Safety Assessment of Organic High-Protein Bars during Storage at Ambient and Refrigerated Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of High-Protein Bar
2.2. Microbiological Quality Evaluation
2.3. Water Activity Evaluation
2.4. Mycotoxin Evaluation
2.5. Biogenic Amines Content
2.6. Analysis of Lipid Oxidation TBARS
2.7. Presence of Pests
2.8. Statistical Analysis
3. Results
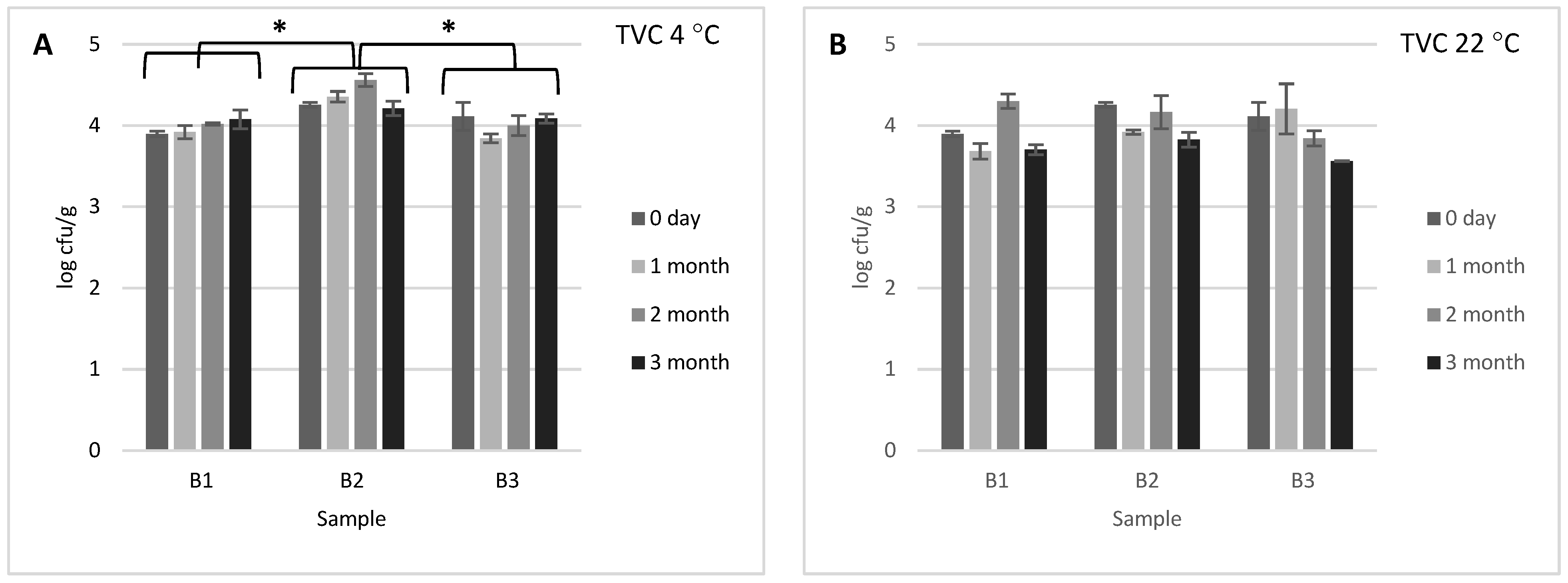
3.1. Microbiological Quality Evaluation
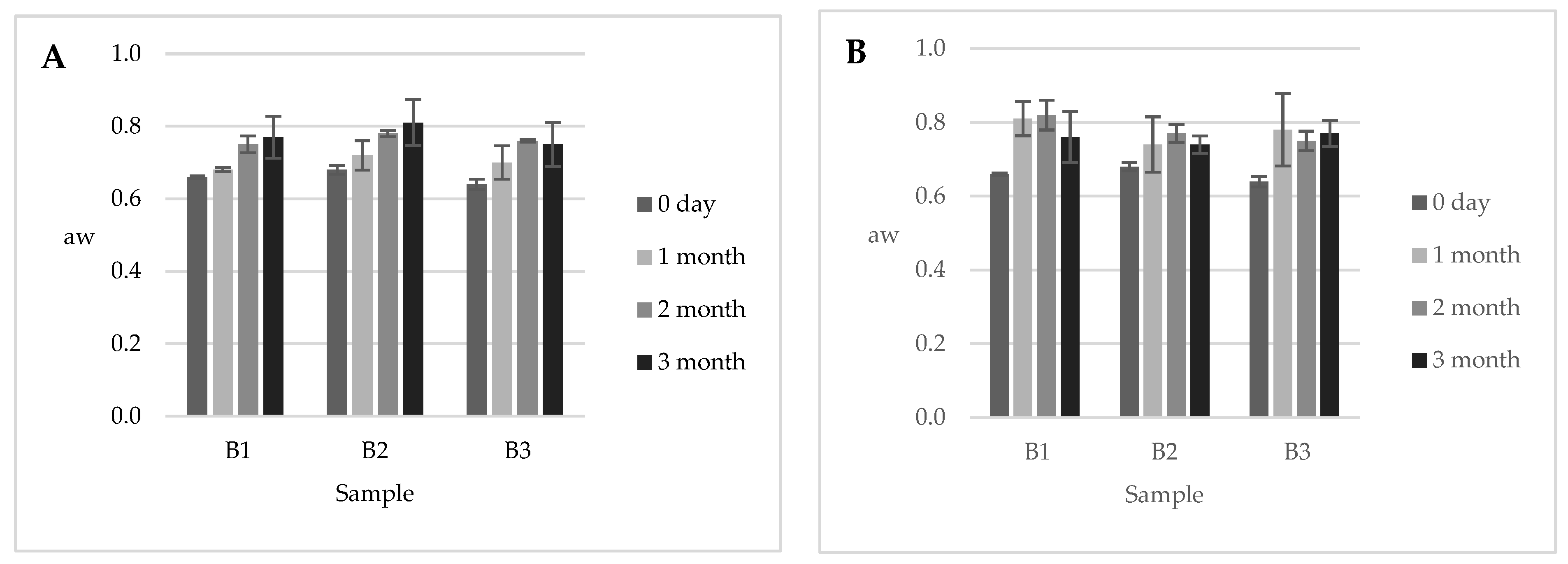
3.2. Water Activity Evaluation
3.3. Mycotoxin Content
3.4. Biogenic Amines Content
3.5. Analysis of Lipid Oxidation as TBARS
3.6. Presence of Pests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Annunziato, R.A.; Timko, C.A.; Crerand, C.E.; Didie, E.R.; Bellace, D.L.; Phelan, S.; Kerzhnerman, I.; Lowe, M.R. A randomized trial examining differential meal replacement adherence in a weight loss maintenance program after one-year follow-up. Eat. Behav. 2009, 10, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Miraballes, M.; Fiszman, S.; Gámbaro, A.; Varela, P. Consumer perceptions of satiating and meal replacement bars, built up from cues in packaging information, health claims and nutritional claims. Food Res. Int. 2014, 64, 456–464. [Google Scholar] [CrossRef]
