Study on the Effect of Different Transcranial Pulse Current Stimulation Intervention Programs for Eliminating Physical Fatigue
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
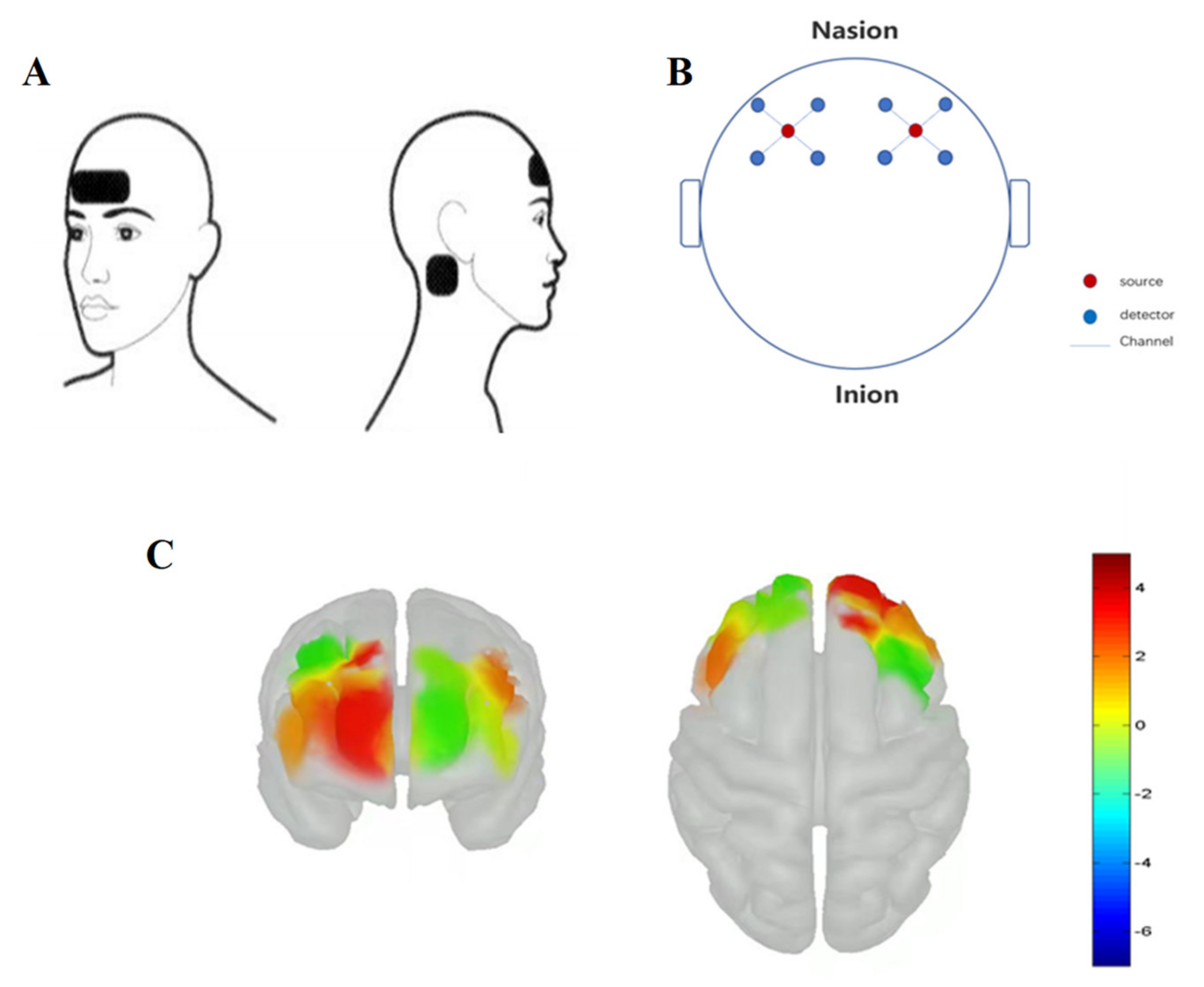
2.2. Experimental Equipment
2.2.1. Transcranial Pulsed Current Stimulation
2.2.2. HRV Test
2.2.3. Functional Near-Infrared Spectroscopy
2.2.4. Treadmill
2.2.5. RPE Questionnaire
2.3. tPCS Intervention Program
2.4. Index Test
2.5. Experimental Flow
2.6. Data Processing and Analysis
3. Results
3.1. The Results of the Time Domain Index
3.2. The Results of the Frequency Domain Index
3.3. The Results of the HbO2
4. Discussion
4.1. Effects of Different tPCS Programs on Moderate Physical Fatigue
4.2. Effects of Different tPCS Intervention Programs on Severe Physical Fatigue
4.3. Mechanism Exploration of tPCS Intervention on HRV and HbO2
5. Limitations
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, J.L.; Amann, M.; Duchateau, J.; Meeusen, R.; Rice, C.L. Neural contributions to muscle fatigue: From the brain to the muscle and back again. Med. Sci. Sports Exerc. 2016, 48, 2294. [Google Scholar] [CrossRef] [PubMed]
- Ricci, J.A.; Chee, E.; Lorandeau, A.L.; Berger, J. Fatigue in the U.S. Workforce: Prevalence and Implications for Lost Productive Work Time. J. Occup. Environ. Med. 2007, 49, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McCracken, L.M.; Iverson, G. Disrupted Sleep Patterns and Daily Functioning in Patients with Chronic Pain. Pain Res. Manag. 2002, 7, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Geisser, M.E.; Donnell, C.S.; Petzke, F.; Gracely, R.H.; Clauw, D.J.; Williams, D.A. Comorbid Somatic Symptoms and Functional Status in Patients With Fibromyalgia and Chronic Fatigue Syndrome: Sensory Amplification as a Common Mechanism. J. Psychosom. Res. 2008, 49, 235–242. [Google Scholar] [CrossRef]
- Skapinakis, P.; Lewis, G.; Mavreas, V. Unexplained Fatigue Syndromes in a Multinational Primary Care Sample: Specificity of Definition and Prevalence and Distinctiveness from Depression and Generalized Anxiety. Am. J. Psychiatry 2003, 160, 785–787. [Google Scholar] [CrossRef]
- Bansal, A.; Bradley, A.; Bishop, K.; Kiani-Alikhan, S.; Ford, B. Chronic fatigue syndrome, the immune system and viral infection. Brain, Behav. Immun. 2012, 26, 24–31. [Google Scholar] [CrossRef]
- Angius, L.; Pascual-Leone, A.; Santarnecchi, E. Brain stimulation and physical performance. Prog. Brain Res. 2018, 240, 317–339. [Google Scholar] [CrossRef]
- Simonsmeier, B.A.; Grabner, R.; Hein, J.; Krenz, U.; Schneider, M. Electrical brain stimulation (tES) improves learning more than performance: A meta-analysis. Neurosci. Biobehav. Rev. 2018, 84, 171–181. [Google Scholar] [CrossRef]
- Guleyupoglu, B.; Schestatsky, P.; Edwards, D.; Fregni, F.; Bikson, M. Classification of methods in transcranial Electrical Stimulation (tES) and evolving strategy from historical approaches to contemporary innovations. J. Neurosci. Methods 2013, 219, 297–311. [Google Scholar] [CrossRef]
