Spinel Magnetic Iron Oxide Nanoparticles: Properties, Synthesis and Washing Methods
Abstract
1. Introduction
2. Crystal Structure and Magnetic Properties of Spinel Iron Oxide
- -
- Hematite α-Fe2O3 which crystallizes in a trigonal structure and in a space group Rch. In this compound, iron is at the oxidation state +III.
- -
- Wustite Fe1−xO which presents a cubic structure according to the space group Fmm. This compound is most uncommon and is found almost exclusively in reducing environments. In this one, iron is mainly at the oxidation state +III.
- -
- Magnetite Fe3O4 which crystallizes in a cubic structure according to the space group Fdm. In this crystallographic structure, called spinel, iron is presented at the oxidation state +II and +III.
- -
- Maghemite γ-Fe2O3 which presents a cubic structure (Fdm or P4132) or a tetragonal structure (P41212). The structure of this compound is related to the spinel structure, iron is only presented at the oxidation state +III. Maghemite is obtained by sweet oxidation of magnetite.
2.1. Crystal Structures
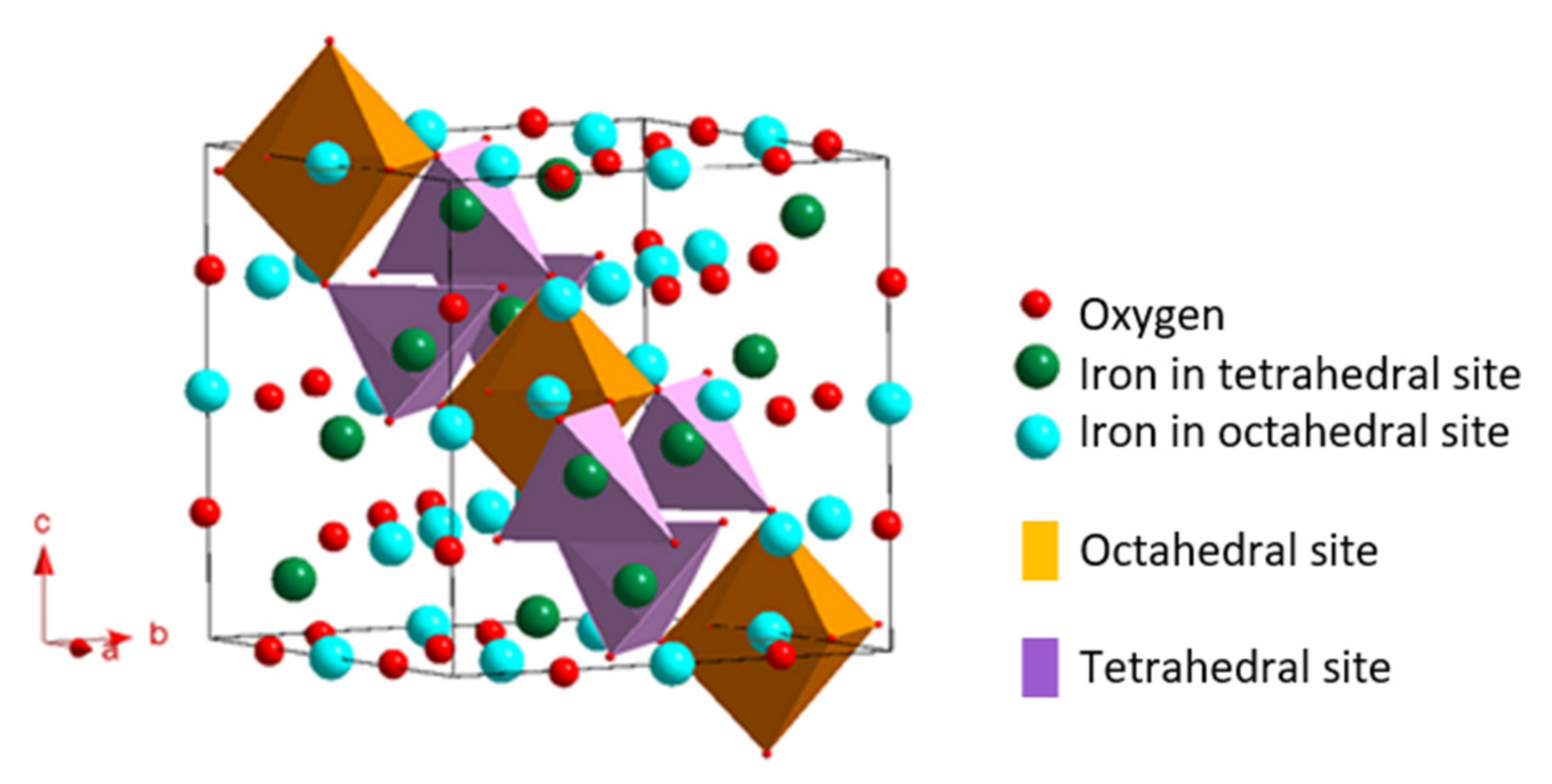
2.1.1. Magnetite Crystal Structure
2.1.2. Maghemite Crystal Structure
- -
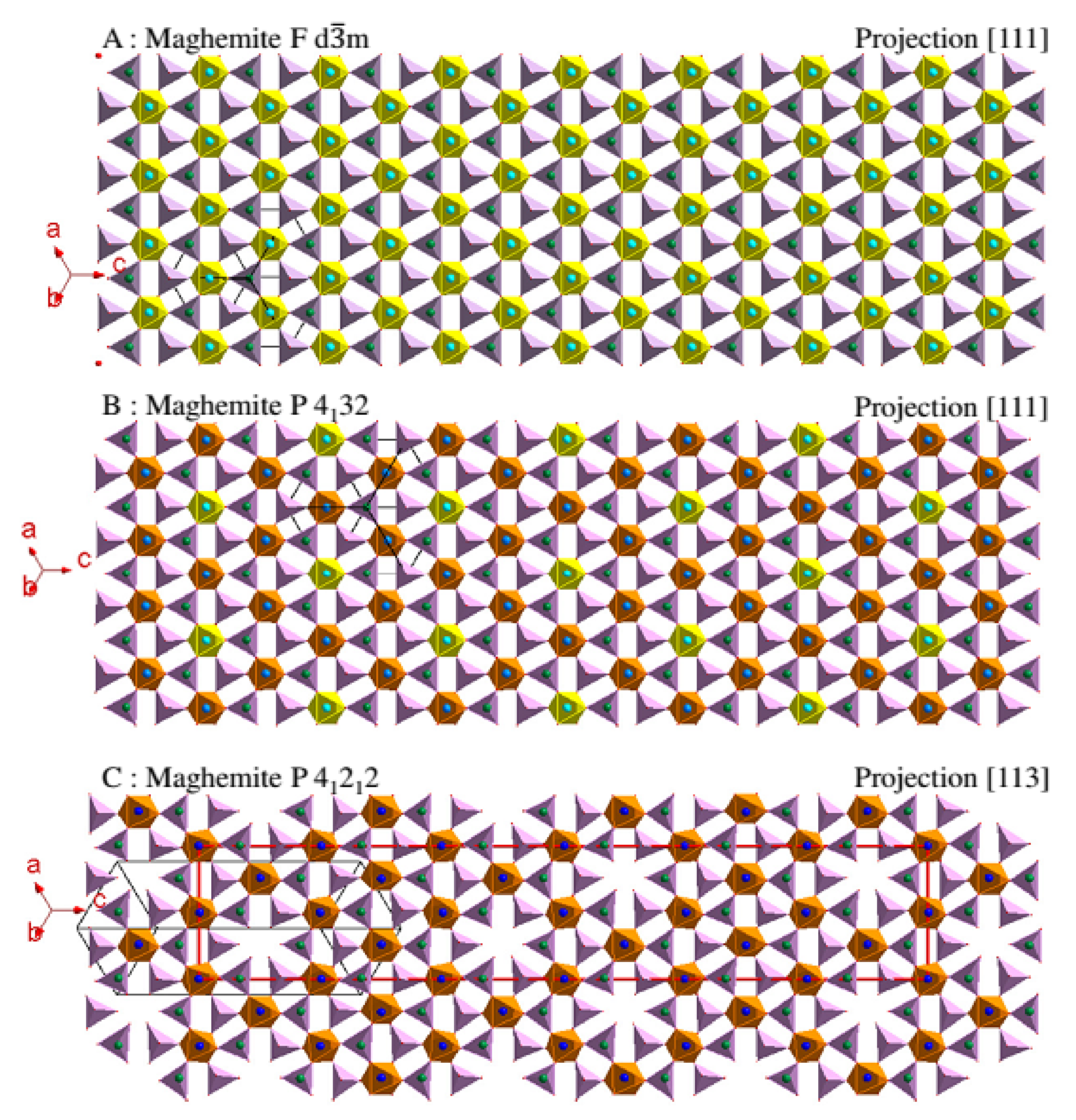
- A random distribution of gaps in the octahedral sites of the mesh without deformation (in yellow on the Figure 2A) with the same probability of presence of the gap and an occupation rate of 5/6. In this case, the crystallographic structure of maghemite stays cubic and describes the space group Fdm. Its lattice parameter of 0.8354 nm at room temperature (JCPDS card 04-013-7114) is slightly reduced (Δ = −0.0039 nm) compared to that of magnetite. This reduction translates the slight contraction of the structure due to the appearance of gaps.
