Fabrication of Carbon Aerogels Derived from Metal-Organic Frameworks/Carbon Nanotubes/Cotton Composites as an Efficient Sorbent for Sustainable Oil–Water Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CZIF-8/CNTs/CCBs
2.3. Physical Characterization
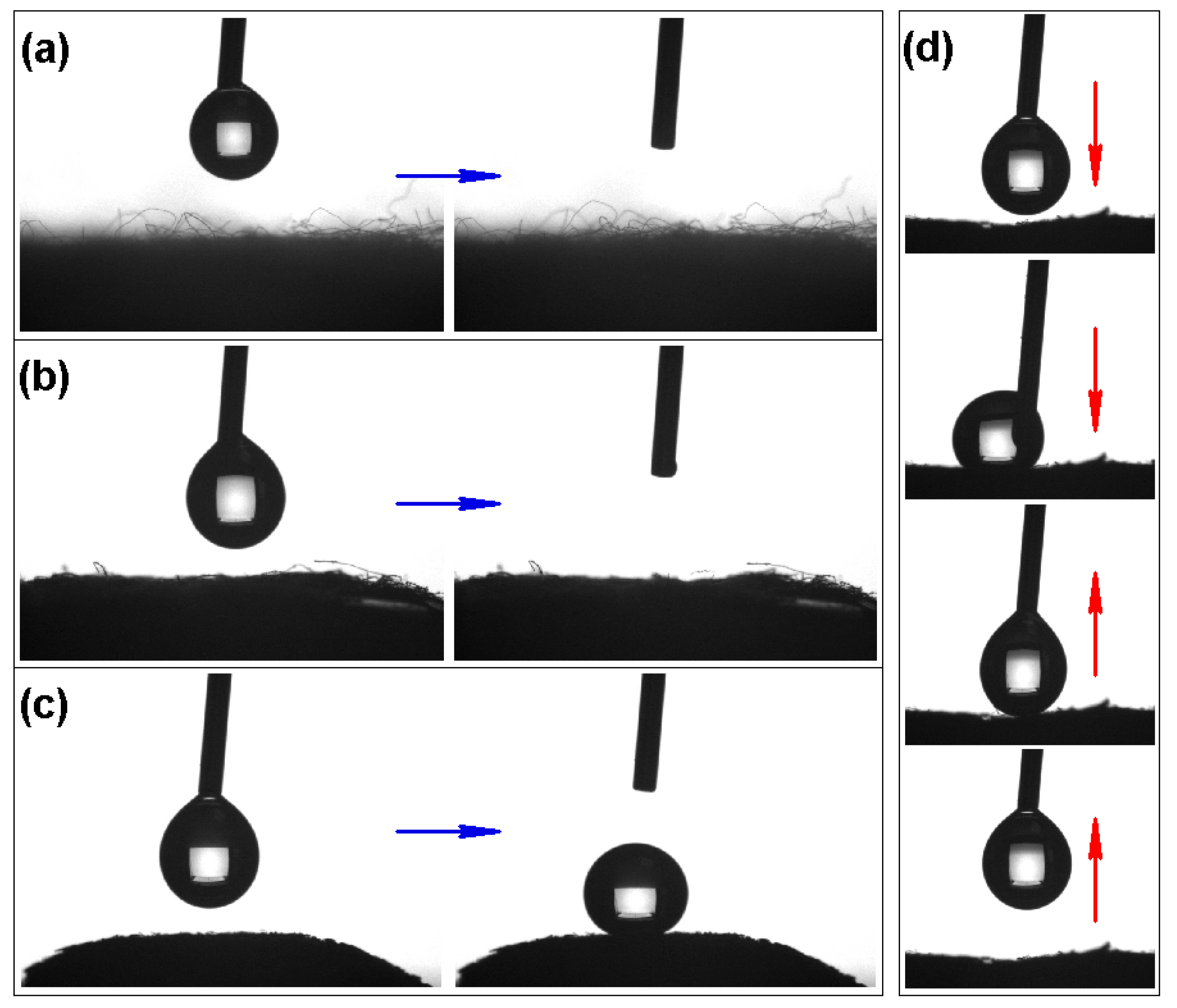
2.4. Oil–Water Separation Test
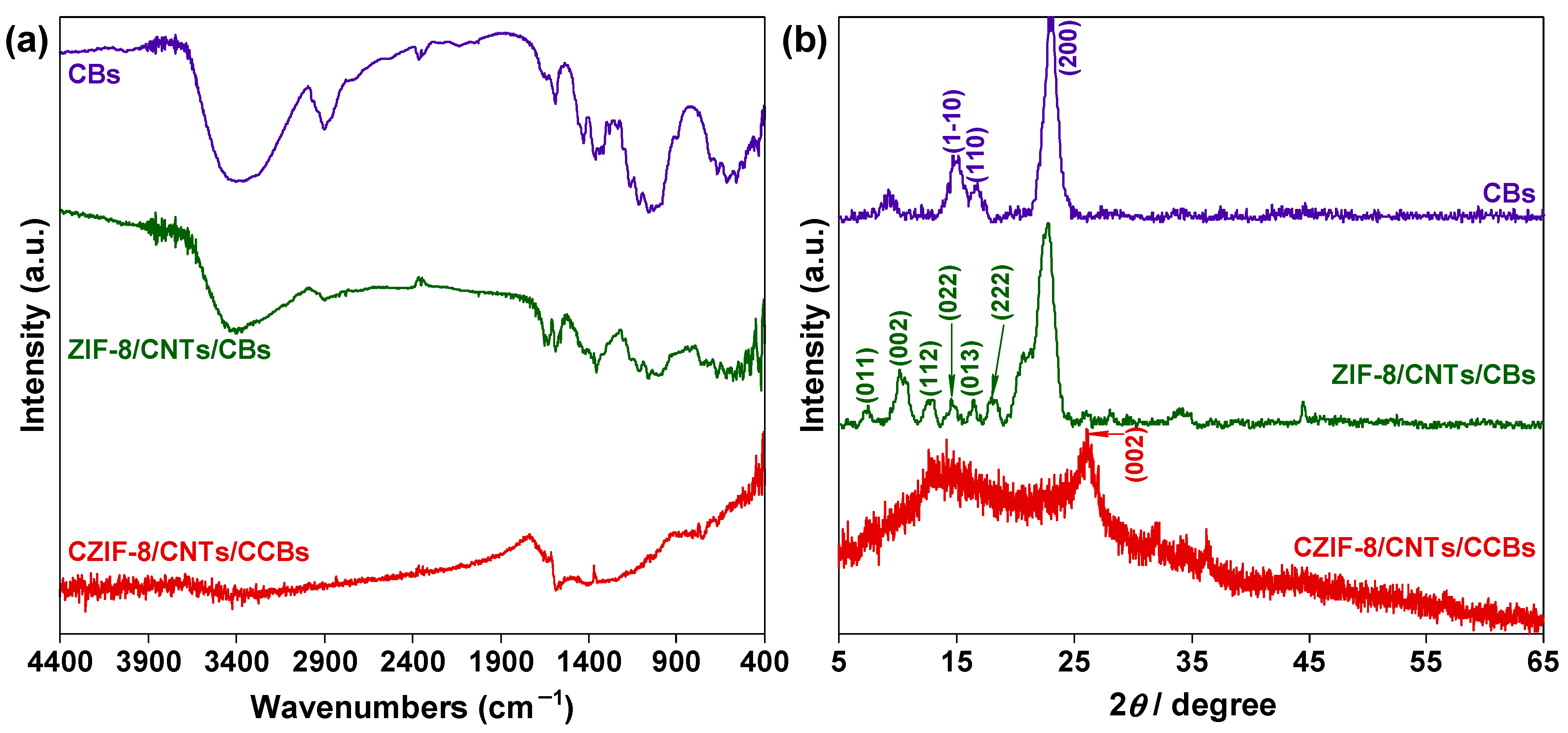
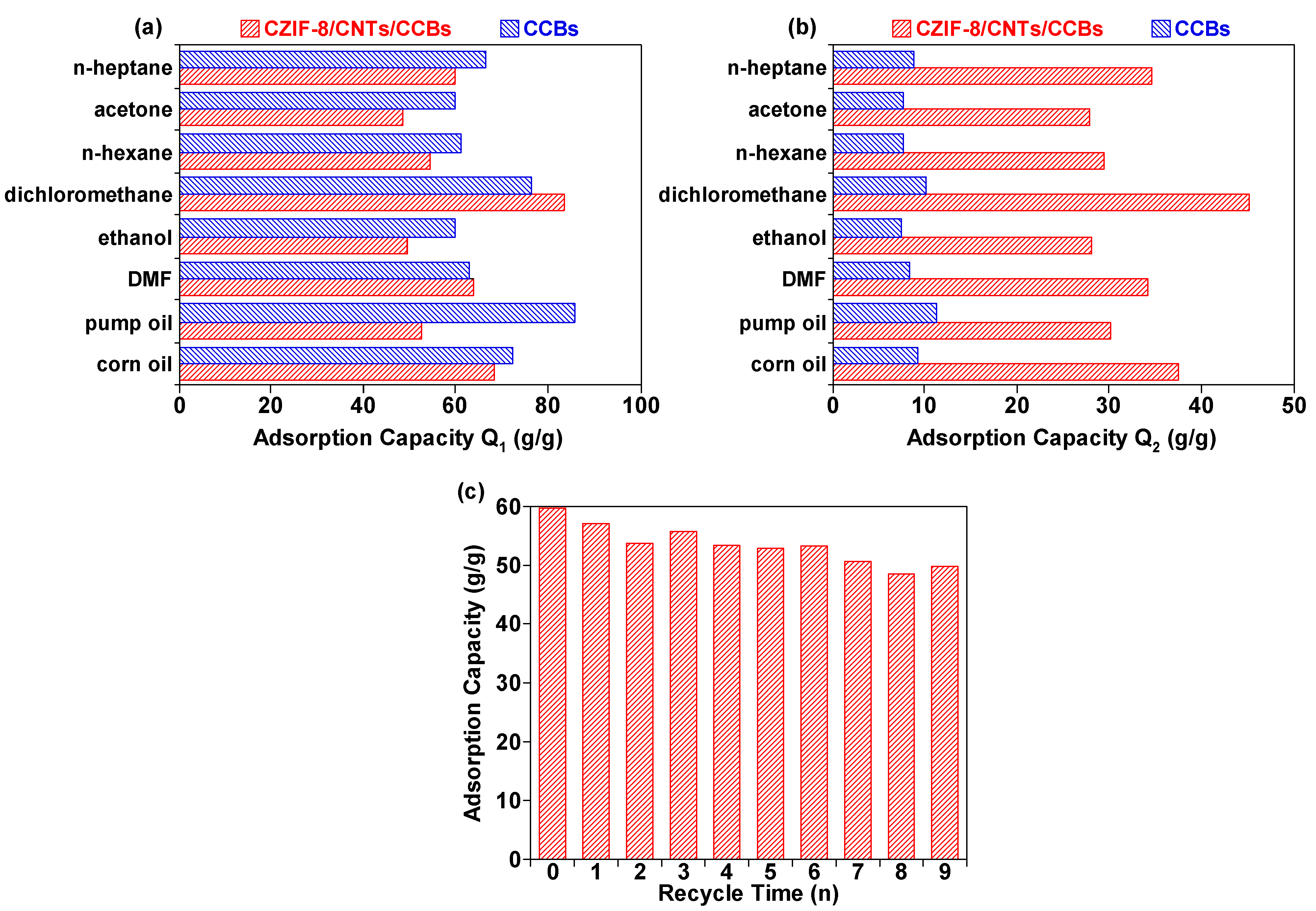
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, X.; Zhang, X.; Xing, Y.; Zhang, H.; Jiang, W.; Zhou, K.; Li, Y. Development of Janus Cellulose Acetate Fiber (CA) Membranes for Highly Efficient Oil-Water Separation. Materials 2021, 14, 5916. [Google Scholar] [CrossRef] [PubMed]
- Sam, E.K.; Liu, J.; Lv, X. Surface Engineering Materials of Superhydrophobic Sponges for Oil/Water Separation: A Review. Ind. Eng. Chem. Res. 2021, 60, 2353–2364. [Google Scholar] [CrossRef]
- Liu, H.; Geng, B.; Chen, Y.; Wang, H. Review on the Aerogel-Type Oil Sorbents Derived from Nanocellulose. ACS Sustain. Chem. Eng. 2016, 5, 49–66. [Google Scholar] [CrossRef]
- Elhenawy, S.; Khraisheh, M.; AlMomani, F.; Hassan, M.K.; Al-Ghouti, M.A.; Selvaraj, R. Recent Developments and Advancements in Graphene-Based Technologies for Oil Spill Cleanup and Oil-Water Separation Processes. Nanomaterials 2021, 12, 87. [Google Scholar] [CrossRef]
- Luo, W.; Sun, D.; Chen, S.; Shanmugam, L.; Xiang, Y.; Yang, J. Robust Microcapsules with Durable Superhydrophobicity and Superoleophilicity for Efficient Oil-Water Separation. ACS Appl. Mater. Interfaces 2020, 12, 57547–57559. [Google Scholar] [CrossRef]
- Han, S.; Song, Q.; Feng, X.; Wang, J.; Zhang, X.; Zhang, Y. Flame-Retardant Silanized Boron Nitride Nanosheet-Infused Superhydrophobic Sponges for Oil/Water Separation. ACS Appl. Nano Mater. 2021, 4, 11809–11819. [Google Scholar] [CrossRef]