- Williams, P.S.; Hoffman, R.L.; Clark, B.C. Preliminary Evidence That Anodal Transcranial Direct Current Stimulation Enhances Time to Task Failure of a Sustained Submaximal Contraction. PLoS ONE 2013, 8, e81418. [Google Scholar] [CrossRef]
- Fresnoza, S.; Christova, M.; Feil, T.; Gallasch, E.; Körner, C.; Zimmer, U.; Ischebeck, A. The effects of transcranial alternating current stimulation (tACS) at individual alpha peak frequency (iAPF) on motor cortex excitability in young and elderly adults. Exp. Brain Res. 2018, 236, 2573–2588. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M.; Stuart, D.G. Neurobiology of muscle fatigue. J. Appl. Physiol. 1992, 72, 1631–1648. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Du, X.; Wang, F.; Ding, R.; Li, Y.; Liu, A.; Wei, L.; Hou, S.; Chen, F.; Hu, Q.; et al. Cortical Plasticity Induced by Anodal Transcranial Pulsed Current Stimulation Investigated by Combining Two-Photon Imaging and Electrophysiological Recording. Front. Cell. Neurosci. 2019, 13, 400. [Google Scholar] [CrossRef] [PubMed]
- Morales-Quezada, L.; Cosmo, C.; Carvalho, S.; Leite, J.; Castillo-Saavedra, L.; Rozisky, J.R.; Fregni, F. Cognitive effects and autonomic responses to transcranial pulsed current stimulation. Exp. Brain Res. 2015, 233, 701–709. [Google Scholar] [CrossRef]
- Moreno-Duarte, I.; Gebodh, N.; Schestatsky, P.; Guleyupoglu, B.; Reato, D.; Bikson, M.; Fregni, F. Transcranial electrical stimulation: Transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), transcranial pulsed current stimulation (tPCS), and transcranial random noise stimulation (tRNS). Stimul. Brain 2014, 35–59. [Google Scholar] [CrossRef]
- Vöröslakos, M.; Takeuchi, Y.; Brinyiczki, K.; Zombori, T.; Oliva, A.; Fernández-Ruiz, A.; Kozák, G.; Kincses, Z.T.; Iványi, B.; Buzsáki, G.; et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat. Commun. 2018, 9, 483. [Google Scholar] [CrossRef]
- Esmaeilpour, Z.; Marangolo, P.; Hampstead, B.M.; Bestmann, S.; Galletta, E.; Knotkova, H.; Bikson, M. Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimul. 2018, 11, 310–321. [Google Scholar] [CrossRef]
- Workman, C.D.; Kamholz, J.; Rudroff, T. Increased leg muscle fatigability during 2 mA and 4 mA transcranial direct current stimulation over the left motor cortex. Exp. Brain Res. 2020, 238, 333–343. [Google Scholar] [CrossRef]
- Horvath, J.C.; Forte, J.; Carter, O. Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations from Single-session Transcranial Direct Current Stimulation (tDCS). Brain Stimul. 2015, 8, 535–550. [Google Scholar] [CrossRef]
- Opitz, A.; Falchier, A.; Yan, C.-G.; Yeagle, E.M.; Linn, G.S.; Megevand, P.; Thielscher, A.; Deborah, A.R.; Milham, M.P.; Mehta, A.D.; et al. Spatiotemporal structure of intracranial electric fields induced by transcranial electric stimulation in humans and nonhuman primates. Sci. Rep. 2016, 6, 31236. [Google Scholar] [CrossRef]
