The Chloroform Extracts of Vietnamese Sophora flavescens Ait. Inhibit the Proliferation of HepG2 Cells through Apoptosis Induction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction and Fractionation
2.2. Solid-Phase Extraction and Prep-HPLC Separation
2.3. Cell Culture and SFA Extract Treatment
2.4. Cell Density Determination
2.5. Flow Cytometry Analysis
2.6. Nuclear Staining
2.7. Real-Time RT-PCR
2.8. Western Blot
2.9. Statistical Analysis
3. Results
3.1. SFA Extraction
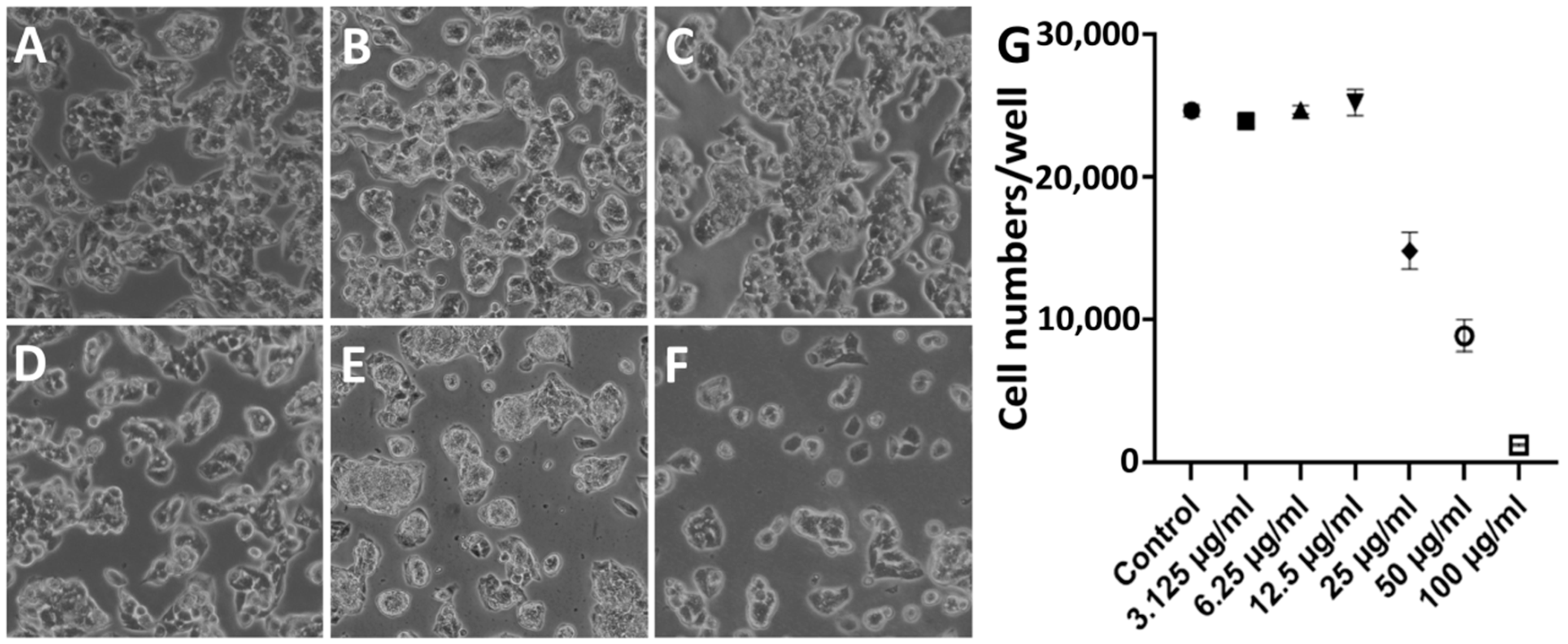
3.2. Effects of SFA-CHCl3 Extract on HepG2 Cell Morphology
3.3. Effects of SFA-CHCl3 Extract on HepG2 Cell Proliferation
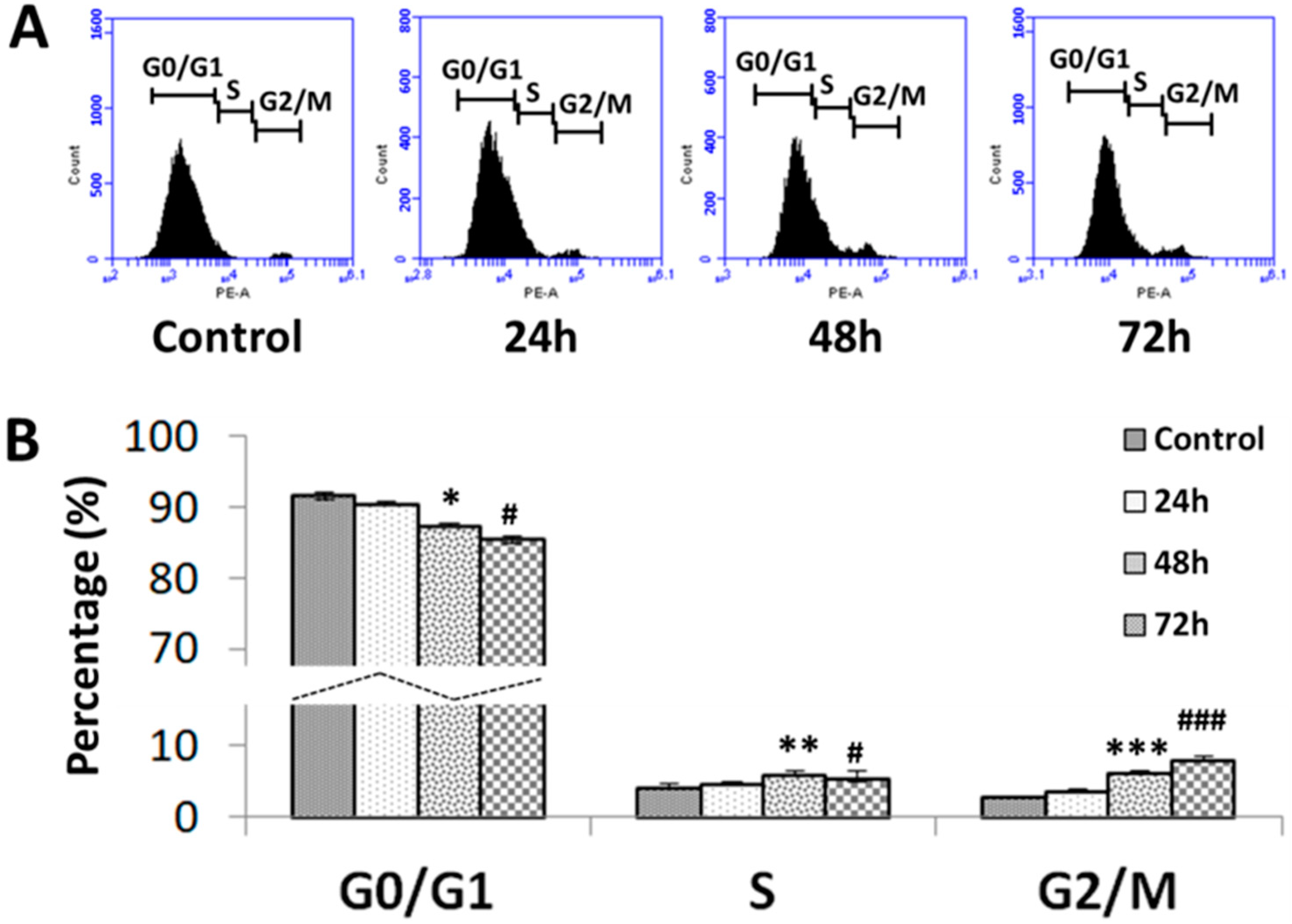
3.4. Effects of SFA-CHCl3 Extract on Cell Cycle Progression of HepG2 Cells
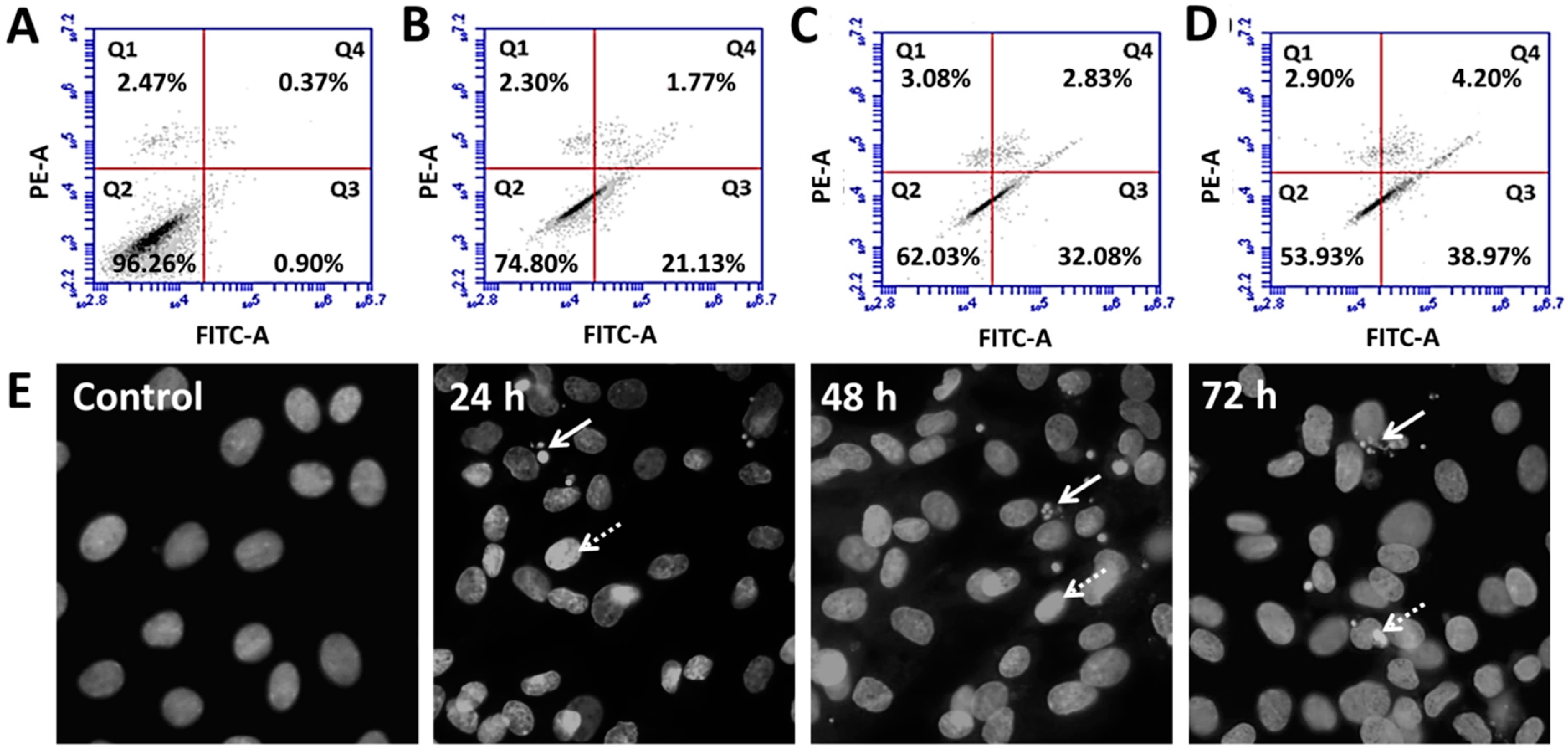
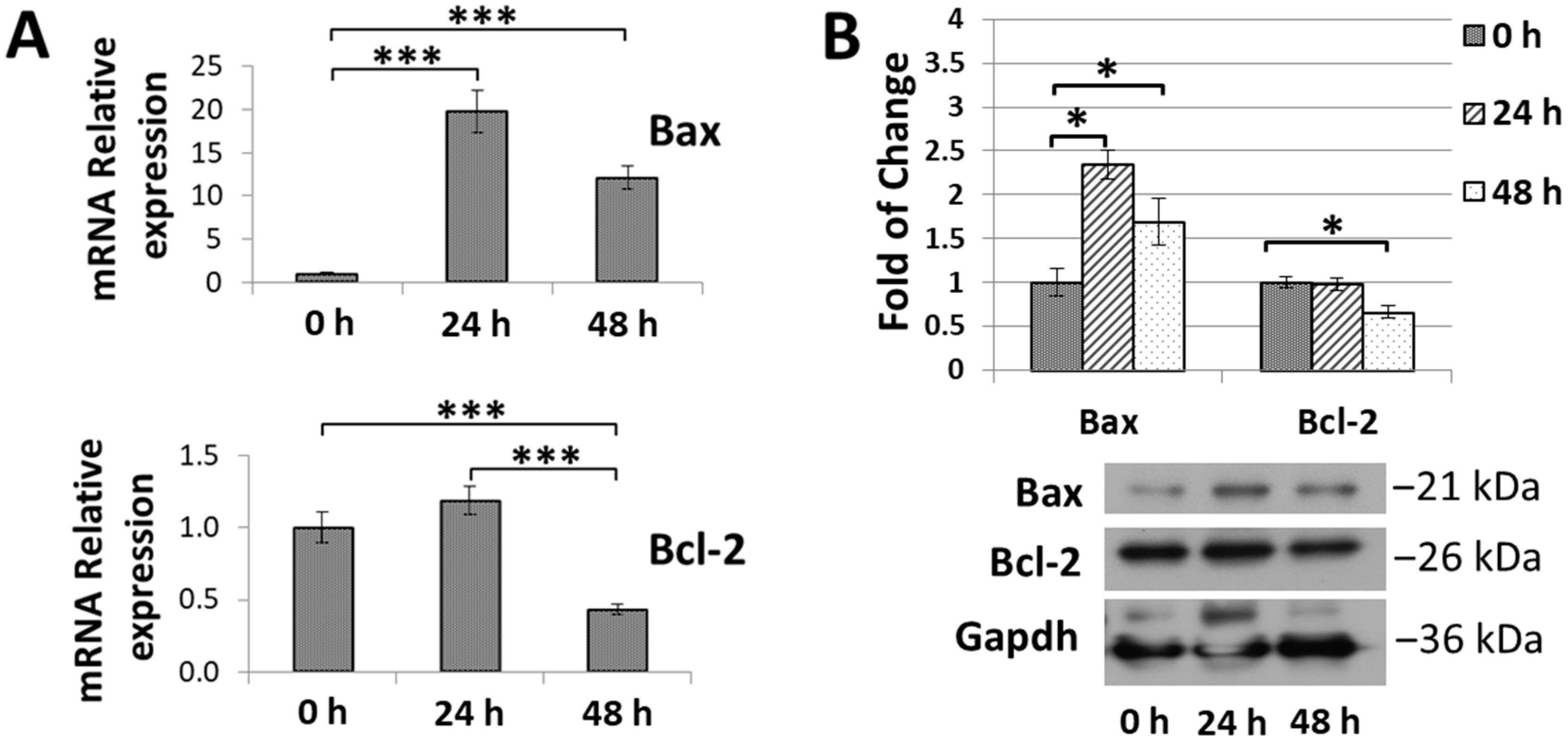
3.5. SFA-CHCl3 Extract Induced Apoptosis of HepG2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal bovine serum |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HPLC | High-performance liquid chromatography |
| HRESIMS | High-resolution electrospray ionisation mass spectrometry |
| HRP | Horseradish peroxidase |
| IgG | Immunoglobulin G |
| O.D. | Optical density |
| PBS | Phosphate-buffered saline |
| Pen/Strep | Penicillin/streptomycin |
| PI | Propidium iodide |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| TBST | Tris-buffered saline, 0.1% Tween 20 |
| UFLC | Ultra-fast liquid chromatography |