- -
- A partially organized division of gaps in the defined octahedral sites of the mesh (in yellow on the Figure 2B) always without deformation with an occupation rate of these octahedra of 2/3. The gaps are situated preferentially in the defined octahedral of the mesh with a probability of presence of the gap of 1/3. The cubic system is always preserved but the space group P4132 translates a lowering of symmetry. Its lattice parameter is 0.8346 nm at room temperature (JCPDS card 04-016-4344).
- -
- A totally organized distribution of gaps. The symmetry drops from cubic to tetragonal (P43212) and the ordering of the gaps is carried out in a superstructure built on three superimposed meshes (Figure 2C). In some works [2], the space group P43212 is indicated instead of P41212. This difference translates the direction of rotation chosen for the helical axis 43 or 41 but the structure is identical. The lattice parameters of this structure are a = b = 0.83296 nm and c = 0.83221 nm at room temperature (JCPDS card 04-007-2135).
- -
- For the space group Fdm: (Fe3+)tetra[Fe3+5/3□1/3]octa(O2−)4
- -
- For the space group P4132: (Fe3+8)tetra[Fe3+4/3□8/3Fe3+12]octa(O2−)32
- -
- For the space group P41212: (Fe3+24)tetra[Fe3+40□8]octa(O2−)96.
2.2. Magnetic Properties
2.2.1. General Information on Magnetism
- -
- Their magnetic moments () which can be assimilated at electric dipoles from orbital atomic moments and spin of materials. Under the effect of an imposed external magnetic field (), they tend to line up in the direction of the field which induces a magnetization within the material.
- -
- Their magnetic susceptibility (χ) representing the trend of magnetic moments of the material to be aligned by the presence of an external magnetic field and which can be defined by the magnetization ratio on the external field /.
- -
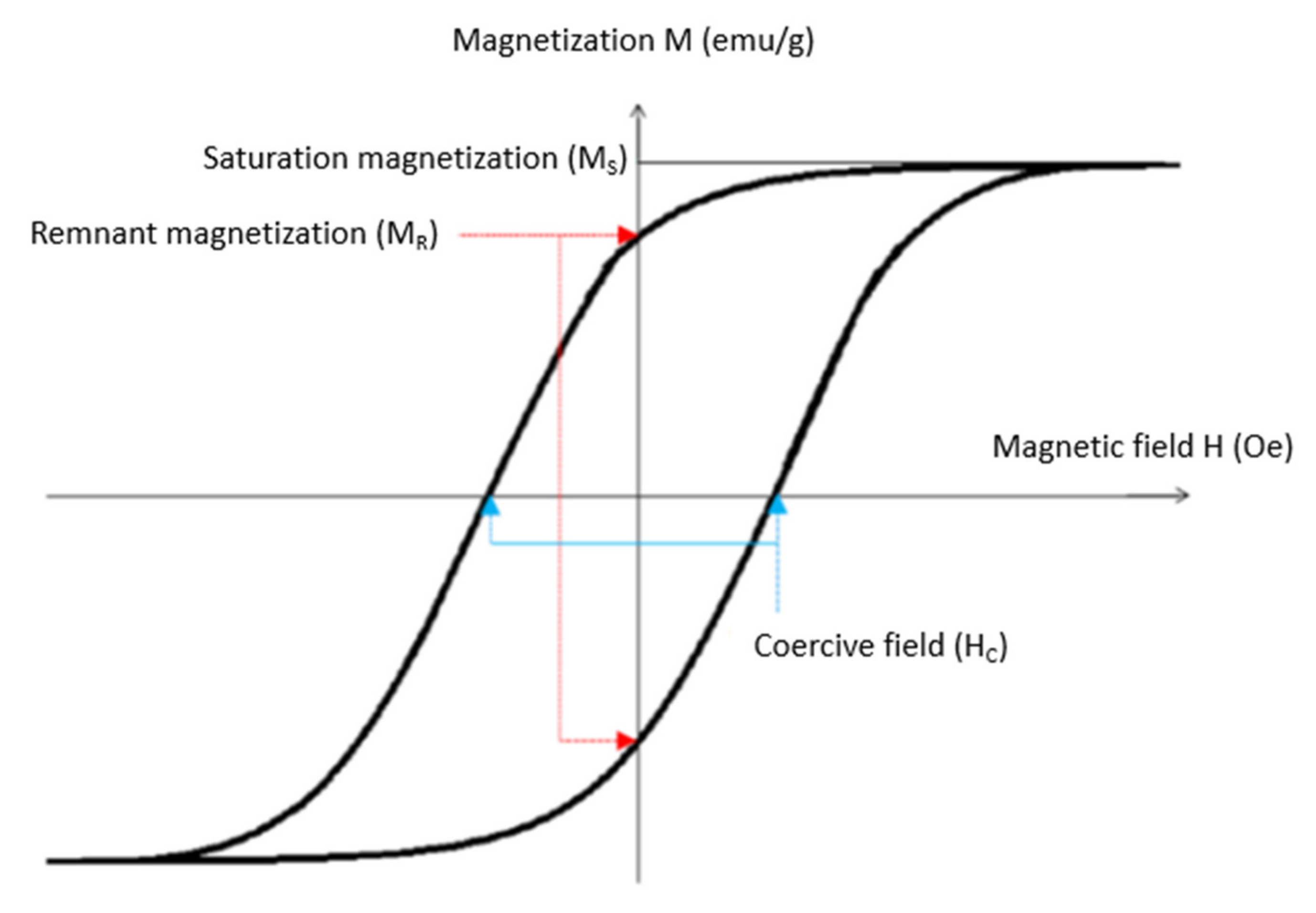
- Their saturation magnetization (Ms) representing the maximum value of the magnetization that a material can reach when the external magnetic field increases: it is given for a defined temperature.