- Dmochowski, J.P.; Bikson, M.; Parra, L.C. The point spread function of the human head and its implications for transcranial current stimulation. Phys. Med. Biol. 2012, 57, 6459–6477. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.; Kamholz, J.; Rudroff, T. The Tolerability and Efficacy of 4 mA Transcranial Direct Current Stimulation on Leg Muscle Fatigability. Brain Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Kincses, Z.T.; Nitsche, M.A.; Paulus, W. Manipulation of phosphene thresholds by transcranial direct current stimulation in man. Exp. Brain Res. 2003, 150, 375–378. [Google Scholar] [CrossRef]
- Chew, T.; Ho, K.-A.; Loo, C.K. Inter- and Intra-individual Variability in Response to Transcranial Direct Current Stimulation (tDCS) at Varying Current Intensities. Brain Stimul. 2015, 8, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.S.; Rach, S.; Neuling, T.; Strüber, D. Transcranial alternating current stimulation: A review of the underlying mechanisms and modulation of cognitive processes. Front. Hum. Neurosci. 2013, 7, 279. [Google Scholar] [CrossRef]
- Yang, D.; Shin, Y.-I.; Hong, K.-S. Systemic Review on Transcranial Electrical Stimulation Parameters and EEG/fNIRS Features for Brain Diseases. Front. Neurosci. 2021, 15, 274. [Google Scholar] [CrossRef]
- Vasquez, A.C.; Thibaut, A.; Morales-Quezada, L.; Leite, J.; Fregni, F. Patterns of brain oscillations across different electrode montages in transcranial pulsed current stimulation. NeuroReport 2017, 28, 421–425. [Google Scholar] [CrossRef][Green Version]
- Vasquez, A.; Malavera, A.; Doruk, D.; Morales-Quezada, L.; Carvalho, S.; Leite, J.; Fregni, F. Duration Dependent Effects of Transcranial Pulsed Current Stimulation (tPCS) Indexed by Electroencephalography. Neuromodul. Technol. Neural Interface 2016, 19, 679–688. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Fregni, F. Transcranial direct current stimulation-an adjuvant tool for the treatment of neuropsychiatric diseases? Curr. Psychiatry Rev. 2007, 3, 222–232. [Google Scholar] [CrossRef]
- Subudhi, A.W.; Miramon, B.R.; Granger, M.E.; Roach, R. Frontal and motor cortex oxygenation during maximal exercise in normoxia and hypoxia. J. Appl. Physiol. 2009, 106, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Pelowski, M.; Pang, C.; Zhou, Y.; Cai, J. Near-infrared spectroscopy as a tool for driving research. Ergonomics 2016, 59, 368–379. [Google Scholar] [CrossRef]
- Schmitt, L.; Regnard, J.; Desmarets, M.; Mauny, F.; Mourot, L.; Fouillot, J.-P.; Coulmy, N.; Millet, G. Fatigue Shifts and Scatters Heart Rate Variability in Elite Endurance Athletes. PLoS ONE 2013, 8, e71588. [Google Scholar] [CrossRef] [PubMed]
- Ravé, G.; Zouhal, H.; Boullosa, D.; Doyle-Baker, P.K.; Saeidi, A.; Ben Abderrahman, A.; Fortrat, J.-O. Heart Rate Variability is Correlated with Perceived Physical Fitness in Elite Soccer Players. J. Hum. Kinet. 2020, 72, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.S.; Hutsler, R.; Bergsteinová, V.; Buskirk, E.R. The validity and reliability of a rating scale of perceived exertion. Med. Sci. Sports 1973, 5, 94–96. [Google Scholar] [CrossRef]