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Xi, S.Y.; Minuk, G.Y. Role of traditional Chinese medicine in the management of patients with hepatocellular carcinoma. World J. Hepatol. 2018, 10, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Hou, J.; Ma, G.; Liu, J. Network pharmacology identifes the mechanisms of action of Shaoyao gancao decoction in the treatment of osteoarthritis. Med. Sci. Monit. 2019, 25, 6051–6073. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Hou, J. Exploring the mechanism of action Xianlingubao Prescription in the treatment of osteoporosis by network pharmacology. Comput. Biol. Chem. 2020, 85, 107240. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [Green Version]
- Hacker, G. The morphology of apoptosis. Cell Tissue Res. 2000, 301, 5–17. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [Green Version]
- Al-Aamri, H.M.; Irving, H.R.; Bradley, C.; Meehan-Andrews, T. Intrinsic and extrinsic apoptosis responses in leukaemia cells following daunorubicin treatment. BMC Cancer 2021, 21, 438. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X. Endoplasmic reticulum-mitochondria tethering in neurodegenerative dieseaes. Transl. Neurodegener. 2017, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Tang, D.; Yang, T.; Qian, D.; Xu, R. Apoptosis Triggering, an Important Way for Natural Products From Herbal Medicines to Treat Pancreatic Cancers. Front. Pharmacol. 2022, 12, 796300. [Google Scholar] [CrossRef]