- -
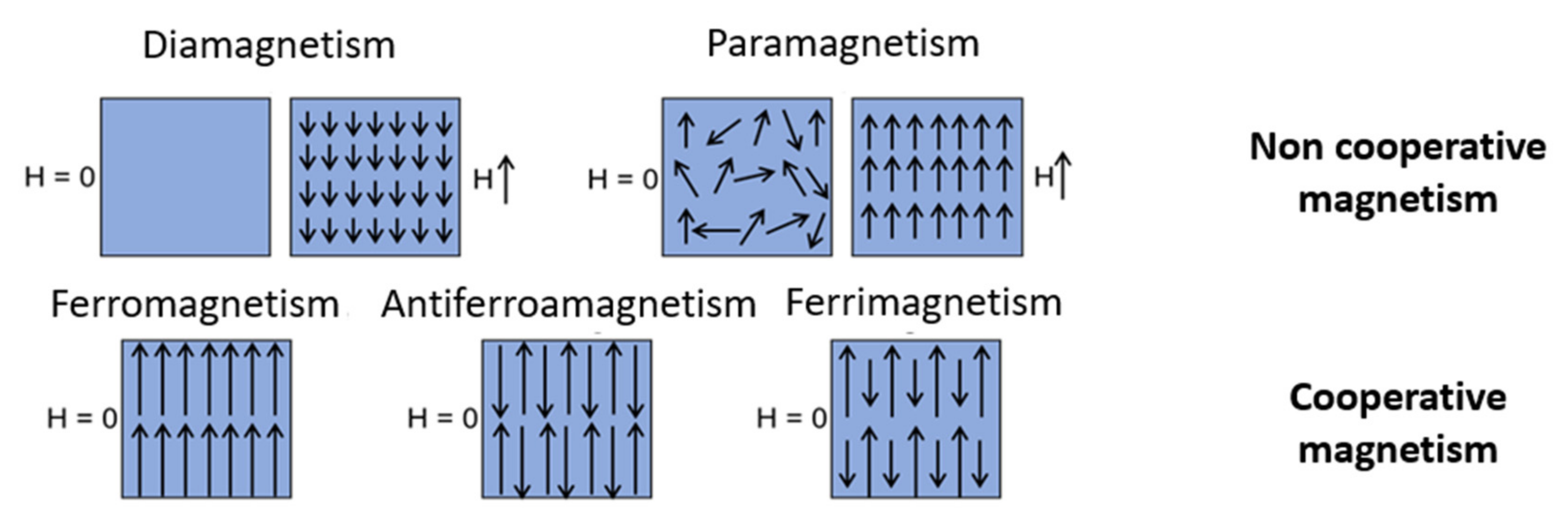
- The diamagnetism which is an intrinsic property of the matter such as χ < 0. The magnetic moments with the application of an external field will tend to align in the opposite direction of this field.
- -
- The paramagnetism which is a property due to free electrons of materials or unpaired electrons of ions such as χ > 0. The magnetic moments will tend to align in the direction of an applied external field.
- -
- Parallel (ferromagnetism); this results in an overall measurable magnetization for the material even in the absence of an external magnetic field.
- -
- Antiparallel with compensation of magnetic moments (antiferromagnetism); there exist two populations of magnetic moments aligned antiparallel to one another. The two populations of magnetic moments fully compensate and there is no overall magnetization measurable in the absence of an external magnetic field in this type of material.
- -
- Antiparallel without compensation of magnetic moments (ferrimagnetism); a global magnetization is measurable for the material even in the absence of an external magnetic field.
2.2.2. Structuration in Magnetic Areas
- -
- The coercive field (HC) which corresponds to the imposed magnetic field when the magnetization of the material is zero.
- -
- The remnant magnetization (MR) which corresponds to the magnetization of the material when the external field is zero.
2.2.3. Main Sources of Magnetic Anisotropy
Magneto-Crystalline Anisotropy
Anisotropy of Surface
Anisotropy of Shape
2.2.4. Evolution of Magnetic Properties in the Case of Nanoparticles
- -
- > Etherm. The magnetic moments of ferrimagnetic domains are blocked in the easy magnetization direction. A rotation of these magnetic moments by application of an external field then causes the mechanical rotation of the entire nanoparticle. The nanoparticle presents a ferrimagnetic comportment in the case of the magnetite.
- -
- < Etherm. The thermal agitation is more important than the total magnetic anisotropy energy and the magnetic moment is free to rotate freely. This magnetic comportment is called superparamagnetism.
2.2.5. Relaxation Time
- -
- If τ >> τm, the magnetic moment of the particle appears as blocked and the particle adopts a ferrimagnetic behavior.
- -
- If τ << τm, the magnetic moment of the particle returns around a lot of time during the measure and the average moment is zero. The particle presents a superparamagnetic behavior.
3. Main Synthesis Methods of Magnetite and Maghemite Nanoparticles
3.1. Hydrothermal Synthesis
3.2. Synthesis by Microemulsion
3.3. Thermal Decomposition
3.4. Polyols Methods
3.5. Sol-Gel Process
3.6. Synthesis by Co-Precipitation
- -
- Stage I: Formation by inorganic polycondensation of zero charge aqua-hydroxo complexes whose concentration will increase with the pH of the solution: [Fe2(OH)4(H2O)8]0 for the Fe2+ ions and [Fe2(OH)6(H2O)6]0 for the Fe3+ ions.
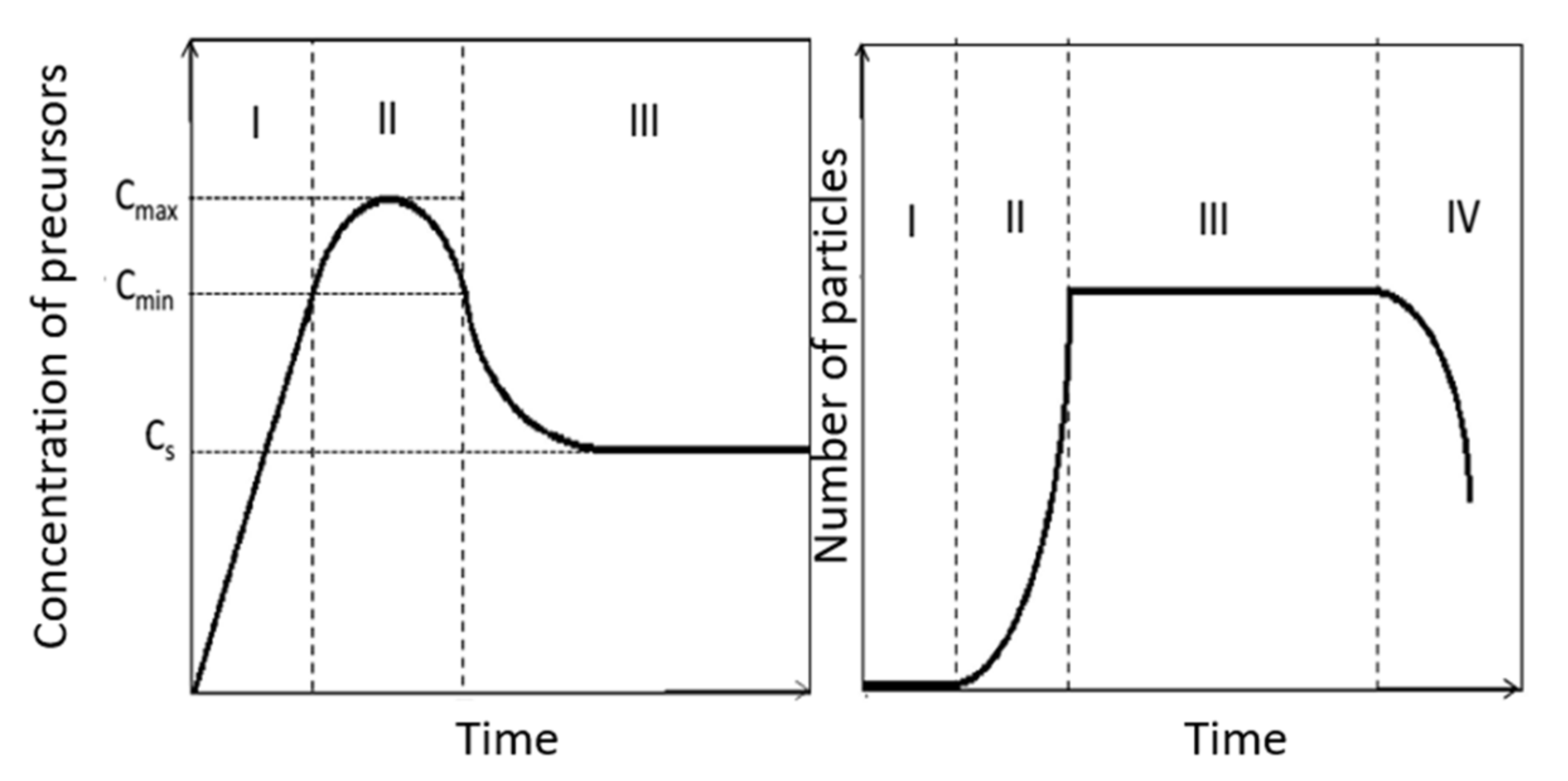
- -
- Stage II: Nucleation which begins when the concentration of precursors reaches a critical value of saturation (Cmin). There is then appearance of germs in the solution. These germs, very small, tend to redissolve easily. The quick germs formation and redissolution process will continue whereas the concentration of precursors increases. When the critical threshold is reached, stable germs are created and there follows a sudden decrease in concentration of precursors in the solution. If the concentration of precursors falls below the minimum value (Cmin), the formation of new germs is blocked.