- Stamford, B.A. Validity and Reliability of Subjective Ratings of Perceived Exertion during Work. Ergonomics 1976, 19, 53–60. [Google Scholar] [CrossRef]
- Coventry, E.; O’Connor, K.M.; Hart, B.A.; Earl, J.E.; Ebersole, K.T. The effect of lower extremity fatigue on shock attenuation during single-leg landing. Clin. Biomech. 2006, 21, 1090–1097. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Ambrus, G.G.; Paulus, W.; Antal, A. Cutaneous perception thresholds of electrical stimulation methods: Comparison of tDCS and tRNS. Clin. Neurophysiol. 2010, 121, 1908–1914. [Google Scholar] [CrossRef]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.R.; Chen, R.; Cohen, L.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef]
- Quammen, D.; Cortes, N.; Van Lunen, B.L.; Lucci, S.; Ringleb, S.I.; Onate, J. Two Different Fatigue Protocols and Lower Extremity Motion Patterns during a Stop-Jump Task. J. Athl. Train. 2012, 47, 32–41. [Google Scholar] [CrossRef]
- Xia, Z. Research on the effect of background interference in children’s motor skills learning. Sports Sci. 2014, 34, 39–51. [Google Scholar]
- Yücel, M.A.; Selb, J.; Cooper, R.; Boas, D.A. Targeted principle component analysis: A new motion artifact correction approach for near-infrared spectroscopy. J. Innov. Opt. Health Sci. 2014, 7, 1350066. [Google Scholar] [CrossRef] [PubMed]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.-F.; Nitsche, M.A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef]
- Gamboa, O.L.; Antal, A.; Moliadze, V.; Paulus, W. Simply longer is not better: Reversal of theta burst after-effect with prolonged stimulation. Exp. Brain Res. 2010, 204, 181–187. [Google Scholar] [CrossRef]
- Proske, U.; Allen, T. The neural basis of the senses of effort, force and heaviness. Exp. Brain Res. 2019, 237, 589–599. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, B.; Wang, X. Research progress on the role of tight-fitting sports equipment in sports. Chin. J. Sports Med. 2021, 40, 402–416. [Google Scholar]
- Schmidt, L.; Cléry-Melin, M.-L.; LaFargue, G.; Valabregue, R.; Fossati, P.; Dubois, B.; Pessiglione, M. Get Aroused and Be Stronger: Emotional Facilitation of Physical Effort in the Human Brain. J. Neurosci. 2009, 29, 9450–9457. [Google Scholar] [CrossRef]
- Blakemore, R.L.; Neveu, R.; Vuilleumier, P. How emotion context modulates unconscious goal activation during motor force exertion. NeuroImage 2017, 146, 904–917. [Google Scholar] [CrossRef]
- Bienenstock, E.L.; Cooper, L.N.; Munro, P.W. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J. Neurosci. 1982, 2, 32–48. [Google Scholar] [CrossRef]
- Vitor-Costa, M.; Okuno, N.M.; Bortolotti, H.; Bertollo, M.; Boggio, P.S.; Fregni, F.; Altimari, L.R. Improving Cycling Performance: Transcranial Direct Current Stimulation Increases Time to Exhaustion in Cycling. PLoS ONE 2015, 10, e0144916. [Google Scholar] [CrossRef]
