Impact of Rapid pH Changes on Activated Sludge Process
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Inoculum for SBR Reactors
2.3. Water Quality Analyses
2.4. Activated Sludge Properties
2.5. Statistical Analyses
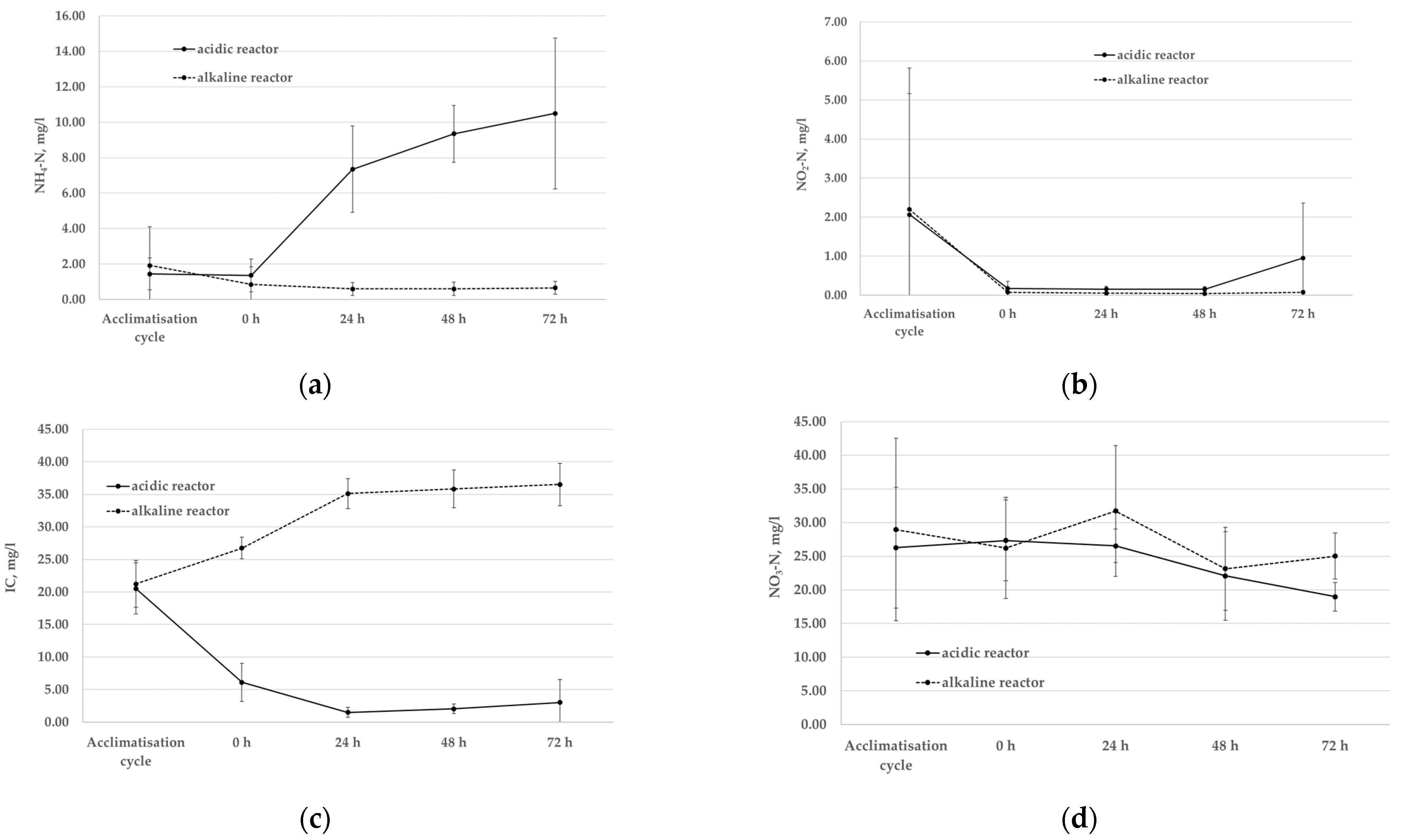
3. Results
3.1. Changes in Influent and Effluent Wastewater Characteristics
3.2. Changes in Activated Sludge Process
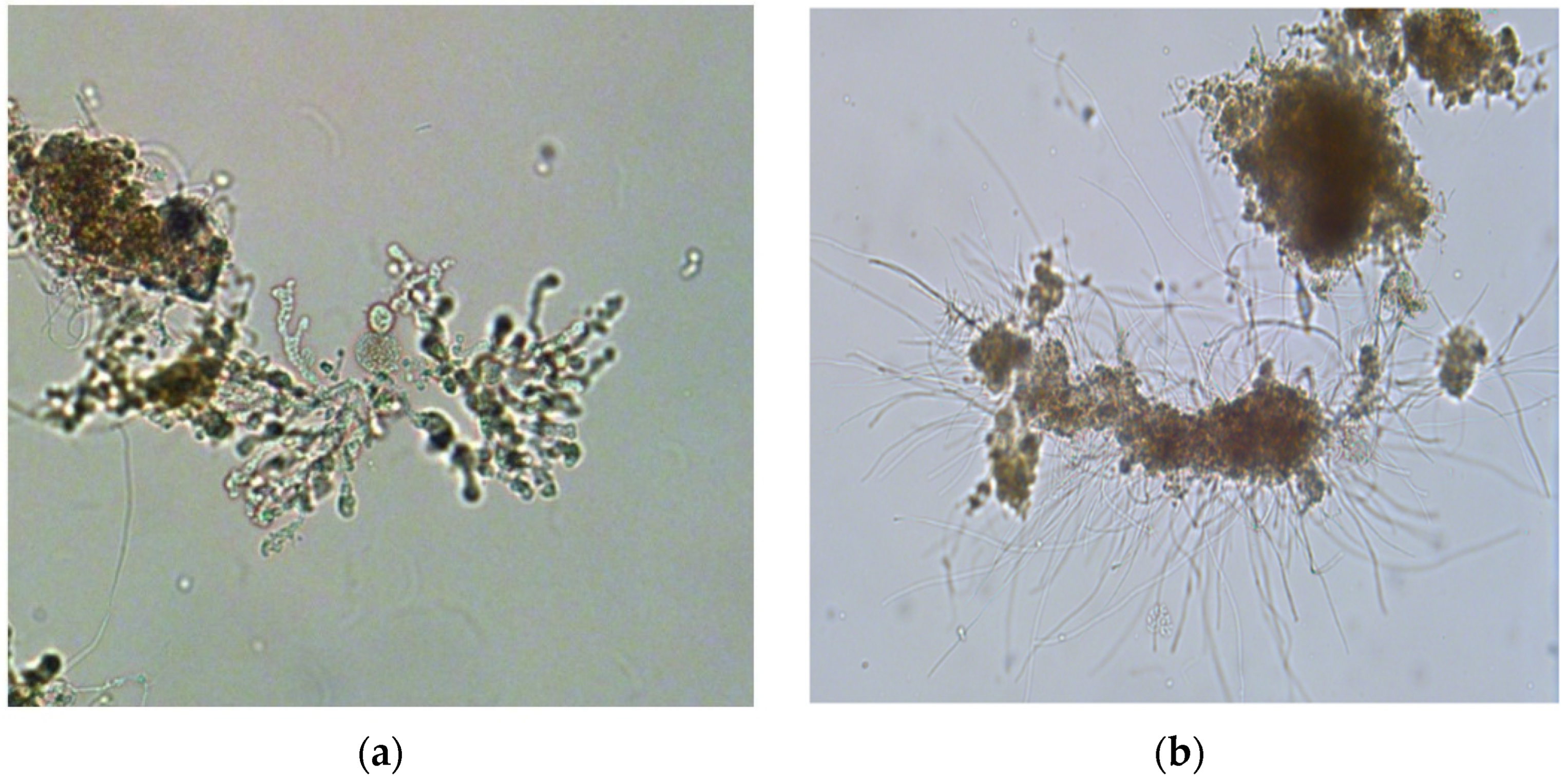
3.3. Sludge Microfauna Changes
4. Discussion
5. Conclusions
- Rapid changes of pH from optimal to acidic had a greater impact on the ASP than alkaline pH.
- Acidic pH affected ammonia conversion to nitrites indicating inhibition of both AOB and NOB; however, this phenomenon may also be attributed to the limitation of inorganic carbon source (due to pH change itself).
- Organic carbon biodegradation also decreased, and increased sludge foaming and sludge washout were noted in acidic pH reactor.
- The slight changes in sludge microfauna population were observed in both reactors. In the acidic reactor, there was a noticeable increase in gymnamoebae, Zoogloea spp., and crawling ciliate Chilodonella sp. In the alkaline pH reactor, the microfauna population decreased and filamentous sludge bulking occurred due to the increase in filamentous flocs.