- Ashley, J.M.; Herzog, H.; Clodfelter, S.; Bovee, V.; Schrage, J.; Pritsos, C. Nutrient adequacy during weight loss interventions: A randomized study in women comparing the dietary intake in a meal replacement group with a traditional food group. Nutr. J. 2007, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, D.A.; Pacanowski, C. Losing weight without dieting. Use of commercial foods as meal replacements for lunch produces an extended energy deficit. Appetite 2011, 57, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Matt, D.; Rembiałkowska, E.; Luik, A.; Peetsmann, E.; Pehme, S. Quality of Organic vs. Conventional Food and Effects on Health: Report; Estonian University of Life Sciences: Tartu, Estonia, 2011. [Google Scholar]
- Parn, O.J.; Bhat, R.; Yeoh, T.K.; Al-Hassan, A.A. Development of novel fruit bars by utilizing date paste. Food Biosci. 2015, 9, 20–27. [Google Scholar] [CrossRef]
- Szydłowska, A.; Zielińska, D.; Łepecka, A.; Trząskowska, M.; Neffe-Skocińska, K.; Kołożyn-Krajewska, D. Development of functional high-protein organic bars with the addition of whey protein concentrate and bioactive ingredients. Agriculture 2020, 10, 390. [Google Scholar] [CrossRef]
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods 2006; Publications Office of the European Union: Luxembourg, 2016.