- Kong, S.M.; Han, Y.; Won, N.I.; Na, Y.H. Polyurethane Sponge with a Modified Specific Surface for Repeatable Oil-Water Separation. ACS Omega 2021, 6, 33969–33975. [Google Scholar] [CrossRef]
- Panickar, R.; Sobhan, C.B.; Chakravorti, S. Highly Efficient Amorphous Carbon Sphere-Based Superhydrophobic and Superoleophilic Sponges for Oil/Water Separation. Langmuir 2021, 37, 12501–12511. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Z.; Liu, Q.; Tong, Q.; Wang, B. Fabrication of PDMS@Fe3O4/MS Composite Materials and Its Application for Oil-Water Separation. Materials 2021, 15, 115. [Google Scholar] [CrossRef]
- Ju, G.; Zhou, L.; Jiao, C.; Shen, J.; Luan, Y.; Zhao, X. One-Step Fabrication of a Functionally Integrated Device Based on Polydimethylsiloxane-Coated SiO2 NPs for Efficient and Continuous Oil Absorption. Materials 2021, 14, 5998. [Google Scholar] [CrossRef]
- Liu, D.; Wang, S.; Wu, T.; Li, Y. A Robust Superhydrophobic Polyurethane Sponge Loaded with Multi-Walled Carbon Nanotubes for Efficient and Selective Oil-Water Separation. Nanomaterials 2021, 11, 3344. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Pickett, K.; Panchal, A.; Liu, M.; Lvov, Y. Superhydrophobic Polyurethane Foam Coated with Polysiloxane-Modified Clay Nanotubes for Efficient and Recyclable Oil Absorption. ACS Appl. Mater. Interfaces 2019, 11, 25445–25456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.D.; Wu, Z.H.; Xia, Q.Q.; Qu, Y.X.; Pan, H.T.; Hu, W.J.; Zhao, L.; Cao, K.; Chen, E.Y.; Yuan, Z.; et al. Ultrafast Flame-Induced Pyrolysis of Poly(dimethylsiloxane) Foam Materials toward Exceptional Superhydrophobic Surfaces and Reliable Mechanical Robustness. ACS Appl. Mater. Interfaces 2021, 13, 23161–23172. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, D.; An, W.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A Robust and Cost-Effective Superhydrophobic Graphene Foam for Efficient Oil and Organic Solvent Recovery. Small 2015, 11, 5222–5229. [Google Scholar] [CrossRef]