- Tergau, F.; Geese, R.; Bauer, A.; Baur, S.; Paulus, W.; Reimers, C.D. Motor cortex fatigue in sports measured by transcranial magnetic double stimulation. Med. Sci. Sports Exerc. 2000, 32, 1942–1948. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moliadze, V.; Atalay, D.; Antal, A.; Paulus, W. Close to threshold transcranial electrical stimulation preferentially activates inhibitory networks before switching to excitation with higher intensities. Brain Stimul. 2012, 5, 505–511. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological Modulation of Cortical Excitability Shifts Induced by Transcranial Direct Current Stimulation in Humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.D.; Fietsam, A.C.; Uc, E.Y.; Rudroff, T. Cerebellar Transcranial Direct Current Stimulation in People with Parkinson’s Disease: A Pilot Study. Brain Sci. 2020, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Priori, A.; Berardelli, A.; Rona, S.; Accornero, N.; Manfredi, M. Polarization of the human motor cortex through the scalp. NeuroReport 1998, 9, 2257–2260. [Google Scholar] [CrossRef]
- Morales-Quezada, L.; Castillo-Saavedra, L.; Cosmo, C.; Doruk, D.; Sharaf, I.; Malavera, A.; Fregni, F. Optimal random frequency range in transcranial pulsed current stimulation indexed by quantitative electroencephalography. NeuroReport 2015, 26, 747–752. [Google Scholar] [CrossRef]
- Morales-Quezada, L.; Saavedra, L.C.; Rozisky, J.; Hadlington, L.; Fregni, F. Intensity dependent effects of transcranial pulsed current stimulation on interhemisphericconnectivity: A high-resolution qEEG, sham-controlled study. NeuroReport 2014, 25, 1054–1058. [Google Scholar] [CrossRef]
- Dominguez, A.; Socas, R.; Marrero, H.; Leon, N.; Llabres, J.; Enriquez, E. Transcranial Direct Current Stimulation improves word production in Conduction Aphasia: Electroencephalographic and behavioral evidences. Int. J. Clin. Health Psychol. 2014, 14, 240–245. [Google Scholar] [CrossRef][Green Version]
- Terney, D.; Chaieb, L.; Moliadze, V.; Antal, A.; Paulus, W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J. Neurosci. 2008, 28, 14147–14155. [Google Scholar] [CrossRef]
- Naegel, S.; Biermann, J.; Theysohn, N.; Kleinschnitz, C.; Diener, H.-C.; Katsarava, Z.; Obermann, M.; Holle, D. Polarity-specific modulation of pain processing by transcranial direct current stimulation—A blinded longitudinal fMRI study. J. Headache Pain 2018, 19, 99. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Behan, P.O. Fatigue and basal ganglia. J. Neurol. Sci. 2000, 179, 34–42. [Google Scholar] [CrossRef]
- Okada, T.; Tanaka, M.; Kuratsune, H.; Watanabe, Y.; Sadato, N. Mechanisms underlying fatigue: A voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol. 2004, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, T.; Zoghi, M.; Farrell, M.; Egan, G.; Jaberzadeh, S. The effects of a single-session cathodal transcranial pulsed current stimulation on corticospinal excitability: A randomized sham-controlled double-blinded study. Eur. J. Neurosci. 2020, 52, 4908–4922. [Google Scholar] [CrossRef] [PubMed]