- Fong, K.; Wang, S. Strain-specific survival of Salmonella enterica in peanut oil, peanut shell, and chia seeds. J. Food Prot. 2016, 79, 361–368. [Google Scholar] [CrossRef]
- Beuchat, L.R.; Komitopoulou, E.; Beckers, H.; Betts, R.P.; Bourdichon, F.; Fanning, S.; Joosten, H.M.; Ter Kuile, B.H. Low-water activity foods: Increased concern as vehicles of foodborne pathogens. J. Food Prot. 2013, 76, 150–172. [Google Scholar] [CrossRef]
- ISO 4833-1; Microbiology of the Food Chain-Horizontal Method for the Enumeration of Microorganisms-Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- ISO 21528-2; Microbiology of Food and Feeding Stuffs-Horizontal Method for the Detection and Enumeration of Enterobacteriaceae-Part 2: Colony Count Method. ISO: Geneva, Switzerland, 2017.
- ISO 21527-1; Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Enumeration of Yeasts and Moulds-Part 1: Colony Count Technique in Products with Water Activity Greater Than 0,95. ISO: Geneva, Switzerland, 2008.
- ISO 6579-1; Microbiology of the Food Chain-Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella-Part 1: Detection of Salmonella spp. ISO: Geneva, Switzerland, 2017.
- ISO 21871; Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Determination of Low Numbers of Presumptive Bacillus Cereus-Most Probable Number Technique and Detection Method. ISO: Geneva, Switzerland, 2006.
- PN-EN ISO 6888-1; Food and Feed Microbiology-Horizontal Method for Determining the Number of Coagulase-Positive Staphylococci (Staphylococcus Aureus and Other Species)-Part 1: Method Using Baird-Parker Agar Medium. PKN: Warsaw, Poland, 2001.
- ISO 21807:2004; Microbiology of Food and Animal Feeding Stuffs—Determination of Water Activity. ISO: Geneva, Switzerland, 2004.
- PN-EN 14123; Foodstuffs-Determination of Aflatoxin B1 and the Sum of Aflatoxin B1, B2, G1 and G2 in Hazelnuts, Peanuts, Pistachios, Figs and Paprika Powder-High Performance Liquid Chromatographic Method with Post-Column Derivatisation and Immunoaffinity Column Cleanup. PKN: Warsaw, Poland, 2008.
- PN-EN 14132; Foodstuffs-Determination of Ochratoxin A in Barley and Roasted Coffe-HPLC Method with Immunoaffinity Column Clean-Up. PKN: Warsaw, Poland, 2010.
- J.S. Hamilton Laboratory Determination of Zearalenone Content; Range: 10÷4000 μg/kg by High-Performance Liquid Chromatography-PB-44/HPLC wyd. III z dn. 28.02.2009. Available online: https://hamilton.com.pl/wp-content/uploads/2017/03/Zatwierdzony_przez_MON_Zakres_Akredytacji_OiB_20.10.2016.pdf (accessed on 21 August 2022).
- J.S. Hamilton Laboratory Determination of Deoxynivalenol Content; 100÷20 000 μg/kg by High-Performance Liquid Chromatography-PB-226/LC wyd. III z dn. 02.01.2015. Available online: https://hamilton.com.pl/wp-content/uploads/2017/03/Zatwierdzony_przez_MON_Zakres_Akredytacji_OiB_20.10.2016.pdf (accessed on 21 August 2022).