- Bi, H.; Yin, Z.; Cao, X.; Xie, X.; Tan, C.; Huang, X.; Chen, B.; Chen, F.; Yang, Q.; Bu, X.; et al. Carbon fiber aerogel made from raw cotton: A novel, efficient and recyclable sorbent for oils and organic solvents. Adv. Mater. 2013, 25, 5916–5921. [Google Scholar] [CrossRef]
- White, R.J.; Brun, N.; Budarin, V.L.; Clark, J.H.; Titirici, M.M. Always look on the “light” side of life: Sustainable carbon aerogels. ChemSusChem 2014, 7, 670–689. [Google Scholar] [CrossRef]
- Yang, Y.; Tong, Z.; Ngai, T.; Wang, C. Nitrogen-rich and fire-resistant carbon aerogels for the removal of oil contaminants from water. ACS Appl. Mater. Interfaces 2014, 6, 6351–6360. [Google Scholar] [CrossRef]
- Fu, C.; Gu, L.; Zeng, Z.; Xue, Q. One-Step Transformation of Metal Meshes to Robust Superhydrophobic and Superoleophilic Meshes for Highly Efficient Oil Spill Cleanup and Oil/Water Separation. ACS Appl. Mater. Interfaces 2020, 12, 1850–1857. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, H.; Zhang, L.; Sun, Y.; Xu, L.; Sun, Z.; Gu, W.; Chen, Z.; Yang, H. Novel One-Step, in Situ Thermal Polymerization Fabrication of Robust Superhydrophobic Mesh for Efficient Oil/Water Separation. Ind. Eng. Chem. Res. 2017, 56, 11817–11826. [Google Scholar] [CrossRef]
- Zhou, B.; Bashir, B.H.; Liu, Y.; Zhang, B. Facile Construction and Fabrication of a Superhydrophobic Copper Mesh for Ultraefficient Oil/Water Separation. Ind. Eng. Chem. Res. 2021, 60, 8139–8146. [Google Scholar] [CrossRef]
- Lu, Z.; He, Y. Fabrication of superhydrophobic surfaces with tunable water droplet adhesion force by a two-step method. Ceram. Int. 2019, 45, 14389–14396. [Google Scholar] [CrossRef]