- Ding, P.L.; He, C.M.; Cheng, Z.H.; Chen, D.F. Flavonoids rather than alkaloids as the diagnostic constituents to distinguish Sophorae Flavescentis Radix from Sophorae Tonkinensis Radix et Rhizoma: An HPLC fngerprint study. Chin. J. Nat. Med. 2018, 16, 951–960. [Google Scholar] [CrossRef]
- Zhu, N.; Hou, J. Molecular mechanism of the anti-inflammatory effects of Sophorae Flavescentis Aiton identified by network pharmacology. Sci. Rep. 2021, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Song, L.Y.; Ma, Y.T.; Fang, W.J.; He, Y.; Wu, J.L.; Zuo, S.R.; Deng, Z.Z.; Wang, S.F.; Liu, S.K. Inhibitory efects of oxymatrine on hepatic stellate cells activation through TGF-beta/miR-195/Smad signaling pathway. BMC Complement. Altern. Med. 2019, 19, 138. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. Meyler’s Side Effects of Drugs, 16th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 229–236. [Google Scholar]
- Qin, X.G.; Hua, Z.; Shuang, W.; Wang, Y.H.; Cui, Y.D. Effects of matrine on HepG2 cell proliferation and expression of tumor relevant proteins in vitro. Pharm. Biol. 2010, 48, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lin, Y.; He, L.; Ou, R.; Chen, T.; Zhang, X.; Li, Q.; Zeng, Z.; Long, Q. Two New Isoprenoid Flavonoids from Sophora flavescens with Antioxidant and Cytotoxic Activities. Molecules 2021, 26, 7228. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Zhao, F.; Ma, J.X.; Gan, H.Y.; Xie, X.F.; Qiao, Z.L.; Zhao, J. A New Prenylated Flavanonol from the Roots of Sophora flavescens. Chem. Nat. Compd. 