- -
- Stage III: Growth of stable germs on the solution. It is carried out by addition of precursors in surface of germs by olation/oxolation. The growth will continue if the concentration of precursor is greater than the solubility of the solid (nanoparticles) in the solution. It should be noted that recently, D. Faivre et al. [51] proposed a growth model of magnetite nanoparticles. According to this model, primary nanoparticles around the nanometer size will be formed at an intermediate stage. These nanoparticles will aggregate then to form the finals nanoparticles.
- -
- Stage IV: Ripening. This is the last important step for the final characterization of the synthesized nanoparticles. A restructuring of nanoparticles formed by the crystallization of hitherto amorphous phases can occur during this stage. The possible aggregation of nanoparticles as well as the Ostwald ripening during which the smallest nanoparticles are dissolved in favor of the larger ones can also intervene during this stage. These two processes are both driven by the reduction of the surface energy of nanoparticles.
3.7. Synthesis by Microwave
4. Main Washing and Size Selection Methods for Nanoparticles
4.1. Dialysis
4.2. Centrifugation
4.3. Centrifugation with Viscosity Gradient
4.4. Ultrafiltration
4.5. Size selection Precipitation (SSP)
4.6. Extraction
4.7. Magnetic Separation
5. Stabilization of Magnetite and Maghemite Nanoparticles in Solution
5.1. Iron oxide Nanoparticles Behaviour in Solution
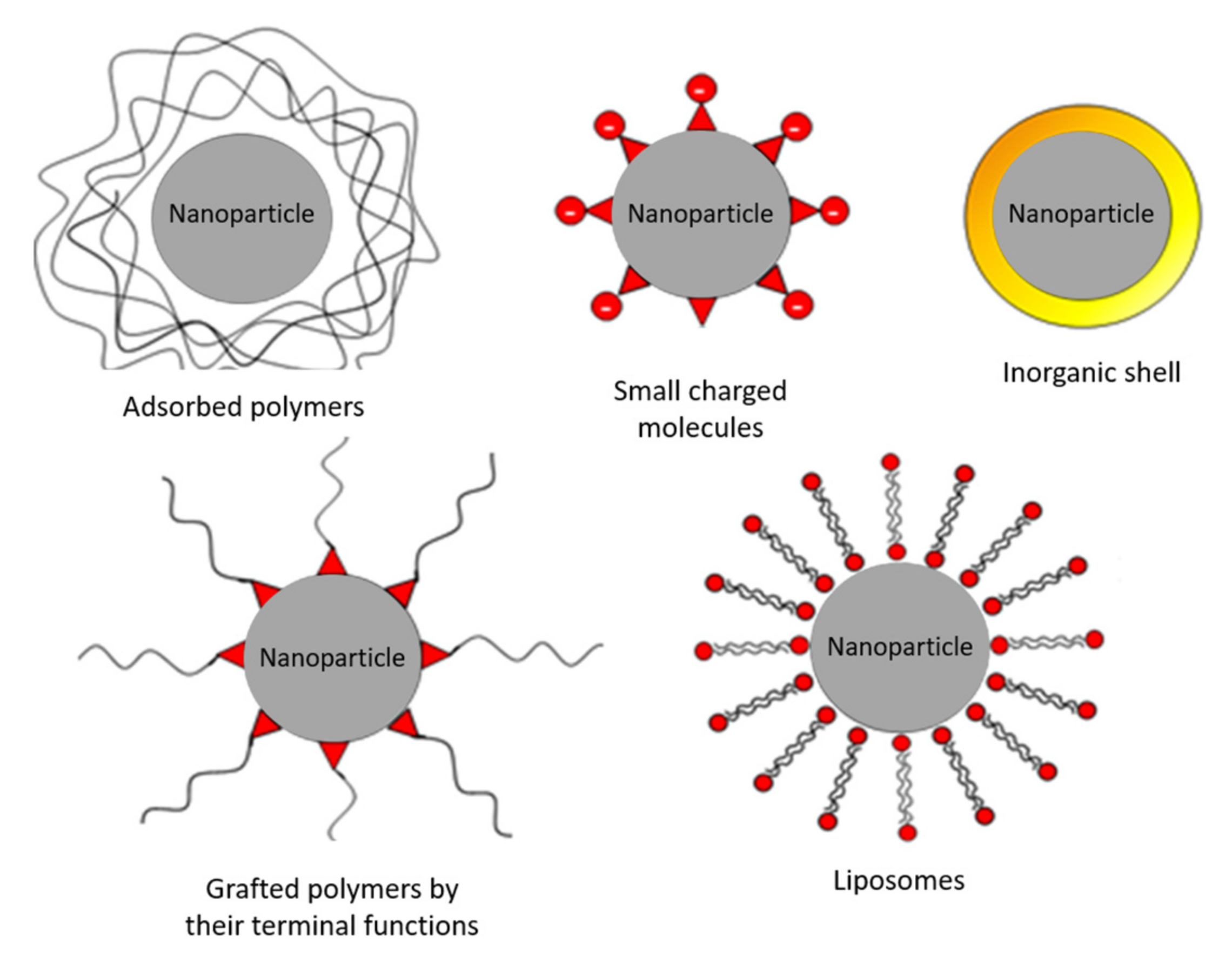
5.2. Main Stabilization Methods
- -
- -
- -
- -
- -
- The encapsulation in liposomes in the case of a double layer of fatty acids (oleic acid for example) [101].