- Kang, R.H.; Kim, D. Thermally Induced Silane Dehydrocoupling: Hydrophobic and Oleophilic Filter Paper Preparation for Water Separation and Removal from Organic Solvents. Materials 2021, 14, 5775. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Zhao, L.; Yao, S.; Duan, C. A new architecture of super-hydrophilic β-SiAlON/graphene oxide ceramic membrane for enhanced anti-fouling and separation of water/oil emulsion. Ceram. Int. 2019, 45, 16717–16721. [Google Scholar] [CrossRef]
- Han, X.; Liu, S.; Shi, L.; Li, S.; Li, Y.; Wang, Y.; Chen, W.; Zhao, X.; Zhao, Y. ZIF-derived wrinkled Co3O4 polyhedra supported on 3D macroporous carbon sponge for supercapacitor electrode. Ceram. Int. 2019, 45, 14634–14641. [Google Scholar] [CrossRef]
- Si, Y.; Dong, Z.; Jiang, L. Bioinspired Designs of Superhydrophobic and Superhydrophilic Materials. ACS Cent. Sci. 2018, 4, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Silviana, S.; Darmawan, A.; Dalanta, F.; Subagio, A.; Hermawan, F.; Milen Santoso, H. Superhydrophobic Coating Derived from Geothermal Silica to Enhance Material Durability of Bamboo Using Hexadimethylsilazane (HMDS) and Trimethylchlorosilane (TMCS). Materials 2021, 14, 530. [Google Scholar] [CrossRef]
- Tudu, B.K.; Sinhamahapatra, A.; Kumar, A. Surface Modification of Cotton Fabric Using TiO2 Nanoparticles for Self-Cleaning, Oil-Water Separation, Antistain, Anti-Water Absorption, and Antibacterial Properties. ACS Omega 2020, 5, 7850–7860. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Zhao, J.; Li, H.; Liu, L.; Li, X.; Huang, X.; Liu, H.; Yu, Y. One-Pot Synthesis and Combined Use of Modified Cotton Adsorbent and Flocculant for Purifying Dyeing Wastewater. ACS Sustain. Chem. Eng. 2018, 6, 6876–6888. [Google Scholar] [CrossRef]