- dos Santos FR, M.; Bazán, P.R.; Balardin, J.B.; de Aratanha, M.A.; Rodrigues, M.; Lacerda, S.; Negi, L.T.; Kozasa, E.H. Changes in Prefrontal fNIRS Activation and Heart Rate Variability during Self-Compassionate Thinking Related to Stressful Memories. Mindfulness 2022, 13, 326–338. [Google Scholar] [CrossRef]
- Merzagora, A.C.; Izzetoglu, M.; Polikar, R.; Weisser, V.; Onaral, B.; Schultheis, M.T. Functional Near-Infrared Spectroscopy and Electroencephalography: A Multimodal Imaging Approach. In International Conference on Foundations of Augmented Cognition; Springer: Berlin/Heidelberg, Germany, 2009; pp. 417–426. [Google Scholar]
- Tomasi, D.; Ernst, T.; Caparelli, E.C.; Chang, L. Common deactivation patterns during working memory and visual attention tasks: An intra-subject fMRI study at 4 Tesla. Hum. Brain Mapp. 2006, 27, 694–705. [Google Scholar] [CrossRef]
- Nguyen, T.; Ahn, S.; Jang, H.; Jun, S.C.; Kim, J.G. Utilization of a combined EEG/NIRS system to predict driver drowsiness. Sci. Rep. 2017, 7, 43933. [Google Scholar] [CrossRef]
- Saavedra, L.C.; Morales-Quezada, L.; Doruk, D.; Rozinsky, J.; Coutinho, L.; Faria, P.; Perissinotti, I.; Wang, Q.-M.; Fregni, F. QEEG indexed frontal connectivity effects of transcranial pulsed current stimulation (tPCS): A sham-controlled mechanistic trial. Neurosci. Lett. 2014, 577, 61–65. [Google Scholar] [CrossRef]
- Gao, N.; Jiang, J.-J.; Gu, S.-H.; Lu, J.-Z.; Xu, L. Morphological Detection and Functional Assessment of Regenerated Nerve after Neural Prosthesis with a PGLA Nerve Conduit. Sci. Rep. 2017, 7, 46403. [Google Scholar] [CrossRef]
- Gianaros, P.J.; Van Der Veen, F.M.; Jennings, J.R. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiol. 2004, 41, 521–530. [Google Scholar] [CrossRef]
- Nikolin, S.; Boonstra, T.W.; Loo, C.K.; Martin, D. Combined effect of prefrontal transcranial direct current stimulation and a working memory task on heart rate variability. PLoS ONE 2017, 12, e0181833. [Google Scholar] [CrossRef]
- Barbas, H.; Saha, S.; Rempel-Clower, N.; Ghashghaei, T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, A.; Sajdyk, T.J.; Gehlert, D.R. The amygdala, panic disorder, and cardiovascular responses. Ann. N. Y. Acad. Sci. 2003, 985, 308–325. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.D.; Saul, J.P.; Cohen, R.J. Transfer function analysis of autonomic regulation. I. Canine atrial rate response. Am. J. Physiol. Heart Circ. Physiol. 1989, 256, H142–H152. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.; Siebner, H.R.; Ward, N.S.; Lee, L.; Nitsche, M.A.; Paulus, W.; Rothwell, J.C.; Lemon, R.N.; Frackowiak, R.S. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur. J. Neurosci. 2005, 22, 495–504. [Google Scholar] [CrossRef]
- Jo, H.; Song, C.; Ikei, H.; Enomoto, S.; Kobayashi, H.; Miyazaki, Y. Physiological and Psychological Effects of Forest and Urban Sounds Using High-Resolution Sound Sources. Int. J. Environ. Res. Public Health 2019, 16, 2649. [Google Scholar] [CrossRef]
- Saul, J. Beat-To-Beat Variations of Heart Rate Reflect Modulation of Cardiac Autonomic Outflow. Physiology 1990, 5, 32–37. [Google Scholar] [CrossRef]