2021, 57, 20–22. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Solid-phase extraction of matrine and oxymatrine from Sophora flavescens Ait using amino-imidazolium polymer. J. Sep. Sci. 2010, 33, 1739–1745. [Google Scholar] [CrossRef]
- Tian, M.; Row, K.H. Simultaneous extraction and separation of martrine, sophoridine and sophocarpine from Sophora flavescens ait by rp-hplc with analytical and preparative chromatography. J. Liq. Chromatogr. Relat. Technol. 2009, 33, 259–269. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Yuan, B.; Cao, C.; Qin, S.; Cao, X.; Bian, G.; Wang, Z.; Jiang, J. Methylated actinomycin D, a novel actinomycin D analog induces apoptosis in HepG2 cells through Fas- and mitochondria-mediated pathways. Mol. Carcinog. 2013, 52, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-Time Quantitative PCR and the 2−∆∆Ct Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. In Protocols in In Vitro Hepatocyte Research. Methods in Molecular Biology (Methods and Protocols); Vinken, M., Rogiers, V., Eds.; Humana Press: New York, NY, USA, 2015; Volume 1250. [Google Scholar]
- Sormunen, R.; Eskelinen, S.; Lehto, V.P. Bile canaliculus formation in cultured HEPG2 cells. Lab. Investig. 1993, 68, 652–662. [Google Scholar] [PubMed]
