |
| Volatile matter | % | 59.4 ± 1.2 |
| Fixed carbon | % | 21.4 ± 0.4 |
| Ash | % | 8.8 ± 0.2 |
| Surface area | m2 g−1 | 0.24 ± 0.01 |
| C | % | 41.5 ± 1.0 |
| N | % | 1.69 ± 0.11 |
| H | % | 6.2 ± 0.2 |
| O * | % | 41.6 ± 1.0 |
| S | % | 0.20 ± 0.04 |
| Empiric formula | - | CH1.79N0.03O0.75 |
| H/C molar ratio | 1.79 ± 0.07 | |
| O/C molar ratio | 0.75 ± 0.03 | |
| HHV ** | MJ kg−1 | 17.3 ± 0.4 |
| Hemicellulose *** | % | 21.2 ± 0.4 |
| Cellulose *** | % | 23.2 ± 0.5 |
| Lignin *** | % | 15.0 ± 0.3 |
| pH | - | 5.7 ± 0.1 |
| Electrical conductivity | mS | 6.7 ± 0.2 |
| Parameter | ||||
|---|---|---|---|---|
| (s−1) | 1.87 × 109 | 2.74 × 1013 | 1.41 × 1040 | 1.20 × 107 |
| 12,527.41 | 19,130.53 | 54,889.86 | 15,032.63 | |
| (kJmol−1) | 104.16 | 159.06 | 456.38 | 124.99 |
| 4.20 | 2.80 | 8.40 | 2.64 | |
| 0.18 | 0.37 | 0.32 | 0.14 |
| Parameter | ||||
|---|---|---|---|---|
| (s−1) | 2.95 × 108 | 3.29 × 107 | 4.77 × 1049 | 9.39 × 106 |
| 13,333.08 | 16,066.52 | 85,362.67 | 18,950.29 | |
| (kJmol−1) | 110.86 | 133.58 | 709.75 | 157.56 |
| 2.49 | 1.13 | 0.65 | 1.37 | |
| 0.61 | 0.33 | 0.02 | 0.04 |
| T process (°C) | Compound | % Area * |
|---|---|---|
| CO2 | 17 | |
| water | 22 | |
| acetic acid | 8.3 | |
| 2-propanone,1-hydroxy- | 3.3 | |
| 2-cyclopenten-1-one, 2-hydroxy- | 1.4 | |
| 150–350 | phenol,2-methoxy-4-(1-propenyl)- | 1.8 |
| phenol,2,6-di-methoxy-4-(2-propenyl)- | 4.9 | |
| hexadecanoic acid | 1.6 | |
| 9-octadecenoic acid | 1.3 | |
| octadecanoic acid | 1.2 | |
| CO2 | 9.4 | |
| water | 12 | |
| 350–500 | furan, 2-methyl | 1.0 |
| toluene | 1.4 | |
| phenol,2-methoxy | 1.6 | |
| CO2 | 22 | |
| acetic acid | 6.5 | |
| 2-propanone,1-hydroxy- | 4.3 | |
| 2-cyclopenten-1-one + furfural | 1.1 | |
| 2-cyclopenten-1-one, 2-hydroxy- | 1.3 | |
| 400 (flash) | 2-methoxy-4-vinylphenol | 1.0 |
| phenol,2-methoxy-4-(1-propenyl)- | 1.0 | |
| phenol,2,6-di-methoxy-4-(2-propenyl)- | 1.0 | |
| hexadecanoic acid | 3.2 | |
| 9-octadecenoic acid | 1.9 | |
| octadecanoic acid | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Londoño-Larrea, P.; Villamarin-Barriga, E.; García, A.N.; Marcilla, A. Study of Cocoa Pod Husks Thermal Decomposition. Appl. Sci. 2022, 12, 9318. https://doi.org/10.3390/app12189318
Londoño-Larrea P, Villamarin-Barriga E, García AN, Marcilla A. Study of Cocoa Pod Husks Thermal Decomposition. Applied Sciences. 2022; 12(18):9318. https://doi.org/10.3390/app12189318
Chicago/Turabian StyleLondoño-Larrea, Pablo, Estefania Villamarin-Barriga, Angela N. García, and Antonio Marcilla. 2022. "Study of Cocoa Pod Husks Thermal Decomposition" Applied Sciences 12, no. 18: 9318. https://doi.org/10.3390/app12189318
APA StyleLondoño-Larrea, P., Villamarin-Barriga, E., García, A. N., & Marcilla, A. (2022). Study of Cocoa Pod Husks Thermal Decomposition. Applied Sciences, 12(18), 9318. https://doi.org/10.3390/app12189318