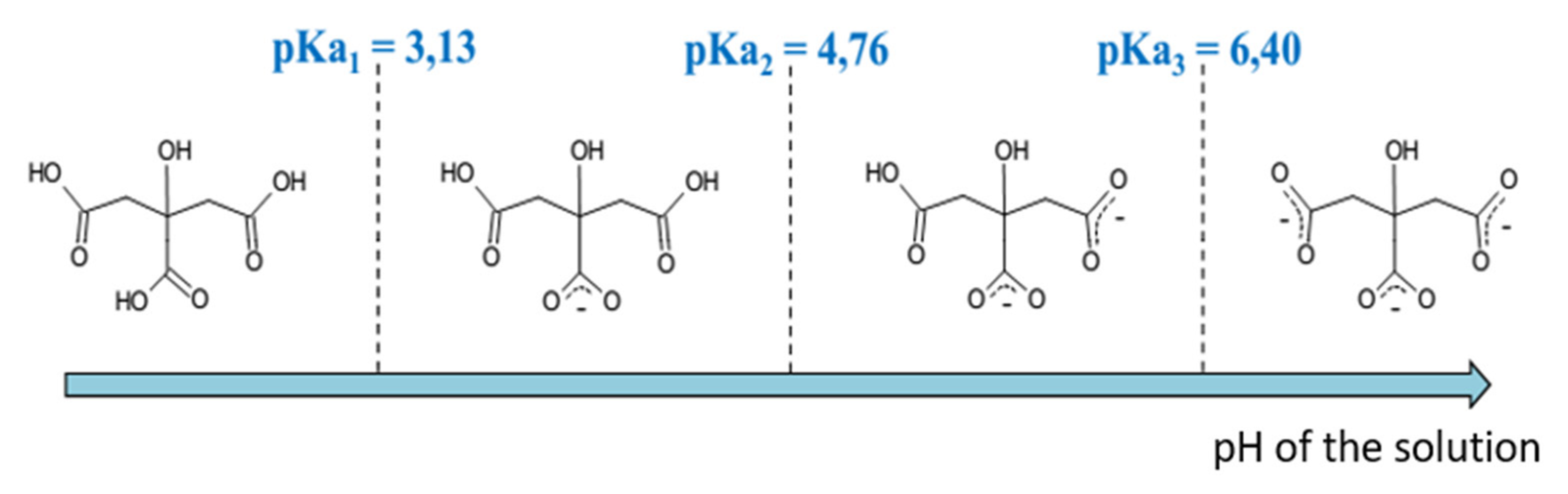
5.3. Stabilization by Citrate Ligands
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Teja, A.S.; Koh, P.-Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Basly, B. Conception et Caractérisation de Nano-Objets Magnétiques Pour L’imagerie par Résonance Magnétique (IRM). Strasbourg. 2010. Available online: http://theses.unistra.fr/ori-oai-search/notice.html?id=oai:EPrintsUneraTest01:2113&printable=true (accessed on 15 July 2022).
- Bragg, W.H. The Structure of Magnetite and the Spinels. Nature 1915, 95, 561. [Google Scholar] [CrossRef]
- Baaziz, W. Synthèse et Caractérisation des Nanoparticules Spinelles et Coeur-Coquille à Base D’oxyde de fer et de Cobalt. Starsbourg. 2011. Available online: https://www.semanticscholar.org/paper/Synth%C3%A8se-et-caract%C3%A9risation-des-nanoparticules-et-%C3%A0-Baaziz/2c604493355223c8a21532718b63438a6ea8ee43 (accessed on 15 July 2022).
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory: Preparation and Characterization; Wiley-VCH: Hoboken, NJ, USA, 2000. [Google Scholar]
- Ho, D.; Sun, X.; Sun, S. Monodisperse Magnetic Nanoparticles for Theranostic Applications. Acc. Chem. Res. 2011, 44, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Hillion, A. Études des propriétés magnétiques d’assemblées de nanoparticules de Co, FeRh et FeAu. Ph.D. Thesis, Claude Bernard University Lyon 1, Villeurbanne, France, 5 October 2012. [Google Scholar]
- Skumryev, V.; Blythe, H.J.; Cullen, J.; Coey, J.M.D. AC susceptibility of a magnetite crystal. J. Magn. Magn. Mater. 1999, 196, 515–517. [Google Scholar] [CrossRef]
- Ozdemir, O.D.D. Rock Magnetism: Fundamentals and Frontiers; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Valstyn, E.; Morrish, A.; Hanton, J. Ferromagnetic Resonance of Single-Domain Particles. Phys. Rev. 1962, 128, 2078. [Google Scholar] [CrossRef]
- Neel, L. Anisotropie Magnetique Superficielle Et Surstructures Dorientation. J. Phys. Radium 1954, 15, 225–239. [Google Scholar] [CrossRef]
- Zijlstra, H. Permanent magnet; theory. In Ferromagnetic Materials; Wohlfarth, E.P., Ed.; North Holland: Amsterdam, The Netherlands, 1982; Volume 3. [Google Scholar]
- Chatterjee, J.; Haik, Y.; Chen, C.-J. Size dependent magnetic properties of iron oxide nanoparticles. J. Magn. Magn. Mater. 2003, 257, 113–118. [Google Scholar] [CrossRef]
- Sreeja, V.; Joy, P.A. Effect of inter-particle interactions on the magnetic properties of magnetite nanoparticles after coating with dextran. Int. J. Nanotechnol. 2011, 8, 907–915. [Google Scholar] [CrossRef]
- Sreeja, V.; Jayaprabha, K.N.; Joy, P.A. Water-dispersible ascorbic-acid-coated magnetite nanoparticles for contrast enhancement in MRI. Appl. Nanosci. 2015, 5, 435–441. [Google Scholar] [CrossRef]
- Jayaprabha, K.N.; Joy, P.A. Citrate modified beta-cyclodextrin functionalized magnetite nanoparticles: A biocompatible platform for hydrophobic drug delivery. Rsc. Adv. 2015, 5, 22117–22125. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Sheng-Nan, S.; Chao, W.; Zan-Zan, Z.; Yang-Long, H.; Venkatraman, S.S.; Zhi-Chuan, X. Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B 2014, 23, 037503. [Google Scholar]
- Massart, R. Preparation of Aqueous Magnetic Liquids in Alkaline and Acidic Media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Lefebure, S.; Dubois, E.; Cabuil, V.; Neveu, S.; Massart, R. Monodisperse magnetic nanoparticles: Preparation and dispersion in water and oils. J. Mater. Res. 1998, 13, 2975–2981. [Google Scholar] [CrossRef]
- Lee, J.; Isobe, T.; Senna, M. Preparation of ultrafine Fe3O4 particles by precipitation in the presence of PVA at high pH. J. Colloid Interface Sci. 1996, 177, 490–494. [Google Scholar] [CrossRef]
- Vayssieres, L.; Chaneac, C.; Tronc, E.; Jolivet, J.P. Size tailoring of magnetite particles formed by aqueous precipitation: An example of thermodynamic stability of nanometric oxide particles. J. Colloid Interface Sci. 1998, 205, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Shi, X.; Sun, K.; Li, C.; Uher, C.; Baker, J.R.; Holl, M.M.B.; Orr, B.G. Facile Hydrothermal Synthesis of Iron Oxide Nanoparticles with Tunable Magnetic Properties. J. Phys. Chem. C 2009, 113, 13593–13599. [Google Scholar] [CrossRef]
- Daou, T.J.; Pourroy, G.; Begin-Colin, S.; Greneche, J.M.; Ulhaq-Bouillet, C.; Legare, P.; Bernhardt, P.; Leuvrey, C.; Rogez, G. Hydrothermal synthesis of monodisperse magnetite nanoparticles. Chem. Mater. 2006, 18, 4399–4404. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, J.; Liu, X.; Chan, H.S.O. Synthesis of Fe3O4 nanoparticles from emulsions. J. Mater. Chem. 2001, 11, 1704–1709. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Bae, C.J.; Park, J.G.; Noh, H.J.; Park, J.H.; Hyeon, T. Large-scale synthesis of uniform and crystalline magnetite nanoparticles using reverse micelles as nanoreactors under reflux conditions. Adv. Funct. Mater. 2005, 15, 503–509. [Google Scholar] [CrossRef]
- Hyeon, T.; Lee, S.S.; Park, J.; Chung, Y.; Bin Na, H. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J. Am. Chem. Soc. 2001, 123, 12798–12801. [Google Scholar] [CrossRef] [PubMed]
- Pauly, M.; Pichon, B.P.; Albouy, P.-A.; Fleutot, S.; Leuvrey, C.; Trassin, M.; Gallani, J.-L.; Begin-Colin, S. Monolayer and multilayer assemblies of spherically and cubic-shaped iron oxide nanoparticles. J. Mater. Chem. 2011, 21, 16018–16027. [Google Scholar] [CrossRef]
- Baaziz, W.; Pichon, B.P.; Fleutot, S.; Liu, Y.; Lefevre, C.; Greneche, J.-M.; Toumi, M.; Mhiri, T.; Begin-Colin, S. Magnetic Iron Oxide Nanoparticles: Reproducible Tuning of the Size and Nanosized-Dependent Composition, Defects, and Spin Canting. J. Phys. Chem. C 2014, 118, 3795–3810. [Google Scholar] [CrossRef]
- Cai, W.; Wan, J. Facile synthesis of superparamagnetic magnetite nanoparticles in liquid polyols. J. Colloid Interface Sci. 2007, 305, 366–370. [Google Scholar] [CrossRef]
- Arndt, D.; Zielasek, V.; Dreher, W.; Baeumer, M. Ethylene diamine-assisted synthesis of iron oxide nanoparticles in high-boiling polyolys. J. Colloid Interface Sci. 2014, 417, 188–198. [Google Scholar] [CrossRef]
