Abstract
The intrinsic muscles of the hand are responsible for finger flexion and extension. The purpose of this study was to investigate the usefulness of stimulating the intrinsic muscles of the hand using repetitive peripheral magnetic stimulation (rPMS). We evaluated angular changes in the finger joints by studying active motion and rPMS. Ten healthy adults were instructed to perform the following tests in random order: (1) maximum active metacarpophalangeal joint flexion; (2) maximum active metacarpophalangeal joint abduction; and (3) repetitive peripheral magnetic stimulation for 2 s at maximum stimulation intensity. A three-dimensional motion analysis system was used to measure angular changes. Pain during stimulation was graded on a numerical rating scale (NRS). The maximum flexion and abduction of the metacarpophalangeal joint were not significantly different between active motion and rPMS. The proximal interphalangeal joint (p = 0.009) and distal interphalangeal joint (p = 0.005) were significantly extended by rPMS. The median NRS score for pain during rPMS was 2. rPMS can produce the same extent of metacarpophalangeal joint flexion and abduction as active movement with less pain. This technique can effectively stimulate the intrinsic muscles of the hand and may be used as a treatment for various diseases that cause immobility of the metacarpophalangeal joints.
1. Introduction
The interosseous and lumbrical muscles are among the intrinsic muscles of the hand that are responsible for flexion of the metacarpophalangeal (MCP) joint and extension of the proximal interphalangeal (PIP) and distal interphalangeal (DIP) joints. These muscles play important roles in grasping and pinching [1,2]. The lumbrical muscles have abundant muscle spindles and nerve distribution that provide the sensory information necessary for fine finger movements [3]. Impairment of the intrinsic muscles of the hand results in a claw hand or intrinsic minus hand with the MCP joint extended and the PIP and DIP joints flexed [2,4]. Hand dysfunction affects not only activities of daily living (ADL) [5,6], but also participation in work, family roles, and autonomy outdoors [7].
In a previous study, percutaneous electrodes were implanted into the intrinsic muscles of the hand to provide electrical stimulation (ES), and the paralyzed hand with spinal cord injury generated 80–90% of the flexion moments attained by able-bodied participants [8]. Another study found that in a patient with cervical spinal cord injury, the flexion force of the MCP joint increased after 2 weeks of ES of the lumbrical muscles via surface electrodes from the palm of the hand [9]. Thus, ES of the intrinsic muscles of the hand promotes flexion of the MCP joint and may be an effective therapeutic procedure. However, implanted electrodes require invasion of the intrinsic muscles, and surface electrodes may cause pain during ES [10,11].
Repetitive peripheral magnetic stimulation (rPMS) is a surface system that induces eddy currents via electromagnetic induction, which stimulates the peripheral nerves and muscles without stimulating skin nociceptors, resulting in the activation of the parieto-premotor network [12,13]. Furthermore, rPMS drives M1 plasticity and sensorimotor improvements [14]. Pain caused by rPMS is significantly lower than ES-induced pain, even when using the same intensity of stimulation [15]. Therefore, rPMS provides stronger stimulation than ES with limited pain [16,17,18]. Pain suppresses neuromuscular activity from the cerebrum [10,11]. Hence, limited pain during stimulation is a major advantage of rPMS, which has been applied to various somatosensory and motor disorders in recent years [19,20]. However, there are no reports available on the application of rPMS to movement disorders of the intrinsic muscles of the hand. Here, we evaluated the effectiveness of applying rPMS to the intrinsic muscles of the hand by analyzing the angular changes of the MCP, PIP, and DIP joints with a three-dimensional motion analysis system kinematically.
2. Materials and Methods
2.1. Study Participants and Trial Design
This prospective exploratory study was conducted according to the guidelines of the Declaration of Helsinki, approved by the Certified Clinical Research Review Board in our institution, and was registered with the Japan Registry of Clinical Trials (no. jRCTs042180062). All participants provided written informed consent.
The study included 10 right-handed healthy adults (5 women) with no history of neurological problems or bone and joint disease in the upper limb. The mean age of the study participants was 25 years (range: 23–29 years), and their mean hand length was 17.6 cm (range: 16.0–19.5 cm). The dominant right hand was used frequently in ADL, hobby activities, sports, etc., depending on the individuals. Therefore, the left hand, which is the non-dominant hand, was targeted for verification under the same conditions as much as possible.