- Fearn, R.A.; Hirst, B.H. Predicting oral drug absorption and hepatobiliary clearance: Human intestinal and hepatic in vitro cell models. Environ. Toxicol. Pharmacol. 2006, 21, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Ocker, M.; Höpfner, M. Apoptosis-Modulating Drugs for Improved Cancer Therapy. Eur. Surg. Res. 2012, 48, 111–120. [Google Scholar] [CrossRef] [PubMed]
- An, W.; Lai, H.; Zhang, Y.; Liu, M.; Lin, X.; Cao, S. Apoptotic Pathway as the Therapeutic Target for Anticancer Traditional Chinese Medicines. Front. Pharmacol. 2019, 10, 758. [Google Scholar] [CrossRef] [Green Version]
- Chao, X.; Wang, G.; Tang, Y.; Dong, C.; Li, H.; Wang, B.; Wu, J.; Zhao, J. The effects and mechanism of peiminine-induced apoptosis in human hepatocellular carcinoma HepG2 cells. PLoS ONE 2019, 14, e0201864. [Google Scholar] [CrossRef] [Green Version]
- Obeng, E. Apoptosis (programmed cell death) and its signals—A review. Braz. J. Biol. 2021, 81, 1133–1143. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Han, S.I.; Kim, Y.S.; Kim, T.H. Role of apoptotic and necrotic cell death under physiologic conditions. BMB Rep. 2008, 41, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Alenzi, F.Q. Links between apoptosis, proliferation and the cell cycle. Br. J. Biomed. Sci. 2004, 61, 99–102. [Google Scholar] [CrossRef]
- Bai, J.; Li, Y.; Zhang, G. Cell cycle regulation and anticancer drug discovery. Cancer Biol. Med. 2017, 14, 348–362. [Google Scholar]