- Thomas, G.; Demoisson, F.; Boudon, J.; Millot, N. Efficient functionalization of magnetite nanoparticles with phosphonate using a one-step continuous hydrothermal process. Dalton Trans. 2016, 45, 10821–10829. [Google Scholar] [CrossRef]
- Fouineau, J.; Brymora, K.; Ourry, L.; Mammeri, F.; Yaacoub, N.; Calvayrac, F.; Ammar-Merah, S.; Greneche, J.-M. Synthesis, Mössbauer Characterization, and Ab Initio Modeling of Iron Oxide Nanoparticles of Medical Interest Functionalized by Dopamine. J. Phys. Chem. C 2013, 117, 14295–14302. [Google Scholar] [CrossRef]
- HongZhang, Q.; Biao, Y.; ChengKui, L.; Wei, L. Synthesis and characterization of water-soluble magnetite nanocrystals via one-step sol-gel pathway. Sci. China Phys. Mech. Astron. 2011, 54, 1239–1243. [Google Scholar]
- Raja, K.; Jaculine, M.M.; Jose, M.; Verma, S.; Prince, A.A.M.; Ilangovan, K.; Sethusankar, K.; Das, S.J. Sol-gel synthesis and characterization of alpha-Fe2O3 nanoparticles. Superlattices Microstruct. 2015, 86, 306–312. [Google Scholar] [CrossRef]
- Xu, Y.; Zhuang, L.; Lin, H.; Shen, H.; Li, J.W. Preparation and characterization of polyacrylic acid coated magnetite nanoparticles functionalized with amino acids. Thin Solid Films 2013, 544, 368–373. [Google Scholar] [CrossRef]
- Sciancalepore, C.; Rosa, R.; Barrera, G.; Tiberto, P.; Allia, P.; Bondioli, F. Microwave-assisted nonaqueous sol-gel synthesis of highly crystalline magnetite nanocrystals. Mater. Chem. Phys. 2014, 148, 117–124. [Google Scholar] [CrossRef]
- Ramos Guivar, J.A.; Sanches, E.A.; Bruns, F.; Sadrollahi, E.; Morales, M.A.; López, E.O.; Litterst, F.J. Vacancy ordered γ-Fe2O3 nanoparticles functionalized with nanohydroxyapatite: XRD, FTIR, TEM, XPS and Mössbauer studies. Appl. Surf. Sci. 2016, 389, 721–734. [Google Scholar] [CrossRef]
- Saikia, C.; Das, M.K.; Ramteke, A.; Maji, T.K. Effect of crosslinker on drug delivery properties of curcumin loaded starch coated iron oxide nanoparticles. Int. J. Biol. Macromol. 2016, 93, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large pH Window with Different Bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [PubMed]
- El Ghandoor, H.; Zidan, H.M.; Khalil, M.M.H.; Ismail, M.I.M. Synthesis and Some Physical Properties of Magnetite (Fe3O4) Nanoparticles. Int. J. Electrochem. Sci. 2012, 7, 5734–5745. [Google Scholar]
- Drbohlavova, J.; Hrdy, R.; Adam, V.; Kizek, R.; Schneeweiss, O.; Hubalek, J. Preparation and Properties of Various Magnetic Nanoparticles. Sensors 2009, 9, 2352–2362. [Google Scholar] [CrossRef]
- Nath, S.; Kaittanis, C.; Ramachandran, V.; Dalal, N.S.; Perez, J.M. Synthesis, Magnetic Characterization, and Sensing Applications of Novel Dextran-Coated Iron Oxide Nanorods. Chem. Mater. 2009, 21, 1761–1767. [Google Scholar] [CrossRef]
- Fazilati, M. Folate decorated magnetite nanoparticles: Synthesis and targeted therapy against ovarian cancer. Cell Biol. Int. 2014, 38, 154–163. [Google Scholar] [CrossRef]
- Illes, E.; Szekeres, M.; Kupcsik, E.; Toth, I.Y.; Farkas, K.; Jedlovszky-Hajdu, A.; Tombacz, E. PEGylation of surfacted magnetite core-shell nanoparticles for biomedical application. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 429–440. [Google Scholar] [CrossRef]
- Maurizi, L.; Bisht, H.; Bouyer, F.; Millot, N. Easy route to functionalize iron oxide nanoparticles via long-term stable thiol groups. Langmuir ACS J. Surf. Colloids 2009, 25, 8857–8859. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, I.; Motte, L.; Aoun, B.; Li, T.; Ren, Y.; Sun, C.; Saboungi, M.-L. Effects of coating spherical iron oxide nanoparticles. Biochim. Biophys. Acta BBA-Gen. Subj. 2017, 1861, 3621–3626. [Google Scholar] [CrossRef] [PubMed]
- Mazuel, F.; Espinosa, A.; Luciani, N.; Reffay, M.; Le Borgne, R.; Motte, L.; Desboeufs, K.; Michel, A.; Pellegrino, T.; Lalatonne, Y.; et al. Massive Intracellular Biodegradation of Iron Oxide Nanoparticles Evidenced Magnetically at Single-Endosome and Tissue Levels. ACS Nano 2016, 10, 7627–7638. [Google Scholar] [CrossRef]
- Lamer, V.; Dinegar, R. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Baumgartner, J.; Dey, A.; Bomans, P.H.H.; Le Coadou, C.; Fratzl, P.; Sommerdijk, N.A.J.M.; Faivre, D. Nucleation and growth of magnetite from solution. Nat. Mater. 2013, 12, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Vreeland, E.C.; Watt, J.; Schober, G.B.; Hance, B.G.; Austin, M.J.; Price, A.D.; Fellows, B.D.; Monson, T.C.; Hudak, N.S.; Maldonado-Camargo, L.; et al. Enhanced Nanoparticle Size Control by Extending LaMer’s Mechanism. Chem. Mater. 2015, 27, 6059–6066. [Google Scholar] [CrossRef]
- Massart, R.; Dubois, E.; Cabuil, V.; Hasmonay, E. Preparation and Properties of Monodisperse Magnetic Fluids. J. Magn. Magn. Mater. 1995, 149, 1–5. [Google Scholar] [CrossRef]
- Jolivet, J.; Belleville, P.; Tronc, E.; Livage, J. Influence of Fe(ii) on the Formation of the Spinel Iron-Oxide in Alkaline-Medium. Clays Clay Miner. 1992, 40, 531–539. [Google Scholar] [CrossRef]
- Babes, L.; Denizot, B.; Tanguy, G.; Le Jeune, J.J.; Jallet, P. Synthesis of iron oxide nanoparticles used as MRI contrast agents: A parametric study. J. Colloid Interface Sci. 1999, 212, 474–482. [Google Scholar] [CrossRef]
- Kim, D.K.; Zhang, Y.; Voit, W.; Rao, K.V.; Muhammed, M. Synthesis and characterization of surfactant-coated superparamagnetic monodispersed iron oxide nanoparticles. J. Magn. Magn. Mater. 2001, 225, 30–36. [Google Scholar] [CrossRef]
- Liang, Y.-J.; Zhang, Y.; Guo, Z.; Xie, J.; Bai, T.; Zou, J.; Gu, N. Ultrafast Preparation of Monodisperse Fe3O4 Nanoparticles by Microwave-Assisted Thermal Decomposition. Chem.—Eur. J. 2016, 22, 11807–11815. [Google Scholar] [CrossRef] [PubMed]