Seven colored markers (10 mm in diameter) were attached to the radial styloid process; the radial side of the MCP, PIP, and DIP joints; the tips of the index and middle fingers; and the base of the third metacarpal bone (Figure 1a). To eliminate the effect of gravity, the participants sat on a chair with their forearm neutral and the MCP, PIP, and DIP joints set at 0° (Figure 1b). The participants were instructed to perform the following tests three times each in random order: (1) maximum active MCP joint flexion in the PIP and DIP extended position; (2) maximum active MCP joint abduction in the PIP and DIP extended position; and (3) rPMS for 2 s at a frequency of 30 Hz at maximum stimulation intensity (magnetic flux density of 0.9 T). Stimulus parameters were set with reference to previous studies of rPMS for upper limb motor dysfunction [17,21]. Nine trials were performed with a 60 s interval using a random number table. A three-dimensional motion analysis system (KinemaTracer; KISSEI COMTEC, Matsumoto, Japan), at a frequency of 60 Hz, was used to measure angular changes in the MCP, PIP, and DIP joints. The MCP joint flexion angle of the index finger was calculated as the angle formed by the radial styloid process, MCP joint, and PIP joint markers. The PIP joint angle of the index finger was calculated as the angle formed by the MCP, PIP, and DIP joint markers. The DIP joint angle of the index finger was calculated as the angle formed by the PIP, DIP, and tip of the index finger markers. The MCP joint abduction angle was defined as the angle formed by the line connecting the tip of the index finger to the base of the third metacarpal bone and the line connecting the tip of the middle finger to the marker at the base of the third metacarpal bone. In the three trials for each participant, data on the maximum flexion of the MCP joint angle were used for subsequent analyses. Furthermore, pain during rPMS was graded on a scale of 0–10 using a numerical rating scale (NRS, with 0 representing no pain and 10 representing the most severe pain) [22].
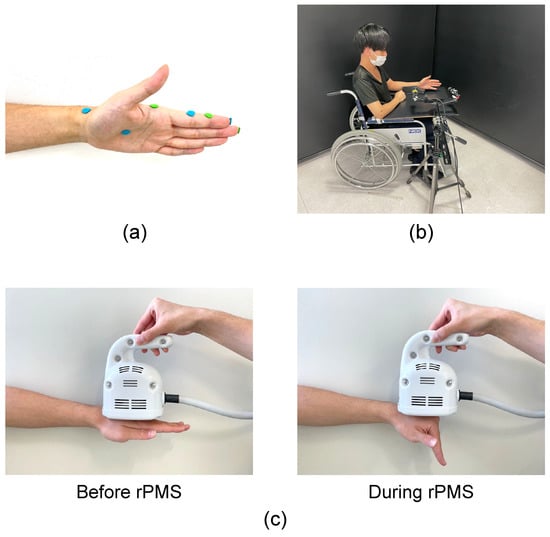
Figure 1.
(a) Marker placements. Markers were placed at the radial styloid process; the radial side of the MCP, PIP, and DIP joints of the index finger; the tips of the index and middle fingers; and the base of the third metacarpal bone. (b) Setting. the participant sat on a chair with their forearm neutral and the MCP, PIP, and DIP joints set at 0°. (c) Stimulation of the intrinsic muscles of the hand from the dorsum using repetitive peripheral magnetic stimulation (rPMS). To stimulate intrinsic muscles, the center of the coil for rPMS was placed between the second and third metacarpal bones on the dorsum of the hand. During rPMS, the MCP joint was flexed while the PIP and DIP joints were extended.
2.2. rPMS Technique
We used a peripheral magnetic stimulator (PathleaderTM; IFG, Sendai, Japan) to generate a biphasic 350 μs magnetic gradient of up to 15 kT/s. The circular coil in this system changes the magnetic field by up to 2 cm in depth, thereby stimulating skeletal muscles and peripheral nerves. The center of the rPMS coil was placed between the second and third metacarpal bones on the dorsum of the hand to stimulate the intrinsic muscles. Accordingly, the position of the coil did not interfere with the flexion of the MCP joint (Figure 1c). By placing the echo probe on the palm of the hand, we confirmed that rPMS can also stimulate the lumbrical muscles via the dorsum of the hand.
2.3. Statistical Analysis
The Shapiro–Wilk test was used to determine normality. The paired t-test was used to compare the maximum change in joint angles between active motion and rPMS. The values are expressed as means ± SDs. SPSS Statistics version 27 (IBM, Armonk, NY, USA) was used for statistical analyses. The statistical significance level was set at p < 0.05.