- Smělá, D.; Pechová, P.; Komprda, T.; Klejdus, B.; Kubáň, V. Liquid chromatographic determination of biogenic amines in a meat product during fermentation and long-term storage. Czech J. Food Sci. 2003, 21, 167–175. [Google Scholar] [CrossRef]
- Pikul, J.; Leszczynski, D.E.; Kummerow, F.A. Evaluation of three modified TBA methods for measuring lipid oxidation in chicken meat. J. Agric. Food Chem. 1989, 37, 1309–1313. [Google Scholar] [CrossRef]
- Magkos, F.; Arvaniti, F.; Zampelas, A. Organic food: Buying more safety or just peace of mind? A critical review of the literature. Crit. Rev. Food Sci. Nutr. 2006, 46, 23–56. [Google Scholar] [CrossRef] [PubMed]
- Gomiero, T. Food quality assessment in organic vs. conventional agricultural produce: Findings and issues. Appl. Soil Ecol. 2018, 123, 714–728. [Google Scholar] [CrossRef]
- Hubert, J.; Stejskal, V.; Athanassiou, C.G.; Throne, J.E. Health hazards associated with arthropod infestation of stored products. Annu. Rev. Entomol. 2018, 63, 553–573. [Google Scholar] [CrossRef] [PubMed]
- Munshi, R.; Kochhar, A.; Kaur, A. Nutrient selection and optimization to formulate a nutrient bar stable on storage and specific to women at risk of osteoporosis. J. Food Sci. Technol. 2020, 57, 3099–3107. [Google Scholar] [CrossRef] [PubMed]
- Bhakha, T.; Ramasawmy, B.; Toorabally, Z.; Neetoo, H.; Bhakha, T.; Ramasawmy, B.; Toorabally, Z.; Neetoo, H. Development, characterization and shelf-life testing of a novel pulse-based snack bar. AIMS Agric. Food 2019, 4, 756–777. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O. Fungal mycotoxins in foods: A review. Cogent Food Agric. 2016, 2, 1213127. [Google Scholar] [CrossRef]
- Jin, J.; Beekmann, K.; Ringø, E.; Rietjens, I.M.C.M.; Xing, F. Interaction between food-borne mycotoxins and gut microbiota: A review. Food Control 2021, 126, 107998. [Google Scholar] [CrossRef]
- Singh, R.; Singh, K.; Nain, M.S. Nutritional evaluation and storage stability of popped pearl millet bar. Curr. Sci. 2021, 120, 1374–1381. [Google Scholar] [CrossRef]
- Regulation (EU) No 1441/Commission Regulation (EC) No 1441/2007 of 5 December 2007 Amending Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs 2007; Publications Office of the European Union: Luxembourg, 2017.
- EFSA Panel on Biological Hazards. Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016, 14, e04524. [Google Scholar] [CrossRef]
- Hamanaka, D.; Uchino, T.; Hu, W.; Yasunaga, E. Effects of infrared radiation on inactivation of Bacillus subtilis spore and Aspergillus niger spore. J. Jpn. Soc. Agric. Mach. 2002, 64, 69–75. [Google Scholar] [CrossRef]
- Hamanaka, D.; Norimura, N.; Baba, N.; Mano, K.; Kakiuchi, M.; Tanaka, F.; Uchino, T. Surface decontamination of fig fruit by combination of infrared radiation heating with ultraviolet irradiation. Food Control 2011, 22, 375–380. [Google Scholar] [CrossRef]
- Tabanelli, G. Biogenic amines and food quality: Emerging challenges and public health concerns. Foods 2020, 9, 859. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.P.; Rodrigues, B.L.; Frasao, B.S.; Conte-Junior, C.A. Biogenic amines as food quality index and chemical risk for human consumption. In Food Quality: Balancing Health and Disease; Handbook of Food Bioengineering; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 75–108. ISBN 978-0-12-811442-1. [Google Scholar]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef]