- Ide, K.; Boushel, R.; Sørensen, H.M.; Fernandes, A.; Cai, Y.; Pott, F.; Secher, N.H. Middle cerebral artery blood velocity during exercise with beta-1 adrenergic and unilateral stellate ganglion blockade in humans. Acta Physiol. Scand. 2000, 170, 33–38. [Google Scholar] [CrossRef]
- González-Alonso, J.; Dalsgaard, M.K.; Osada, T.; Volianitis, S.; Dawson, E.; Yoshiga, C.C.; Secher, N.H. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J. Physiol. 2004, 557, 331–342. [Google Scholar] [CrossRef]

 means moderate physical fatigue;
means moderate physical fatigue;  means severe physical fatigue. (A) Difference of different tPCS intervention programs on RMSSD before and after intervention. (B) Difference of different tPCS intervention programs on SDNN before and after intervention. (C) Difference of different tPCS intervention programs on LF before and after intervention. (D) Difference of different tPCS intervention programs on HF before and after intervention. (E) Difference of different tPCS intervention programs on HbO2 before and after intervention.
means severe physical fatigue. (A) Difference of different tPCS intervention programs on RMSSD before and after intervention. (B) Difference of different tPCS intervention programs on SDNN before and after intervention. (C) Difference of different tPCS intervention programs on LF before and after intervention. (D) Difference of different tPCS intervention programs on HF before and after intervention. (E) Difference of different tPCS intervention programs on HbO2 before and after intervention.
 means moderate physical fatigue;
means moderate physical fatigue;  means severe physical fatigue. (A) Difference of different tPCS intervention programs on RMSSD before and after intervention. (B) Difference of different tPCS intervention programs on SDNN before and after intervention. (C) Difference of different tPCS intervention programs on LF before and after intervention. (D) Difference of different tPCS intervention programs on HF before and after intervention. (E) Difference of different tPCS intervention programs on HbO2 before and after intervention.
means severe physical fatigue. (A) Difference of different tPCS intervention programs on RMSSD before and after intervention. (B) Difference of different tPCS intervention programs on SDNN before and after intervention. (C) Difference of different tPCS intervention programs on LF before and after intervention. (D) Difference of different tPCS intervention programs on HF before and after intervention. (E) Difference of different tPCS intervention programs on HbO2 before and after intervention.
 means before intervention;
means before intervention;  means after intervention; 1 means moderate physical fatigue (group A); 2 means severe physical fatigue (group B).
means after intervention; 1 means moderate physical fatigue (group A); 2 means severe physical fatigue (group B).
 means before intervention;
means before intervention;  means after intervention; 1 means moderate physical fatigue (group A); 2 means severe physical fatigue (group B).