- SVI30 index is not the main indicator of the formation of high FA concentration in this case. Although, SVI5 changed more than SVI30 and can be specified as the first sign of the inhibition.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, Q.; Peng, D.C. Nitrite oxidizing bacteria (NOB) dominating in nitrifying community in full-scale biological nutrient removal wastewater treatment plants. AMB Express 2017, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Golovko, K.; Tihomirova, K.; Neilands, R.; Resetilovs, J.; Mezule, L.; Juhna, T. Short-term effect of shock ammonium nitrogen load on activated sludge properties. Water Sci. Technol. 2019, 80, 2191–2199. [Google Scholar] [CrossRef]
- Rodriguez-Caballero, A.; Ribera, A.; Balcázar, J.L.; Pijuan, M. Nitritation versus full nitrification of ammonium-rich wastewater: Comparison in terms of nitrous and nitric oxides emissions. Bioresour. Technol. 2013, 139, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.S.; Srinath, E.G. Inhibition of nitrification by ammonia and nitrous acid. Water Pollut. Control. Fed. 1976, 48, 835–852. [Google Scholar]
- Chung, J.; Shim, H.; Park, S.-J.; Kim, S.-J.; Bae, W. Optimization of free ammonia concentration for nitrite accumulation in shortcut biological nitrogen removal process. Bioprocess Biosyst. Eng. 2006, 28, 275–282. [Google Scholar] [CrossRef]
- Andreadakis, D.; Noutsopoulos, C.; Fragkiskatos, G.; Mamais, D.; Misirli, T.; Argyropoulou, K.; Themeli, E.; Malamis, S. Inhibition of free nitrous acid and free ammonia on polyphosphate accumulating organisms: Evidence of insufficient phosphorus removal through nitritation-denitritation. J. Environ. Manag. 2021, 297, 113390. [Google Scholar] [CrossRef]
- Magri, A.; Flotats, X. Modelling of biological nitrogen removal from the liquid fraction of pig slurry in a sequencing batch reactor. Biosyst. Eng. 2008, 101, 239–259. [Google Scholar] [CrossRef]
- Popa, P.; Timofti, M.; Voiculescu, M.; Dragan, S.; Trif, C.; Georgescu, L.P. Study of physico-chemical characteristics of wastewater in an urban agglomeration in Romania. Sci. World J. 2012, 549028. [Google Scholar] [CrossRef] [Green Version]
- Henrikson, L.; Brodin, Y.W. Liming of Acidified Surface Waters: A Swedish Synthesis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; p. 458. [Google Scholar]
- Liu, Y.; Ngo, H.H.; Guo, W.; Peng, L.; Wang, D.; Ni, B. The roles of free ammonia (FA) in biological wastewater treatment processes: A review. Environ. Int. 2019, 123, 10–19. [Google Scholar] [CrossRef]
- Gray, N.F. Biology Of Wastewater Treatment, 2nd ed.; Imperial College Press: London, UK, 2004; p. 1420. [Google Scholar]
- Baldwin, D.; Campbell, C. Short-Term Effects of Low pH on the Microfauna of an Activated Sludge Wastewater Treatment System. Water Qual. Res. J. Can. 2001, 36, 519–535. [Google Scholar] [CrossRef]
- Puigagut, J.; Salvado, H.; Garcia, J. Short-term harmful effects of ammonia nitrogen on activated sludge microfauna. Water Res. 2005, 39, 4397–4404. [Google Scholar] [CrossRef]
- Denisova, V.; Tihomirova, K.; Gruskevica, K.; Mežule, L.; Juhna, T. Comparison of three strategies for phosphorus removal from wastewater in sequencing batch reactors. In Proceedings of the Granular Sludge System Conference: Book of Abstracts 2018, Delft, The Netherlands, 18–21 March 2018. [Google Scholar]
- LVS EN 1484:2000; Water Analysis—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC). International Organization for Standardization, European Committee for Standardization: Brussels, Belgium, 2000.