- Waqas, A.; Mohammed, G.I.; Al-Eryani, D.A.; Saigl, Z.M.; Alyoubi, A.O.; Alwael, H.; Bashammakh, A.S.; O’Sullivan, C.K.; El-Shahawi, M.S. Biogenic amines formation mechanism and determination strategies: Future challenges and limitations. Crit. Rev. Anal. Chem. 2020, 50, 485–500. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards. Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Etemadian, Y.; Shabanpour, B.; Ramzanpour, Z.; Shaviklo, A.R.; Kordjazi, M. Production of the corn snack seasoned with brown seaweeds and their characteristics. J. Food Meas. Charact. 2018, 12, 2068–2079. [Google Scholar] [CrossRef]
- Vinson, J.A.; Zubik, L.; Bose, P.; Samman, N.; Proch, J. Dried fruits: Excellent in vitro and in vivo antioxidants. J. Am. Coll. Nutr. 2005, 24, 44–50. [Google Scholar] [CrossRef]
- Ryan, L.; Thondre, P.S.; Henry, C.J.K. Oat-based breakfast cereals are a rich source of polyphenols and high in antioxidant potential. J. Food Compos. Anal. 2011, 24, 929–934. [Google Scholar] [CrossRef]

| Ingredient (g) | Bar Symbol | ||
|---|---|---|---|
| B1 | B2 | B3 | |
| Whey protein concentrate (WPC) | 15.9 | 16.7 | 16.3 |
| Pumpkin seeds | 14.3 | 15.0 | 14.6 |
| Spelt flakes | 11.9 | 12.5 | 12.2 |
| Prune | 7.9 | 16.7 | 0.0 |
| Dried apricots | 7.9 | 0.0 | 16.3 |
| Oat flakes | 0.0 | 8.3 | 8.1 |
| Coconut shreds | 7.9 | 0.0 | 0.0 |
| Honey | 3.2 | 3.3 | 3.3 |
| Sunflower oil | 2.4 | 2.5 | 2.4 |
| Inulin | 2.4 | 2.5 | 2.4 |
| Dried cherries | 1.6 | 1.7 | 1.6 |
| Freeze-dried raspberries | 0.8 | 0.8 | 0.8 |
| Water | 7.9 | 3.3 | 5.7 |
| Chocolate | 15.9 | 16.7 | 16.3 |
| Sum | 100 | 100 | 100 |
| Time | Temperature of Storage (°C) | Pathogen | B. cereus | Salmonella sp. | S. aureus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Day/Month) | Sample | B1 | B2 | B3 | B1 | B2 | B3 | B1 | B2 | B3 | |
| (Presence) | (log CFU/g) | ||||||||||
| 0 | + | + | − | − | − | − | <2.00 | <2.00 | <2.00 | ||
| 1 | 4 | + | + | + | − | − | − | 3.05 | 3.06 | 2.50 | |
| 22 | + | + | − | − | − | − | 3.05 | 2.36 | <2.00 | ||
| 2 | 4 | + | + | + | − | − | − | 2.83 | 3.04 | <2.00 | |
| 22 | + | − | + | − | − | − | <2.00 | <2.00 | <2.00 | ||
| 3 | 4 | + | + | + | − | − | − | 2.80 | 2.06 | <2.00 | |
| 22 | + | + | + | − | − | − | <2.00 | <2.00 | <2.00 | ||
| Mycotoxin (μg/kg) | Products at Day 0 | Products after Storage at 4 °C | Products after Storage at 22 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | B3 | B1/4 | B2/4 | B3/4 | B1/22 | B2/22 | B3/22 | |
| Aflatoxin B1 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Aflatoxin B2 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Aflatoxin G1 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Aflatoxin G2 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Sum of aflatoxin B1, B2, G1, G2 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Ochratoxin A | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 |
| Zearalenone | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| Deoxynivalenol | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