- Alimbetov, D.; Askarova, S.; Umbayev, B.; Davis, T.; Kipling, D. Pharmacological Targeting of Cell Cycle, Apoptotic and Cell Adhesion Signaling Pathways Implicated in Chemoresistance of Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadok, V.A.; Bratton, D.L.; Frasch, S.C.; Warner, M.L.; Henson, P.M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998, 5, 551–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shlomovitz, I.; Speir, M.; Gerlic, M. Flipping the dogma—Phosphatidylserine in non-apoptotic cell death. Cell Commun. Signal. 2019, 17, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, E.H.; Wei, M.C.; Weiler, S.; Flavell, R.A.; Mak, T.W.; Lindsten, T.; Korsmeyer, S.J. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell. 2001, 8, 705–711. [Google Scholar] [CrossRef]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.; Liu, Q.; Liu, K.; Yagasaki, K.; Wu, E.; Zhang, G. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NFkappaB signaling. Cytotechnology 2009, 59, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Ran, Z.H.; Xu, Q.; Xiao, S.D. Experimental study of the killing effects of oxymatrine on human colon cancer cell line SW1116. Chin. J. Dig. Dis. 2005, 6, 15–20. [Google Scholar] [CrossRef]
- Jin, H.; Sun, Y.; Wang, S.; Cheng, X. Matrine activates PTEN to induce growth inhibition and apoptosis in V600EBRAF harboring melanoma cells. Int. J. Mol. Sci. 2013, 14, 16040–16057. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, J.; Sun, L.; Cheng, B.; Pan, S.; Zhou, M.; Sun, X. Matrine induces programmed cell death and regulates expression of relevant genes based on PCR array analysis in C6 glioma cells. Mol. Biol. Rep. 2009, 36, 791–799. [Google Scholar] [CrossRef]