- Chikan, V.; McLaurin, E. Rapid Nanoparticle Synthesis by Magnetic and Microwave Heating. Nanomaterials 2016, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Pascu, O.; Carenza, E.; Gich, M.; Estradé, S.; Peiro, F.; Herranz, G.; Roig, A. Surface Reactivity of Iron Oxide Nanoparticles by Microwave-Assisted Synthesis; Comparison with the Thermal Decomposition Route. J. Phys. Chem. C 2012, 116, 15108–15116. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent Progress on Magnetic Iron Oxide Nanoparticles: Synthesis, Surface Functional Strategies and Biomedical Applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Brollo, M.E.F.; Veintemillas-Verdaguer, S.; Salván, C.M.; del Puerto Morales, M. Key Parameters on the Microwave Assisted Synthesis of Magnetic Nanoparticles for MRI Contrast Agents. Contrast Media Mol. Imaging 2017, 2017, 8902424. [Google Scholar] [CrossRef]
- Ai, Z.; Deng, K.; Wan, Q.; Zhang, L.; Lee, S. Facile Microwave-Assisted Synthesis and Magnetic and Gas Sensing Properties of Fe 3 O 4 Nanoroses. J. Phys. Chem. C 2010, 114, 6237–6242. [Google Scholar] [CrossRef]
- Wu, L.; Yao, H.; Hu, B.; Yu, S.-H. Unique Lamellar Sodium/Potassium Iron Oxide Nanosheets: Facile Microwave-Assisted Synthesis and Magnetic and Electrochemical Properties. Chem. Mater. 2011, 23, 3946–3952. [Google Scholar] [CrossRef]
- Hu, L.; Percheron, A.; Chaumont, D.; Brachais, C.-H. Microwave-Assisted One-Step Hydrothermal Synthesis of Pure Iron Oxide Nanoparticles: Magnetite, Maghemite and Hematite. J. Sol-Gel Sci. Technol. 2011, 60, 198–205. [Google Scholar] [CrossRef]
- Liu, Z.; Miao, F.; Hua, W.; Zhao, F. Fe3O4 Nanoparticles: Microwave-Assisted Synthesis and Mechanism. Mater. Lett. 2012, 67, 358–361. [Google Scholar] [CrossRef]
- Kowalczyk, B.; Lagzi, I.; Grzybowski, B.A. Nanoseparations: Strategies for size and/or shape-selective purification of nanoparticles. Curr. Opin. Colloid Interface Sci. 2011, 16, 135–148. [Google Scholar] [CrossRef]
- Mori, Y. Size-Selective Separation Techniques for Nanoparticles in Liquid. KONA Powder Part. J. 2015, 32, 102–114. [Google Scholar] [CrossRef]
- Robertson, J.D.; Rizzello, L.; Avila-Olias, M.; Gaitzsch, J.; Contini, C.; Magon, M.S.; Renshaw, S.A.; Battaglia, G. Purification of Nanoparticles by Size and Shape. Sci. Rep. 2016, 6, 27494. [Google Scholar] [CrossRef] [PubMed]
- Costo, R.; Bello, V.; González-Carreño, T.; Veintemillas-Verdaguer, S.; Wang, D. Size sorting of ultrasmall magnetic nanoparticles and their aggregates behaviour. Mater. Res. Bull. 2013, 48, 4294–4300. [Google Scholar] [CrossRef][Green Version]
- Mireles, L.-K.; Sacher, E.; Yahia, L.; Laurent, S.; Stanicki, D. Washing effect on superparamagnetic iron oxide nanoparticles. Data Brief 2016, 7, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Lassenberger, A.; Bixner, O.; Gruenewald, T.; Lichtenegger, H.; Zirbs, R.; Reimhult, E. Evaluation of High-Yield Purification Methods on Monodisperse PEG-Grafted Iron Oxide Nanoparticles. Langmuir 2016, 32, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Mao, C. Viscosity Gradient as a Novel Mechanism for the Centrifugation-Based Separation of Nanoparticles. Adv. Mater. 2011, 23, 4880–4885. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Zerbetto, M.; Ferrari, A.C.; Amendola, V. Sorting Nanoparticles by Centrifugal Fields in Clean Media. J. Phys. Chem. C 2013, 117, 13217–13229. [Google Scholar] [CrossRef]
- Akthakul, A.; Hochbaum, A.I.; Stellacci, F.; Mayes, A.M. Size Fractionation of Metal Nanoparticles by Membrane Filtration. Adv. Mater. 2005, 17, 532–535. [Google Scholar] [CrossRef]
- Sweeney, S.F.; Woehrle, G.H.; Hutchison, J.E. Rapid Purification and Size Separation of Gold Nanoparticles via Diafiltration. J. Am. Chem. Soc. 2006, 128, 3190–3197. [Google Scholar] [CrossRef]
- Snoswell, D.R.E.; Duan, J.; Fornasiero, D.; Ralston, J. The selective aggregation and separation of titania from a mixed suspension of silica and titania. Int. J. Miner. Process. 2005, 78, 1–10. [Google Scholar] [CrossRef]
- Goloverda, G.; Jackson, B.; Kidd, C.; Kolesnichenko, V. Synthesis of ultrasmall magnetic iron oxide nanoparticles and study of their colloid and surface chemistry. J. Magn. Magn. Mater. 2009, 321, 1372–1376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hollamby, M.J.; Eastoe, J.; Chemelli, A.; Glatter, O.; Rogers, S.; Heenan, R.K.; Grillo, I. Separation and Purification of Nanoparticles in a Single Step. Langmuir 2010, 26, 6989–6994. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Weeranoppanant, N.; Xie, L.; Chen, Y.; Lusardi, M.R.; Imbrogno, J.; Bawendi, M.G.; Jensen, K.F. Multistage extraction platform for highly efficient and fully continuous purification of nanoparticles. Nanoscale 2017, 9, 7703–7707. [Google Scholar] [CrossRef] [PubMed]
- Forge, D.; Gossuin, Y.; Roch, A.; Laurent, S.; Elst, L.V.; Muller, R.N. Development of magnetic chromatography to sort polydisperse nanoparticles in ferrofluids. Contrast Media Mol. Imaging 2010, 5, 126–132. [Google Scholar] [CrossRef]
- Halverson, D.; Friedman, G. Magnetic Field Assisted Fractionation of Nonmagnetic Colloids in Ferrofluid. IEEE Trans. Magn. 2007, 43, 2692–2694. [Google Scholar] [CrossRef]
- Ogrady, K.; Stewardson, H.; Chantrell, R.; Fletcher, D.; Unwin, D.; Parker, M. Magnetic Filtration of Ferrofluids. IEEE Trans. Magn. 1986, 22, 1134–1136. [Google Scholar] [CrossRef]
- Bacri, J.; Perzynski, R.; Salin, D.; Cabuil, V.; Massart, R. Ionic Ferrofluids—A Crossing of Chemistry and Physics. J. Magn. Magn. Mater. 1990, 85, 27–32. [Google Scholar] [CrossRef]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef]
- Berry, C.C.; Wells, S.; Charles, S.; Curtis, A.S.G. Dextran and albumin derivatised iron oxide nanoparticles: Influence on fibroblasts in vitro. Biomaterials 2003, 24, 4551–4557. [Google Scholar] [CrossRef]
- Jiang, W.Q.; Yang, H.C.; Yang, S.Y.; Horng, H.E.; Hung, J.C.; Chen, Y.C.; Hong, C.Y. Preparation and properties of superparamagnetic nanoparticles with narrow size distribution and biocompatible. J. Magn. Magn. Mater. 2004, 283, 210–214. [Google Scholar] [CrossRef]
- Shan, G.B.; Xing, J.M.; Luo, M.F.; Liu, H.Z.; Chen, J.Y. Immobilization of Pseudomonas delafieldii with magnetic polyvinyl alcohol beads and its application in biodesulfurization. Biotechnol. Lett. 2003, 25, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Milani, A.S.; Stroeve, P. Optimal Design and Characterization of Superparamagnetic Iron Oxide Nanoparticles Coated with Polyvinyl Alcohol for Targeted Delivery and Imaging. J. Phys. Chem. B 2008, 112, 14470–14481. [Google Scholar] [CrossRef] [PubMed]