| Biogenic Amines (mg/kg) | Products at Day 0 | Products after Storage at 4 °C | Products after Storage at 22 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | B3 | B1/4 | B2/4 | B3/4 | B1/22 | B2/22 | B3/22 | |
| 2-phenylethyl amine | 1.24 ± 0.13 aA | 1.31 ± 0.1 aA | 1.14 ± 0.12 aA | n.d. | 2.95 ± 0.65 aA | 1.66 ± 0.37 aA | 1.24 ± 0.27 aA | 1.41 ± 0.31 aA | 1.35 ± 0.30 aA |
| Cadaverine | 2.09 ± 0.24 aA | 2.68 ± 0.21 aA | 2.05 ± 0.27 aA | n.d. | 2.47 ± 0.35 aA | 2.99 ± 0.42 aA | 3.30 ± 0.46 aA | 3.94 ± 0.55 aA | 3.09 ± 0.31 aA |
| Histamine | <1.00 | <1.00 | <1.00 | n.d. | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 |
| Putrescine | 11.8 ± 0.31 aA | 12.2 ± 0.27 aA | 12.7 ± 0.27 aA | n.d. | 14.9 ± 3.9 aA | 13.8 ± 3.60 aA | 8.63 ± 2.24 aB | 7.38 ± 1.92 aB | 8.08 ± 2.10 aB |
| Spermidine | 7.61± 0.21 aA | 9.49 ± 0.29 aA | 7.67 ± 0.25 aA | n.d. | 7.25 ± 1.02 aA | 6.8 ± 0.96 aA | 4.03 ± 0.56 aB | 5.86 ± 0.82 aB | 5.19 ± 0.73 aB |
| Spermine | 3.71 ± 0.15 aA | 4.53 ± 0.17 aA | 3.67 ± 0.26 aA | n.d. | 3.84 ± 0.85 aA | 3.44 ± 0.76 aA | 1.74 ± 0.38 aA | 2.77 ± 0.61 aA | 2.35 ± 0.52 aA |
| Tryptamine | <5.00 | <5.00 | <5.00 | n.d. | <5.00 | <5.00 | <5.00 | <5.00 | <5.00 |
| Tyramine | 2.47 ± 0.35 aA | 2.55 ± 0.31 aA | 2.29 ± 0.32 aA | n.d. | 2.24 ± 0.18 aA | 2.16 ± 0.17 aA | 2.17 ± 0,17 aA | 1.66 ± 0.13 aA | 2.01 ± 0.16 aA |
| Time and Temperature of Storage (Month/°C) | Bar Symbol | ||
|---|---|---|---|
| B1 | B2 | B3 | |
| 0 | 0.47 ± 0.03 aB | 0.38 ± 0.03 aA | 0.48 ± 0.02 abB |
| 1/4 | 0.59 ± 0.03 abC | 0.32 ± 0.02 aA | 0.47 ± 0.02 aB |
| 2/4 | 0.53 ± 0.06 abB | 0.36 ± 0.03 aA | 0.45 ± 0.07 aAB |
| 3/4 | 0.63 ± 0.05 bAB | 0.75 ± 0.06 bB | 0.61 ± 0.06 bA |
| 1/22 | 0.90 ± 0.03 cB | 0.54 ± 0.05 abA | 1.01 ± 0.05 dB |
| 2/22 | 0.81 ± 0.03 cB | 0.45 ± 0.02 abA | 0.74 ± 0.04 cB |
| 3/22 | 1.96 ± 0.17 dA | 1.33 ± 0.41 cA | 1.57 ± 0.11 eA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trząskowska, M.; Neffe-Skocińska, K.; Okoń, A.; Zielińska, D.; Szydłowska, A.; Łepecka, A.; Kołożyn-Krajewska, D. Safety Assessment of Organic High-Protein Bars during Storage at Ambient and Refrigerated Temperatures. Appl. Sci. 2022, 12, 8454. https://doi.org/10.3390/app12178454
Trząskowska M, Neffe-Skocińska K, Okoń A, Zielińska D, Szydłowska A, Łepecka A, Kołożyn-Krajewska D. Safety Assessment of Organic High-Protein Bars during Storage at Ambient and Refrigerated Temperatures. Applied Sciences. 2022; 12(17):8454. https://doi.org/10.3390/app12178454
Chicago/Turabian StyleTrząskowska, Monika, Katarzyna Neffe-Skocińska, Anna Okoń, Dorota Zielińska, Aleksandra Szydłowska, Anna Łepecka, and Danuta Kołożyn-Krajewska. 2022. "Safety Assessment of Organic High-Protein Bars during Storage at Ambient and Refrigerated Temperatures" Applied Sciences 12, no. 17: 8454. https://doi.org/10.3390/app12178454
APA StyleTrząskowska, M., Neffe-Skocińska, K., Okoń, A., Zielińska, D., Szydłowska, A., Łepecka, A., & Kołożyn-Krajewska, D. (2022). Safety Assessment of Organic High-Protein Bars during Storage at Ambient and Refrigerated Temperatures. Applied Sciences, 12(17), 8454. https://doi.org/10.3390/app12178454