- Baird, R.B.; Bridgewater, L.L. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Foissner, W. Protists as bioindicators in activated sludge: Identification, ecology and future needs. Eur. J. Protistol. 2016, 55, 75–94. [Google Scholar] [CrossRef]
- Sowinska, A.; Pawlak, M.; Mazurkiewicz, J.; Pacholska, M. Comparison of the Results from Microscopic Tests Concerning the Quality of Activated Sludge and Effluent. Water 2017, 9, 918. [Google Scholar] [CrossRef] [Green Version]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1963; p. 125. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Wiley-Blackwell: Oxford, UK, 2004; p. 272. [Google Scholar]
- Tora, J.; Lafuente, J.; Baeza, J.A.; Carrera, J. Combined effect of inorganic carbon limitation and inhibition by free ammonia and free nitrous acid on ammonia oxidizing bacteria. Bioresour. Technol. 2010, 101, 6051–6058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, H.; Wang, J.; Liu, Z.; Guan, Q. Effect of free ammonia inhibition on NOB activity in high nitrifying performance of sludge. RSC Adv. 2018, 8, 31987–31995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, B.; Han, H.; Jia, S.; Zhuang, H.; Zhao, Q.; Xu, P. Effect of alkalinity on nitrite accumulation in treatment of coal chemical industry wastewater using moving bed biofilm reactor. J. Environ. Sci. 2014, 26, 1014–1022. [Google Scholar] [CrossRef]
- Belmonte, M.; Hsieh, C.-F.; Campos, J.L.; Guerrero, L.; Méndez, R.; Mosquera-Corral, A.; Vidal, G. Effect of Free Ammonia, Free Nitrous Acid, and Alkalinity on the Partial Nitrification of Pretreated Pig Slurry, Using an Alternating Oxic/Anoxic SBR. BioMed Res. Int. 2017, 2017, 6571671. [Google Scholar] [CrossRef]
- Su, J.; Wang, L.; Zhang, Y.; Gu, Z. A Numerical Study on Influent Flow Rate Variations in a Secondary Settling Tank. Processes 2019, 7, 884. [Google Scholar] [CrossRef]
- Gerardi, M.H. Settleability Problems and Loss of Solids in the Activated Sludge Process; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; p. 192. [Google Scholar]
- Deepnarain, N.; Nasr, M.; Kumari, S.; Stenstrom, T.A.; Reddy, P.; Pillay, K.; Bux, F. Decision tree for identification and prediction of filamentous bulking at full-scale activated sludge wastewater treatment plant. Process Saf. Environ. Prot. 2019, 126, 25–34. [Google Scholar] [CrossRef]
- Mesquita, D.P.; Amaral, A.L.; Ferreira, E.C. Identifying different types of bulking in an activated sludge system through quantitative image analysis. Chemosphere 2011, 85, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Peng, Y.; Wang, S.; Yang, X.; Yuan, Z. Filamentous and non-filamentous bulking of activated sludge encountered under nutrients limitation or deficiency conditions. Chem. Eng. J. 2014, 255, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Farouk, R.Y.; Li, L.; Wang, Y.; Li, Y.; Melak, S. Influence of pretreatment and pH on the enhancement of hydrogen and volatile fatty acids production from food waste in the semi-continuously running reactor. Int. J. Hydrogen Energy 2019, 45, 3729–3738. [Google Scholar] [CrossRef]
- McCarty, P.L. What is the Best Biological Process for Nitrogen Removal: When and Why? Environ. Sci. Technol. 2018, 52, 3835–3841. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-F.; Tay, J.-H.; Liu, Y. Inhibition of free ammonia to the formation of aerobic granules. Biochem. Eng. J. 2004, 17, 41–48. [Google Scholar] [CrossRef]
- Jimenez, E.; Gimenez, J.B.; Seco, A.; Ferrer, J.; Serralta, J. Effect of pH, substrate and free nitrous acid concentrations on ammonium oxidation rate. Bioresour. Technol. 2012, 124, 478–484. [Google Scholar] [CrossRef] [PubMed]