- Unsoy, G.; Yalcin, S.; Khodadust, R.; Gunduz, G.; Gunduz, U. Synthesis optimization and characterization of chitosan-coated iron oxide nanoparticles produced for biomedical applications. J. Nanoparticle Res. 2012, 14, 964. [Google Scholar] [CrossRef]
- Lopez-Cruz, A.; Barrera, C.; Calero-DdelC, V.L.; Rinaldi, C. Water dispersible iron oxide nanoparticles coated with covalently linked chitosan. J. Mater. Chem. 2009, 19, 6870–6876. [Google Scholar] [CrossRef]
- Bee, A.; Massart, R.; Neveu, S. Synthesis of Very Fine Maghemite Particles. J. Magn. Magn. Mater. 1995, 149, 6–9. [Google Scholar] [CrossRef]
- Andreas, K.; Georgieva, R.; Ladwig, M.; Mueller, S.; Notter, M.; Sittinger, M.; Ringe, J. Highly efficient magnetic stem cell labeling with citrate-coated superparamagnetic iron oxide nanoparticles for MRI tracking. Biomaterials 2012, 33, 4515–4525. [Google Scholar] [CrossRef]
- Sahoo, Y.; Pizem, H.; Fried, T.; Golodnitsky, D.; Burstein, L.; Sukenik, C.N.; Markovich, G. Alkyl phosphonate/phosphate coating on magnetite nanoparticles: A comparison with fatty acids. Langmuir 2001, 17, 7907–7911. [Google Scholar] [CrossRef]
- Eskandari, H.; Shariati, M.R. Dodecylbenzene sulfonate-coated magnetite nanoparticles as a new adsorbent for solid phase extraction-spectrophotometric determination of ultra trace amounts of ammonium in water samples. Anal. Chim. Acta 2011, 704, 146–153. [Google Scholar] [CrossRef]
- Xu, Z.; Hou, Y.; Sun, S. Magnetic core/shell Fe3O4/Au and Fe3O4/Au/Ag nanoparticles with tunable plasmonic properties. J. Am. Chem. Soc. 2007, 129, 8698–8699. [Google Scholar] [CrossRef]
- Klotz, M.; Ayral, A.; Guizard, C.; Menager, C.; Cabuil, V. Silica coating on colloidal maghemite particles. J. Colloid Interface Sci. 1999, 220, 357–361. [Google Scholar] [CrossRef]
- Liu, H.-M.; Wu, S.-H.; Lu, C.-W.; Yao, M.; Hsiao, J.-K.; Hung, Y.; Lin, Y.-S.; Mou, C.-Y.; Yang, C.-S.; Huang, D.-M.; et al. Mesoporous silica nanoparticles improve magnetic labeling efficiency in human stem cells. Small 2008, 4, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Wells, S. Surface-modified superparamagnetic nanoparticles for drug delivery: Preparation, characterization, and cytotoxicity studies. IEEE Trans. Nanobiosci. 2004, 3, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kohler, N.; Zhang, M.Q. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials 2002, 23, 1553–1561. [Google Scholar] [CrossRef]
- Schweiger, C.; Pietzonka, C.; Heverhagen, J.; Kissel, T. Novel magnetic iron oxide nanoparticles coated with poly(ethylene imine)-g-poly(ethylene glycol) for potential biomedical application: Synthesis, stability, cytotoxicity and MR imaging. Int. J. Pharm. 2011, 408, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Tombacz, E.; Bica, D.; Hajdu, A.; Illes, E.; Majzik, A.; Vekas, L. Surfactant double layer stabilized magnetic nanofluids for biomedical application. J. Phys.-Condens. Matter 2008, 20, 204103. [Google Scholar] [CrossRef]
- Maurizi, L.; Bouyer, F.; Paris, J.; Demoisson, F.; Saviot, L.; Millot, N. One step continuous hydrothermal synthesis of very fine stabilized superparamagnetic nanoparticles of magnetite. Chem. Commun. Camb. Engl. 2011, 47, 11706–11708. [Google Scholar] [CrossRef]
- Bart, A.; Heldreth, M.M.F. Final Report: On the Safety Assessment of Citric Acid, Innorganic Citrate Salts, and Alkyl Citrate Esters as Used in Cosmetics. 2012. Available online: http://www.cir-safety.org/sites/default/files/citric032012FR.pdf (accessed on 15 July 2022).
- Cheng, C.-M.; Kou, G.; Wang, X.-L.; Wang, S.-H.; Gu, H.-C.; Guo, Y.-J. Synthesis of carboxyl superparamagnetic ultrasmall iron oxide (USPIO) nanoparticles by a novel flocculation-redispersion process. J. Magn. Magn. Mater. 2009, 321, 2663–2669. [Google Scholar] [CrossRef]
- Bishop, L.M.; Yeager, J.C.; Chen, X.; Wheeler, J.N.; Torelli, M.D.; Benson, M.C.; Burke, S.D.; Pedersen, J.A.; Hamers, R.J. A Citric Acid-Derived Ligand for Modular Functionalization of Metal Oxide Surfaces via ‘Click’ Chemistry. Langmuir 2012, 28, 1322–1329. [Google Scholar] [CrossRef]
- Huisgen, R. Kinetics and Mechanism of 1,3-Dipolar Cycloadditions. Angew. Chem. Int. Ed. Engl. 1963, 2, 633–645. [Google Scholar] [CrossRef]


| Type of Oxide | Saturation Magnetization | Mono-Domain Critical Diameter | Limit Diameter Superparamagnetism |
|---|---|---|---|
| Magnetite | 92 emu/g | 30 ± 5 nm | 20 ± 5 nm |
| Maghemite | 74 emu/g | 30 ± 5 nm | 20 ± 5 nm |
| Method | Advantages | Disadvantages | Shape and Size Saturation Magnetization |
|---|---|---|---|
| Co-precipitation [20,21,22,23] |
|
|
|
| Hydrothermal Co-precipitation [24,25] |
|
|
|
| Micro-emulsions [26,27] |
|
|
|
| Thermal decomposition [28,29,30] |
|
|
|
| Polyol method [31,32] |
|
|
|
| Sol-gel [35,36] |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girardet, T.; Venturini, P.; Martinez, H.; Dupin, J.-C.; Cleymand, F.; Fleutot, S. Spinel Magnetic Iron Oxide Nanoparticles: Properties, Synthesis and Washing Methods. Appl. Sci. 2022, 12, 8127. https://doi.org/10.3390/app12168127
Girardet T, Venturini P, Martinez H, Dupin J-C, Cleymand F, Fleutot S. Spinel Magnetic Iron Oxide Nanoparticles: Properties, Synthesis and Washing Methods. Applied Sciences. 2022; 12(16):8127. https://doi.org/10.3390/app12168127
Chicago/Turabian StyleGirardet, Thomas, Pierre Venturini, Hervé Martinez, Jean-Charles Dupin, Franck Cleymand, and Solenne Fleutot. 2022. "Spinel Magnetic Iron Oxide Nanoparticles: Properties, Synthesis and Washing Methods" Applied Sciences 12, no. 16: 8127. https://doi.org/10.3390/app12168127
APA StyleGirardet, T., Venturini, P., Martinez, H., Dupin, J.-C., Cleymand, F., & Fleutot, S. (2022). Spinel Magnetic Iron Oxide Nanoparticles: Properties, Synthesis and Washing Methods. Applied Sciences, 12(16), 8127. https://doi.org/10.3390/app12168127