3. Results
The study was successfully completed by all participants without any adverse events. The temporal changes in the MCP, PIP, and DIP joints of the index finger during active MCP joint flexion and rPMS are shown in Figure 2. In the active movements, the PIP and DIP joints flexed slightly with MCP joint flexion (Figure 2a). Shortly after the initiation of rPMS, the MCP joint abducted and flexed, and the flexion angle increased as the abduction angle of the MCP joint decreased. The PIP and DIP joints extended during rPMS (Figure 2b).
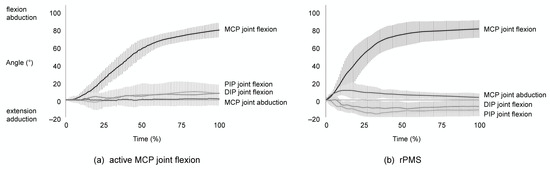
Figure 2.
Temporal changes in the MCP, PIP, and DIP joint angles of the index finger during active MCP joint flexion and rPMS. The solid lines represent the average changes in joint angles of the index finger, while the vertical lines represent the SD. (a) The PIP and DIP joints were slightly flexed with MCP joint flexion in active MCP joint flexion. (b) The MCP joint abducted and flexed shortly after the onset of rPMS, and the flexion angle increased as the abduction angle of the MCP joint decreased. The PIP and DIP joints extended.
The maximum joint angles for each participant are shown in Table 1. They were normally distributed. The maximum active flexion of the MCP joint was 83 ± 4°, with the PIP joint extending by 6 ± 14° and the DIP joint extending by 6 ± 10°. The maximum active abduction of the MCP joint was 16 ± 6°. Using rPMS, the maximum flexion of the MCP, PIP, and DIP joints, as well as the maximum abduction of the MCP joint, were 82 ± 10°, −12 ± 5°, −8 ± 7°, and 15 ± 6°, respectively. The maximum flexion and abduction of the MCP joint were not significantly different between active motion and rPMS. However, the PIP and DIP joints extended significantly more during rPMS (Figure 3). The median NRS for pain during rPMS was 2 (range: 0–4).

Table 1.
Maximum joint angles between active MCP joint flexion and rPMS.
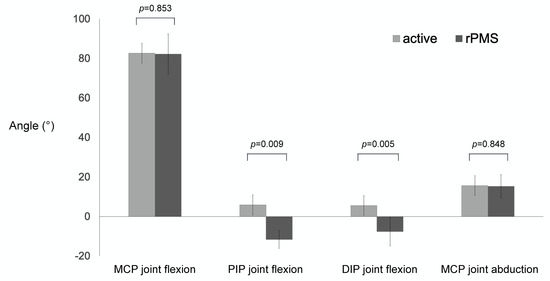
Figure 3.
The maximum angles of the of MCP, PIP, and DIP joints by active movement and rPMS. There were no significant differences in the maximum angles of flexion and abduction of the MCP joint. rPMS extended the PIP and DIP joints significantly more.
4. Discussion
This simple rPMS technique produced the same extent of flexion and abduction of the MCP joint as active movement. Pain during rPMS was lower. Furthermore, the PIP and DIP joints were extended more in rPMS than in active motion, indicating that the interosseous and lumbrical muscles were stimulated. This technique is unique because it stimulates the intrinsic muscles from the dorsum of the hand to flex the MCP joint. Voluntary MCP joint flexion is usually accompanied by the activities of the extrinsic muscles (flexor digitorum superficialis and flexor digitorum profundus) resulting in PIP and DIP joint flexion. In contrast, MCP joint flexion and PIP and DIP joint extension occur by stimulating intrinsic muscles with rPMS. We can activate the intrinsic muscles of the hand selectively by applying rPMS without compensatory movement of the extrinsic muscles.
This technique could be used as a therapeutic procedure for various conditions that cause immobility of the MCP joint secondary to dysfunction of the intrinsic muscles of the hand. When the intrinsic muscles of the hand are not activated, MCP joint contracture in the extended position tends to occur [2]. rPMS may prevent joint contractures from immobility by repeatedly flexing the MCP joint. Furthermore, this procedure may improve paralysis, increase the flexion strength of the MCP joint, and improve pinching or fine finger movements in patients with hemiplegia due to a stroke or incomplete cervical spinal cord injury.