- Ling, Q.; Xu, X.; Wei, X.; Wang, W.; Zhou, B.; Wang, B.; Zheng, S. Oxymatrine induces human pancreatic cancer PANC-1 cells apoptosis via regulating expression of Bcl-2 and IAP families, and releasing of cytochrome c. J. Exp. Clin. Cancer Res. 2011, 30, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, G.; Luo, Q.; Qin, J.; Wang, L.; Shi, Y.; Sun, C. Effects of oxymatrine on proliferation and apoptosis in human hepatoma cells. Colloids Surf. B Biointerfaces 2006, 48, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Li, Y.M.; Liu, T.; He, W.T.; Chen, Y.T.; Chen, X.H.; Li, X.; Zhou, W.C.; Yi, J.F.; Ren, Z.J. Antitumor effect of matrine in human hepatoma G2 cells by inducing apoptosis and autophagy. World J. Gastroenterol. 2010, 16, 4281–4290. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, B.; Yang, W.; Zhang, Q.; Wang, W.; Zhai, W.; Lu, L.; Zheng, Y.; Dang, Z.; Li, B.; et al. Matrine inhibits proliferation and migration of HepG2 cells by downregulating ERK1/2 signaling pathways. J. Cancer Res. Ther. 2020, 16, 209–214. [Google Scholar] [PubMed]
- Chen, K.; Zhu, P.; Ye, J.; Liao, Y.; Du, Z.; Chen, F.; He, J.; Zhang, S.; Zhai, W. Oxymatrine inhibits the migration and invasion of hepatocellular carcinoma cells by reducing the activity of MMP-2/-9 via regulating p38 signaling pathway. J. Cancer 2019, 10, 5397–5403. [Google Scholar] [CrossRef]