- Ferreira, V.C.; Wise, W.R.; Monteiro, O.C. Comparative study of powder and cotton-supported BiOCl particles on the photocatalytic degradation of industrial pollutants. Ceram. Int. 2020, 46, 27508–27516. [Google Scholar] [CrossRef]
- Yang, L.; Zhan, Y.; Yu, R.; Lan, J.; Shang, J.; Dou, B.; Liu, H.; Zou, R.; Lin, S. Facile and Scalable Fabrication of Antibacterial CO2-Responsive Cotton for Ultrafast and Controllable Removal of Anionic Dyes. ACS Appl. Mater. Interfaces 2021, 13, 2694–2709. [Google Scholar] [CrossRef]
- Dalapati, R.; Nandi, S.; Gogoi, C.; Shome, A.; Biswas, S. Metal-Organic Framework (MOF) Derived Recyclable, Superhydrophobic Composite of Cotton Fabrics for the Facile Removal of Oil Spills. ACS Appl. Mater. Interfaces 2021, 13, 8563–8573. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, L.; Hu, Z.; Yu, Y.; Zhang, Y.; Hou, S.; Chen, A. Raw-Cotton-Derived N-Doped Carbon Fiber Aerogel as an Efficient Electrode for Electrochemical Capacitors. ACS Sustain. Chem. Eng. 2018, 6, 4008–4015. [Google Scholar] [CrossRef]
- Yue, Y.; Huang, Y.-L.; Bian, S.-W. Nitrogen-Doped Hierarchical Porous Carbon Films Derived from Metal–Organic Framework/Cotton Composite Fabrics as Freestanding Electrodes for Flexible Supercapacitors. ACS Appl. Electron. Mater. 2021, 3, 2178–2186. [Google Scholar] [CrossRef]
- Singla, G.; Bhange, S.N.; Mahajan, M.; Kurungot, S. Facile synthesis of CNT interconnected PVP-ZIF-8 derived hierarchically porous Zn/N co-doped carbon frameworks for oxygen reduction. Nanoscale 2021, 13, 6248–6258. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, N.; Tan, D.; Qi, Z.; Wu, C. Facile Synthesis of Enzyme-Embedded Metal-Organic Frameworks for Size-Selective Biocatalysis in Organic Solvent. Front. Bioeng. Biotechnol. 2020, 8, 714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, Y.; Li, M.; Hou, L. Influence of the 2-methylimidazole/zinc nitrate hexahydrate molar ratio on the synthesis of zeolitic imidazolate framework-8 crystals at room temperature. Sci. Rep. 2018, 8, 9597. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.; Li, M.; Hu, T.; Xue, Z.; Yu, J.; Chi, R. Preparation of an Adsorbent Based on Amidoxime and Triazole Modified Waste Cotton Fabrics through an Azide–Alkyne Click Reaction with Excellent Adsorption Performance toward Cu(II). ACS Sustain. Chem. Eng. 2018, 7, 1944–1955. [Google Scholar] [CrossRef]
- Mu, J.; Shao, C.; Guo, Z.; Zhang, Z.; Zhang, M.; Zhang, P.; Chen, B.; Liu, Y. High photocatalytic activity of ZnO-carbon nanofiber heteroarchitectures. ACS Appl. Mater. Interfaces 2011, 3, 590–596. [Google Scholar] [CrossRef]
- Zhu, Z.; Fu, S.; Lucia, L.A. A fiber-aligned thermal-managed wood-based superhydrophobic aerogel for efficient oil recovery. ACS Sustain. Chem. Eng. 2019, 7, 16428–16439. [Google Scholar] [CrossRef]
- Guan, H.; Cheng, Z.; Wang, X. Highly compressible wood sponges with a spring-like lamellar structure as effective and reusable oil absorbents. ACS Nano 2018, 12, 10365–10373. [Google Scholar] [CrossRef]
- Gui, X.; Zeng, Z.; Lin, Z.; Gan, Q.; Xiang, R.; Zhu, Y.; Cao, A.; Tang, Z. Magnetic and highly recyclable macroporous carbon nanotubes for spilled oil sorption and separation. ACS Appl. Mater. Interfaces 2013, 5, 5845–5850. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wu, Y.; Yang, F.; Gan, J.; Wang, Y.; Zhang, J. Wood sponge reinforced with polyvinyl alcohol for sustainable oil-water separation. ACS Omega 2021, 6, 12866–12876. [Google Scholar] [CrossRef]
- Feng, C.; Yi, Z.; She, F.; Gao, W.; Peng, Z.; Garvey, C.J.; Dumee, L.F.; Kong, L. Superhydrophobic and superoleophilic micro-wrinkled reduced graphene oxide as a highly portable and recyclable oil sorbent. ACS Appl. Mater. Interfaces 2016, 8, 9977–9985. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.N.; Wang, H.; Wang, Y.Y.; Wei, Y.H.; Si, J.Y.; Yuen, A.C.Y.; Xie, J.S.; Yu, B.; Zhu, S.E.; Lu, H.D.; et al. Robust, lightweight, hydrophobic, and fire-retarded polyimide/MXene aerogels for effective oil/water separation. ACS Appl. Mater. Interfaces 2019, 11, 40512–40523. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. Carbon aerogel from winter melon for highly efficient and recyclable oils and organic solvents absorption. ACS Sustain. Chem. Eng. 2014, 2, 1492–1497. [Google Scholar] [CrossRef]
- Ieamviteevanich, P.; Palaporn, D.; Chanlek, N.; Poo-arporn, Y.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Carbon nanofiber aerogel/magnetic core–shell nanoparticle composites as recyclable oil sorbents. ACS Appl. Nano Mater. 2020, 3, 3939–3950. [Google Scholar] [CrossRef]
- Sun, T.; Hao, S.; Fan, R.; Qin, M.; Chen, W.; Wang, P.; Yang, Y. Hydrophobicity-adjustable MOF constructs superhydrophobic MOF-rGO aerogel for efficient oil-water separation. ACS Appl. Mater. Interfaces 2020, 12, 56435–56444. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Cai, Z.; Yan, N.; Liu, M.; Yu, Y. Highly compressible and hydrophobic anisotropic aerogels for selective oil/organic solvent absorption. ACS Sustain. Chem. Eng. 2018, 7, 332–340. [Google Scholar] [CrossRef]

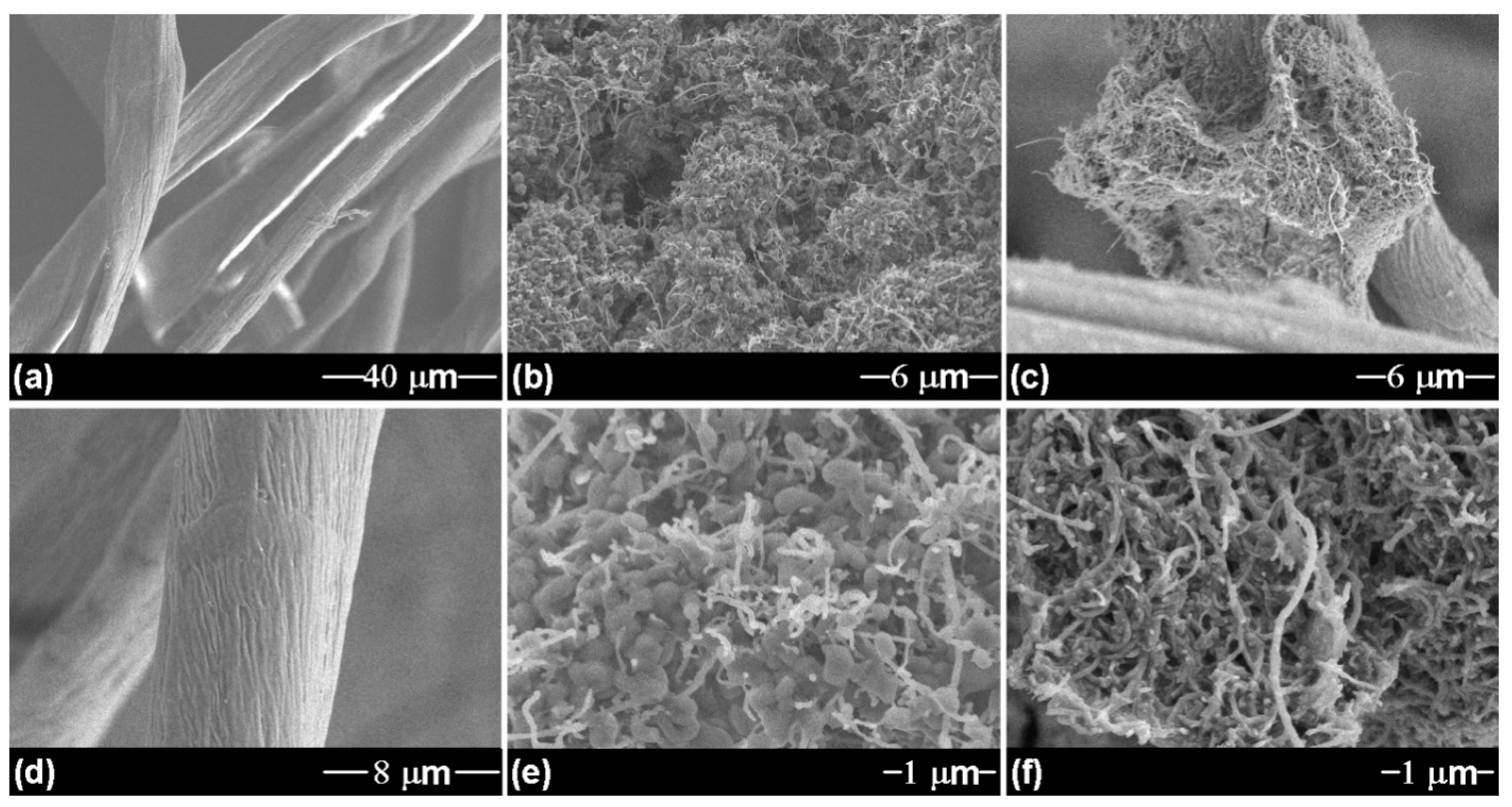
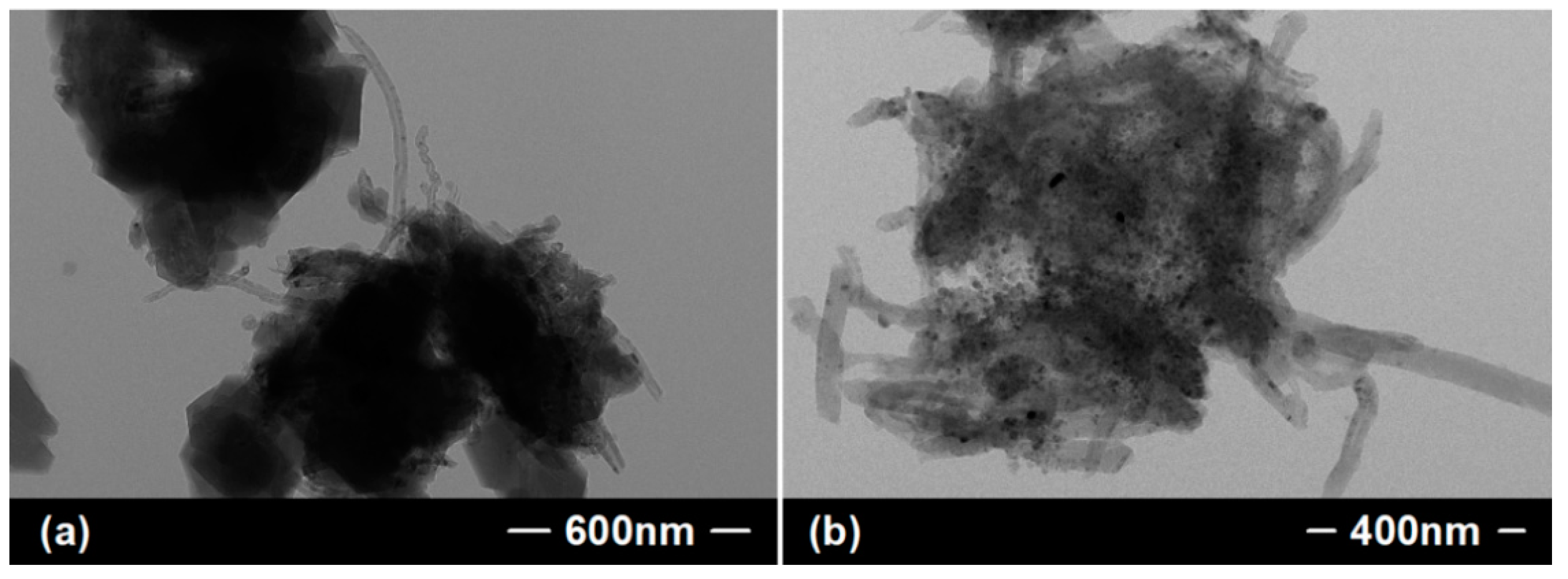
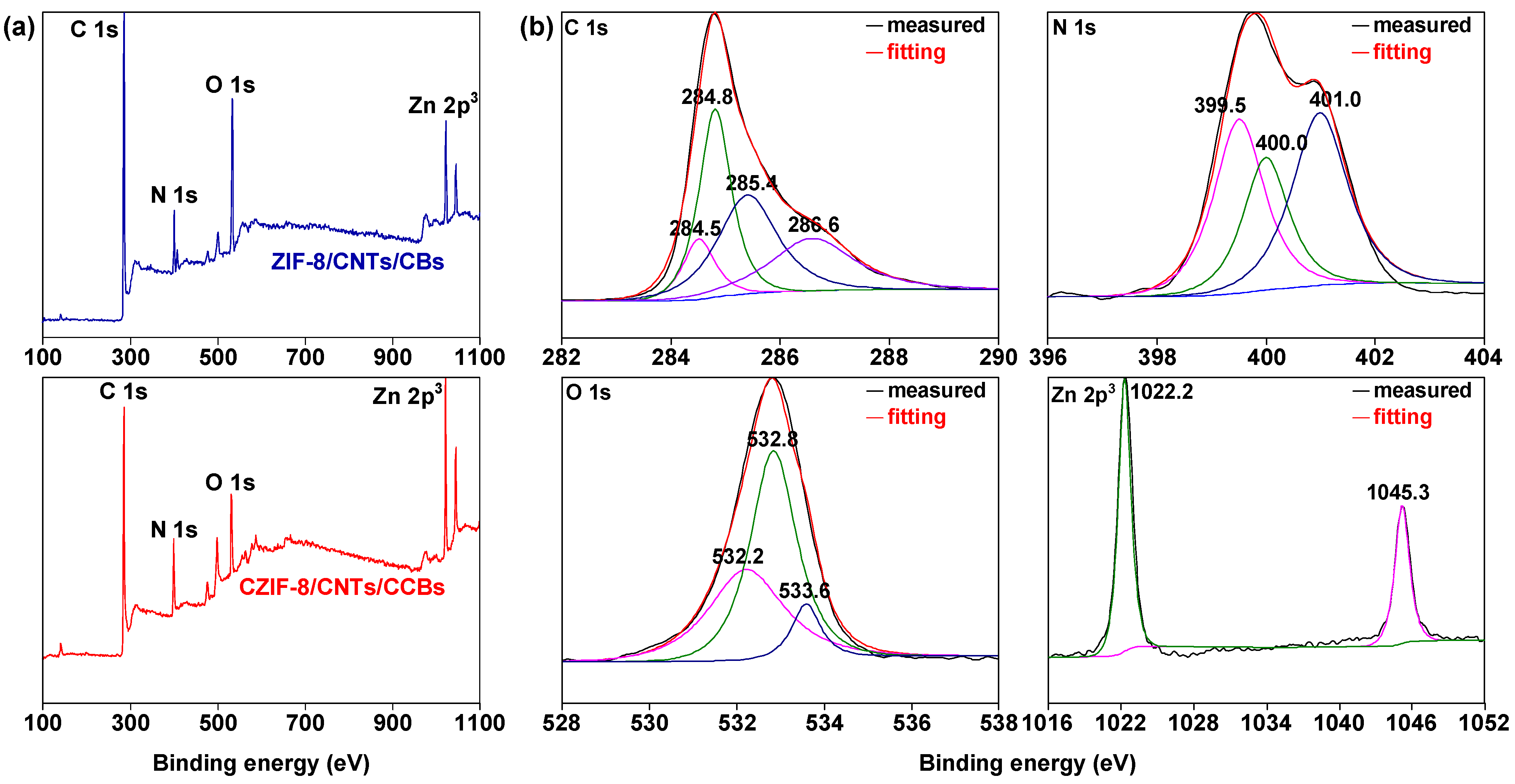
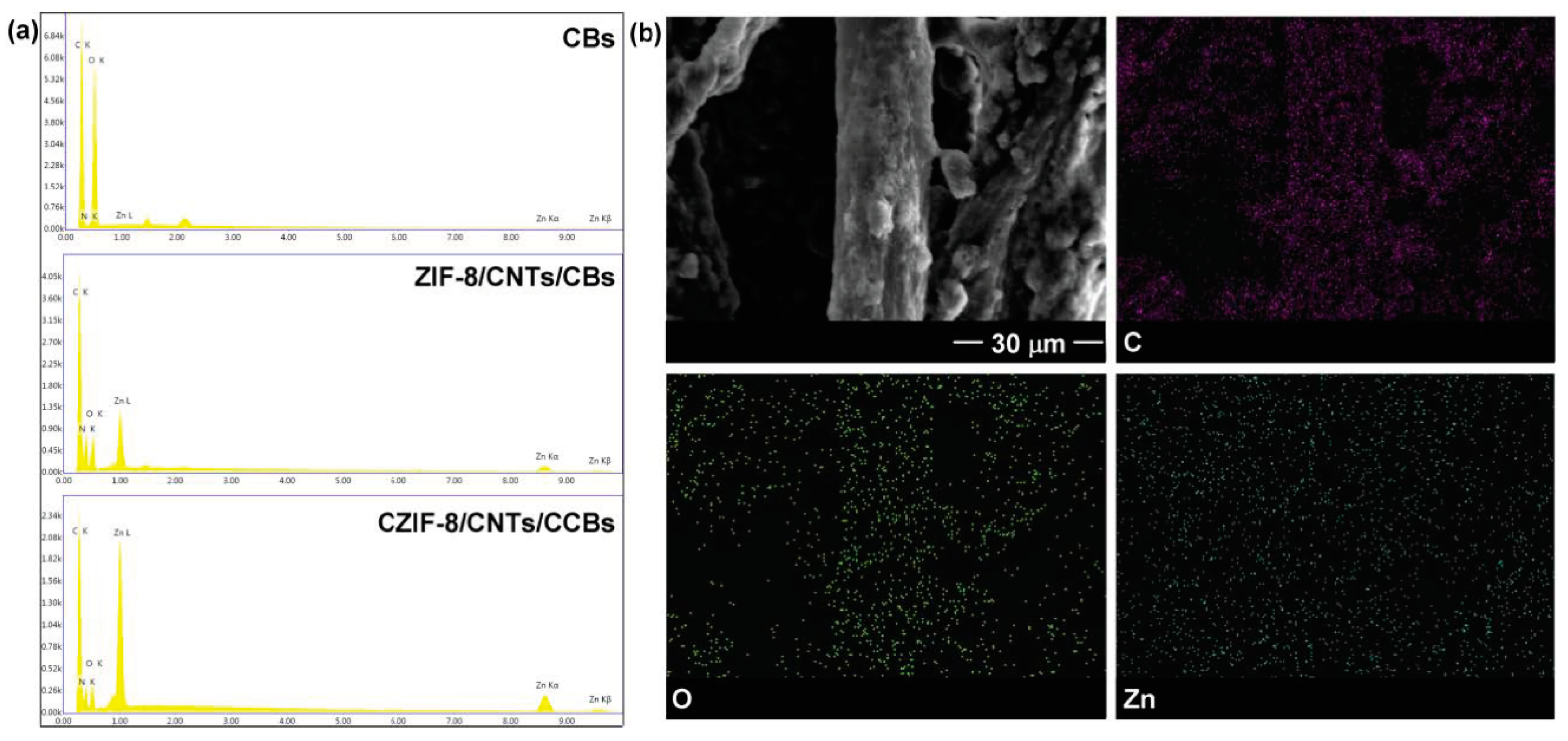

| Sorbent Material | Absorbed Substances | Sorption Capacity (g.g−1) | Ref. |
|---|---|---|---|
| CNT/wood aerogel | oils and organic solvents | 16–39 | [39] |
| Silylated wood sponges | oils and organic solvents | 17–41 | [40] |
| Carbon nanotube sponges | oils | 49–56 | [41] |
| Polyvinyl-alcohol-reinforced wood sponge | oils and organic solvents | 4–27 | [42] |