This study has several limitations. We evaluated hand joint movement by stimulating the intrinsic muscles of the hand using rPMS in healthy subjects. The rPMS coil was the same regardless of the participant’s hand or muscle size. Therefore, depending on the subject’s physical characteristics, the joint movements induced by rPMS vary. In addition, we did not evaluate different age groups nor differences by sex. The sample size was small in this study. Because we could not find any published articles to calculate the sample size for this study, we conducted this study in an exploratory manner. We evaluated 10 subjects and found that the maximum flexion and abduction of the MCP joints were not significantly different between active motion and rPMS, whereas the PIP and DIP joints were significantly extended by rPMS. Based on the results of this study, the estimated sample sizes required to show a significance level of 5% and a detection power of 80% for MCP joint flexion, MCP joint abduction, PIP joint flexion, and DIP joint flexion are 2183, 1604, 5, and 6, respectively, calculated with G*Power 3.1 software [23,24]. Hence, the results in a larger sample would be similar to the results of this study. Moreover, the center of the rPMS coil was placed between the second and third metacarpal bones and the angular changes of the index finger were evaluated in this study. The movement of the ring and little fingers were expected to be smaller than that of the index finger. We assessed only healthy individuals, so it is uncertain whether similar MCP joint movements can be obtained in patients with stroke or cervical spinal cord injury. ES takes place primarily by excitation of the intramuscular nerves, and only 3–7% of the muscle twitch force is produced by direct stimulation of muscle fibers [25]. Thus, ES cannot generate a sufficient level of muscle contraction in patients with severe peripheral nerve injury [26]. Although this study did not compare rPMS with ES, rPMS results in muscle contraction through a similar mechanism as ES. When the dominant nerve of the intrinsic muscles of the hand is affected, rPMS may not be effective. rPMS stimulated the intrinsic muscles of the hand selectively in this study; however, hyperextended PIP and DIP joints may cause damage to some persons.
5. Conclusions
We were able to stimulate the intrinsic muscles of the hand and produce the same extent of MCP joint flexion and abduction as active movement by performing rPMS with healthy individuals. Further studies are needed in different age groups as well as comparisons by sex. Moreover, we plan to evaluate the effect of rPMS in patients with dysfunction, muscle weakness, and/or muscle atrophy of the intrinsic muscles of the hand.
Author Contributions
All authors designed and conducted the study, collected data, and analyzed data. K.F. prepared the manuscript draft with valuable intellectual input from H.K. and H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, approved by the Fujita Health University Ethics Committee, and was registered with the Japan Registry of Clinical Trials (no. jRCTs042180062).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data collected and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Ryoka Itoh (OTR) and Daisuke Yamaguchi (OTR) from the Department of Rehabilitation at Fujita Health University Hospital for their assistance with the measurements used to collect data for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jacobson, M.D.; Raab, R.; Fazeli, B.M.; Abrams, R.A.; Botte, M.J.; Lieber, R.L. Architectural design of the human intrinsic hand muscles. J. Hand Surg. Am. 1992, 17, 804–809. [Google Scholar] [CrossRef]
- Schreuders, T.A.; Brandsma, J.W.; Stam, H.J. The intrinsic muscles of the hand. Phys. Rehab. Kur. Med. 2007, 17, 20–27. [Google Scholar] [CrossRef]
- Soukup, T.; Pedrosa-Domellöf, F.; Thornell, L.E. Intrafusal fiber type composition of muscle spindles in the first human lumbrical muscle. Acta Neuropathol. 2003, 105, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kozin, S.H.; Porter, S.; Clark, P.; Thoder, J.J. The contribution of the intrinsic muscles to grip and pinch strength. J. Hand Surg. Am. 1999, 24, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Shimizu, H. Essential motion of metacarpophalangeal joints during activities of daily living. J. Hand Ther. 2013, 26, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Murai, T.; Uchiyama, S.; Nakamura, K.; Ido, Y.; Hata, Y.; Kato, H. Functional range of motion in the metacarpophalangeal joints of the hand measured by single axis electric goniometers. J. Orthop. Sci. 2018, 23, 504–510. [Google Scholar] [CrossRef]