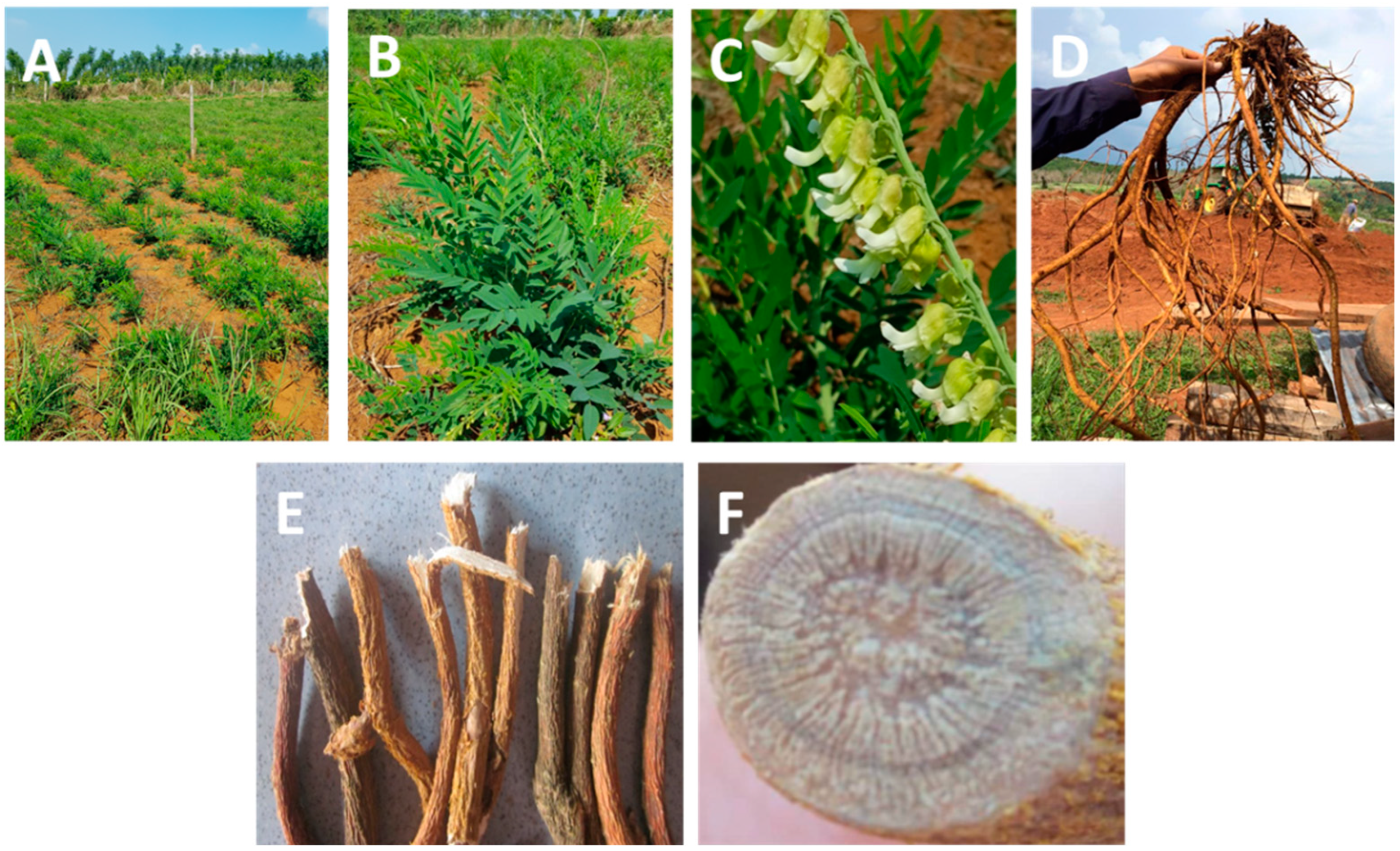
| Solvent | Matrine (mg/g) | Oxymatrine (mg/g) |
|---|---|---|
| Ethanol extract | 28.1 | 1.79 |
| Chloroform extract | 70.1 | 4.2 |
| Ethyl acetate extract | 1.1 | 0.09 |
| Aqueous extract | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trang, C.N.M.; Chi, H.N.Q.; Manh, N.K.; Son, H.N.; Ngo, D.-N.; Long, L.T. The Chloroform Extracts of Vietnamese Sophora flavescens Ait. Inhibit the Proliferation of HepG2 Cells through Apoptosis Induction. Appl. Sci. 2022, 12, 5906. https://doi.org/10.3390/app12125906
Trang CNM, Chi HNQ, Manh NK, Son HN, Ngo D-N, Long LT. The Chloroform Extracts of Vietnamese Sophora flavescens Ait. Inhibit the Proliferation of HepG2 Cells through Apoptosis Induction. Applied Sciences. 2022; 12(12):5906. https://doi.org/10.3390/app12125906
Chicago/Turabian StyleTrang, Cao Ngoc Minh, Ho Nguyen Quynh Chi, Nguyen Khac Manh, Hoang Nghia Son, Dai-Nghiep Ngo, and Le Thanh Long. 2022. "The Chloroform Extracts of Vietnamese Sophora flavescens Ait. Inhibit the Proliferation of HepG2 Cells through Apoptosis Induction" Applied Sciences 12, no. 12: 5906. https://doi.org/10.3390/app12125906
APA StyleTrang, C. N. M., Chi, H. N. Q., Manh, N. K., Son, H. N., Ngo, D.-N., & Long, L. T. (2022). The Chloroform Extracts of Vietnamese Sophora flavescens Ait. Inhibit the Proliferation of HepG2 Cells through Apoptosis Induction. Applied Sciences, 12(12), 5906. https://doi.org/10.3390/app12125906