| Microwrinkled reduced graphene oxide | oils | 36–84 | [43] |
| Cellulose-based aerogels | oils and organic solvents | 45–99 | [48] |
| Winter melon carbon aerogel | oils and organic solvents | 16–50 | [45] |
| Carbon nanofiber aerogel | oils and organic solvents | 37–87 | [46] |
| MOF-coated cotton fiber composite | oils and organic solvents | 25–48 | [31] |
| MOF-reduced graphene oxide aerogel | oils and organic solvents | 45–147 | [47] |
| Polyimide/MXene aerogels | oils and organic solvents | 18–58 | [44] |
| CZIF-8/CNTs/CCBs | oils and organic solvents | 48–84 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Ke, Z.; Shen, C.; Sun, R.; Wei, Q.; Yin, Z.; Yang, W. Fabrication of Carbon Aerogels Derived from Metal-Organic Frameworks/Carbon Nanotubes/Cotton Composites as an Efficient Sorbent for Sustainable Oil–Water Separation. Appl. Sci. 2022, 12, 7285. https://doi.org/10.3390/app12147285
Sun Y, Ke Z, Shen C, Sun R, Wei Q, Yin Z, Yang W. Fabrication of Carbon Aerogels Derived from Metal-Organic Frameworks/Carbon Nanotubes/Cotton Composites as an Efficient Sorbent for Sustainable Oil–Water Separation. Applied Sciences. 2022; 12(14):7285. https://doi.org/10.3390/app12147285
Chicago/Turabian StyleSun, Yinyu, Zhongcheng Ke, Caiyun Shen, Ruikang Sun, Qing Wei, Zihan Yin, and Wei Yang. 2022. "Fabrication of Carbon Aerogels Derived from Metal-Organic Frameworks/Carbon Nanotubes/Cotton Composites as an Efficient Sorbent for Sustainable Oil–Water Separation" Applied Sciences 12, no. 14: 7285. https://doi.org/10.3390/app12147285
APA StyleSun, Y., Ke, Z., Shen, C., Sun, R., Wei, Q., Yin, Z., & Yang, W. (2022). Fabrication of Carbon Aerogels Derived from Metal-Organic Frameworks/Carbon Nanotubes/Cotton Composites as an Efficient Sorbent for Sustainable Oil–Water Separation. Applied Sciences, 12(14), 7285. https://doi.org/10.3390/app12147285