| Initial Wastewater | Acidic Reactor | Alkaline Reactor | ||||
|---|---|---|---|---|---|---|
| Parameters | Average Value | ±sd | Average Value | ±sd | Average Value | ±sd |
| pH | 7.4 | 0.1 | 6.6 | 0.1 | 8.4 | 0.1 |
| EC | 1417 | 94 | 1593 | 107 | 1571 | 148 |
| T, °C | 21.9 | 1.1 | 22.2 | 0.6 | 22.0 | 1.0 |
| TN, mg/L | 60 | 7 | 57 | 5 | 58 | 8 |
| NH4-N, mg/L | 53 | 7 | 54 | 5 | 55 | 7 |
| NO3-N, mg/L | 5.2 | 0.8 | 3.2 | 0.7 | 3.1 | 1.6 |
| NO2-N, mg/L | 0.020 | 0.004 | 0.016 | 0.003 | 0.007 | 0.001 |
| TP, mg/L | 23.4 | 3.0 | 23.4 | 2.6 | 21.9 | 1.6 |
| PO4-P, mg/L | 19.3 | 2.5 | 18.7 | 3.2 | 17.2 | 2.8 |
| COD, mg/L | 287 | 72 | 249 | 67 | 243 | 81 |
| TOC, mg/L | 54.2 | 18.5 | 64.9 | 8.8 | 66.1 | 13.7 |
| IC, mg/L | 106.4 | 8.0 | 70.2 | 6.7 | 123.9 | 12.0 |
| TC, mg/L | 160.6 | 25.4 | 135.1 | 14.2 | 190.0 | 24.2 |
| Average (n = 3) | Acclimatization Cycle | Acidic Reactor, ind/mL | Alkaline Reactor, ind/mL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Microfauna Group | Genus/Species | 0 h | 24 h | 48 h | 72 h | 0 h | 24 h | 48 h | 72 h | |
| gymnamoebae | Amoeba limax | 23 | 33 | 90 | 80 | 123 | 37 | 37 | 57 | 53 |
| Mayorella | 103 | 160 | 310 | 330 | 343 | 197 | 303 | 173 | 267 | |
| testate amoebae | Arcella | 117 | 103 | 90 | 90 | 57 | 157 | 90 | 73 | 60 |
| crawling ciliates | Chilodonella | 27 | 43 | 40 | 57 | 187 | 50 | 40 | 123 | 33 |
| Aspidisca | 140 | 120 | 63 | 93 | 47 | 140 | 57 | 37 | 37 | |
| free swimming ciliates | Litonotus | 330 | 583 | 250 | 60 | 37 | 493 | 247 | 73 | 77 |
| Prorodon | 537 | 567 | 550 | 420 | 217 | 583 | 500 | 287 | 220 | |
| Holophrya | 60 | 30 | 57 | 57 | 23 | 23 | 47 | 60 | 40 | |
| Glaucoma | 50 | 27 | 33 | 77 | 87 | 40 | 43 | 77 | 60 | |
| Spirostomum | 20 | 37 | 13 | 17 | 3 | 17 | 33 | 17 | 13 | |
| stalked ciliates | Epistylis | 436 | 465 | 348 | 156 | 440 | 239 | 396 | 251 | 436 |
| Carchesium | 77 | 30 | 50 | 40 | 20 | 13 | 33 | 47 | 23 | |
| V. convallaria | 93 | 80 | 60 | 73 | 73 | 67 | 133 | 50 | 50 | |
| V. microstoma | 347 | 150 | 193 | 140 | 127 | 187 | 163 | 57 | 30 | |
| carnivorous ciliates | Acineta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 |
| Tokophrya | 3 | 0 | 0 | 3 | 3 | 3 | 3 | 10 | 17 | |
| rotifers | Rotaria | 47 | 40 | 40 | 37 | 37 | 63 | 60 | 50 | 60 |
| Cephalodella | 20 | 30 | 27 | 17 | 10 | 30 | 27 | 20 | 27 | |
| worms | Nematoda | 23 | 20 | 17 | 20 | 7 | 7 | 3 | 10 | 3 |
| Aeolosoma | 3 | 3 | 10 | 13 | 13 | 7 | 13 | 17 | 10 | |
| zoogloea | Z. ramigera | 60 | 70 | 163 | 293 | 383 | 73 | 100 | 150 | 130 |
| Z. uva | 50 | 47 | 87 | 120 | 190 | 43 | 67 | 93 | 70 | |
| Total abundance, ind/mL | 2566 | 2639 | 2492 | 2193 | 2427 | 2469 | 2396 | 1734 | 1716 | |
| Shannon–Weaver index | 2.44 | 2.33 | 2.50 | 2.60 | 2.48 | 2.40 | 2.50 | 2.71 | 2.49 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokina, K.; Mezule, L.; Gruskevica, K.; Neilands, R.; Golovko, K.; Juhna, T. Impact of Rapid pH Changes on Activated Sludge Process. Appl. Sci. 2022, 12, 5754. https://doi.org/10.3390/app12115754
Kokina K, Mezule L, Gruskevica K, Neilands R, Golovko K, Juhna T. Impact of Rapid pH Changes on Activated Sludge Process. Applied Sciences. 2022; 12(11):5754. https://doi.org/10.3390/app12115754
Chicago/Turabian StyleKokina, Kristina, Linda Mezule, Kamila Gruskevica, Romans Neilands, Ksenija Golovko, and Talis Juhna. 2022. "Impact of Rapid pH Changes on Activated Sludge Process" Applied Sciences 12, no. 11: 5754. https://doi.org/10.3390/app12115754
APA StyleKokina, K., Mezule, L., Gruskevica, K., Neilands, R., Golovko, K., & Juhna, T. (2022). Impact of Rapid pH Changes on Activated Sludge Process. Applied Sciences, 12(11), 5754. https://doi.org/10.3390/app12115754