- Videler, A.J.; Beelen, A.; van Schaik, I.N.; de Visser, M.; Nollet, F. Limited upper limb functioning has impact on restrictions in participation and autonomy of patients with hereditary motor and sensory neuropathy 1a. J. Rehabil. Med. 2009, 41, 746–750. [Google Scholar] [CrossRef]
- Lauer, R.T.; Kilgore, K.L.; Peckham, P.H.; Bhadra, N.; Keith, M.W. The function of the finger intrinsic muscles in response to electrical stimulation. IEEE Trans. Rehabil. Eng. 1999, 7, 19–26. [Google Scholar] [CrossRef]
- Carroll, S.G.; Bird, S.F.; Brown, D.J. Electrical stimulation of the lumbrical muscles in an incomplete quadriplegic patient: Case report. Paraplegia 1992, 30, 223–226. [Google Scholar] [CrossRef]
- Kofler, M.; Glocker, F.X.; Leis, A.A.; Seifert, C.; Wissel, J.; Kronenberg, M.F.; Fuhr, P. Modulation of upper extremity motoneurone excitability following noxious finger tip stimulation in man: A study with transcranial magnetic stimulation. Neurosci. Lett. 1998, 246, 97–100. [Google Scholar] [CrossRef]
- Valeriani, M.; Restuccia, D.; Di Lazzaro, V.; Oliviero, A.; Le Pera, D.; Profice, P.; Saturno, E.; Tonali, P. Inhibition of biceps brachii muscle motor area by painful heat stimulation of the skin. Exp. Brain Res. 2001, 139, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Struppler, A.; Angerer, B.; Havel, P. Modulation of sensorimotor performances and cognition abilities induced by RPMS: Clinical and experimental investigations. Suppl. Clin. Neurophysiol. 2003, 56, 358–367. [Google Scholar] [PubMed]
- Struppler, A.; Binkofski, F.; Angerer, B.; Bernhardt, M.; Spiegel, S.; Drzezga, A.; Bartenstein, P. A fronto-parietal network is mediating improvement of motor function related to repetitive peripheral magnetic stimulation: A PET-H2O15 study. Neuroimage 2007, 36, T174–T186. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.D.; Massé-Alarie, H.; Camiré-Bernier, S.; Ribot-Ciscar, É.; Schneider, C. After-effects of peripheral neurostimulation on brain plasticity and ankle function in chronic stroke: The role of afferents recruited. Neurophysiol. Clin. 2017, 47, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Han, T.R.; Shin, H.I.; Kim, I.S. Magnetic stimulation of the quadriceps femoris muscle: Comparison of pain with electrical stimulation. Am. J. Phys. Med. Rehabil. 2006, 85, 593–599. [Google Scholar] [CrossRef]
- Smania, N.; Corato, E.; Fiaschi, A.; Pietropoli, P.; Aglioti, S.M.; Tinazzi, M. Therapeutic effects of peripheral repetitive magnetic stimulation on myofascial pain syndrome. Clin. Neurophysiol. 2003, 114, 350–358. [Google Scholar] [CrossRef]
- Fujimura, K.; Kagaya, H.; Endou, C.; Ishihara, A.; Nishigaya, K.; Muroguchi, K.; Tanikawa, H.; Yamada, M.; Kanada, Y.; Saitoh, E. Effects of repetitive peripheral magnetic stimulation on shoulder subluxations caused by stroke: A preliminary study. Neuromodulation 2020, 23, 847–851. [Google Scholar] [CrossRef]
- Kagaya, H.; Ogawa, M.; Mori, S.; Aoyagi, Y.; Shibata, S.; Inamoto, Y.; Mori, H.; Saitoh, E. Hyoid bone movement at rest by peripheral magnetic stimulation of suprahyoid muscles in normal individuals. Neuromodulation 2019, 22, 593–596. [Google Scholar] [CrossRef]
- Beaulieu, L.D.; Schneider, C. Effects of repetitive peripheral magnetic stimulation on normal or impaired motor control. A review. Neurophysiol. Clin. 2013, 43, 251–260. [Google Scholar] [CrossRef]
- Beaulieu, L.D.; Schneider, C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: A literature review on parameters of application and afferents recruitment. Neurophysiol. Clin. 2015, 45, 223–237. [Google Scholar] [CrossRef]
- Obayashi, S.; Takahashi, R. Repetitive peripheral magnetic stimulation improves severe upper limb paresis in early acute phase stroke survivors. NeuroRehabilitation 2020, 46, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S.; European Palliative Care Research Collaborative (EPCRC). Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: A systematic literature review. J. Pain Symptom. Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis progrAm. for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Pui Kei, C.; Mohd Nordin, N.A.; Abdul Aziz, A.F. The effectiveness of home-based therapy on functional outcome, self-efficacy and anxiety among discharged stroke survivors. Medicine 2020, 99, e23296. [Google Scholar] [CrossRef]
- Crago, P.E.; Peckham, P.H.; Mortimer, J.T.; Van der Meulen, J.P. The choice of pulse duration for chronic electrical stimulation via surface, nerve, and intramuscular electrodes. Ann. BioMed. Eng. 1974, 2, 252–264. [Google Scholar] [CrossRef]
- Kagaya, H.; Shimada, Y.; Sato, K.; Sato, M. Changes in muscle force following therapeutic electrical stimulation in patients with complete paraplegia. Paraplegia 1996, 34, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).