means after intervention; 1 means moderate physical fatigue (group A); 2 means severe physical fatigue (group B).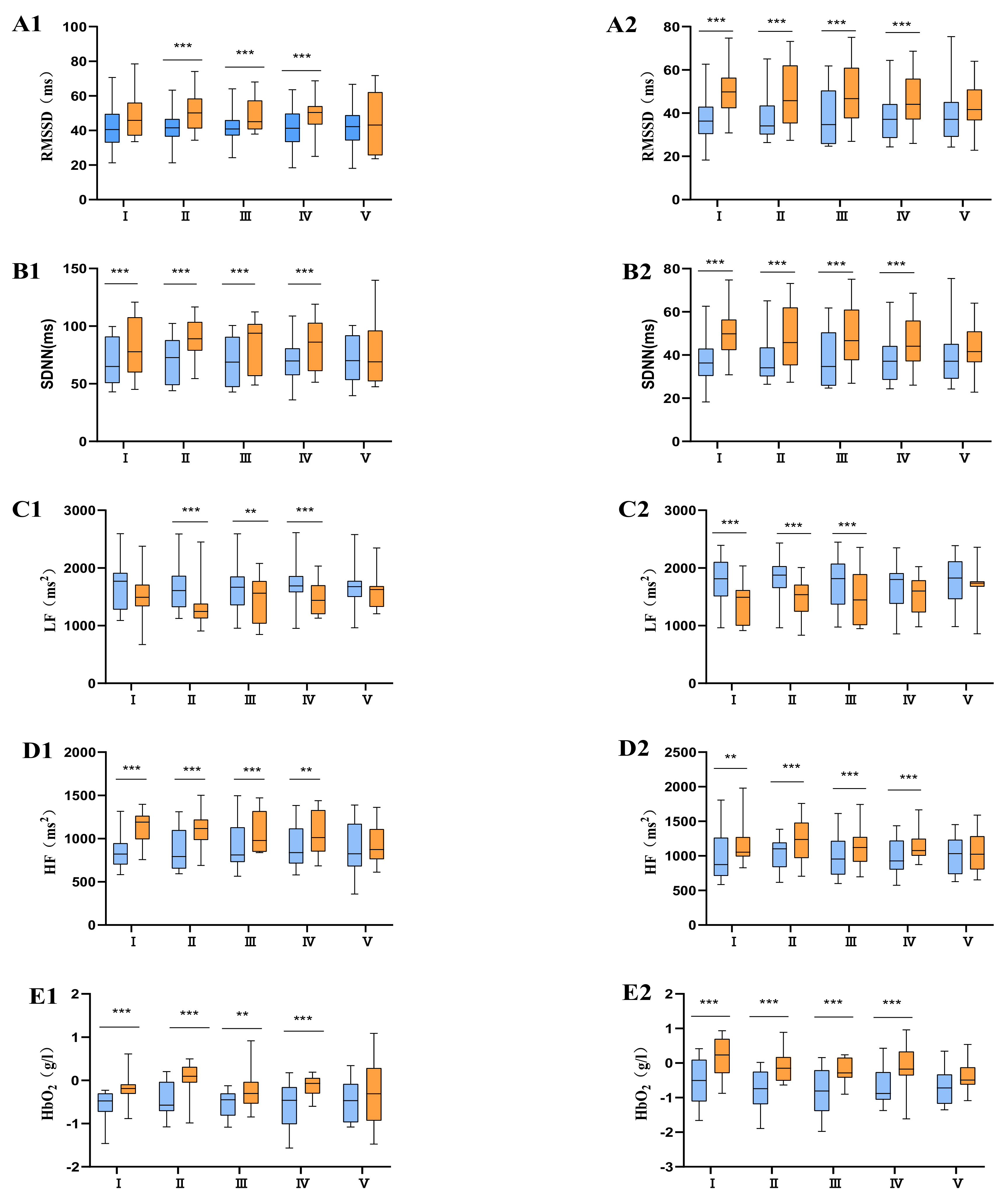

| Age/Years | Height/cm | Weight/kg | Training Years/Years | Number of Males | Number of Females | |
|---|---|---|---|---|---|---|
| Group A | 19.87 ± 1.25 | 172.20 ± 4.30 | 72.87 ± 7.85 | 6.13 ± 0.83 | 12 | 3 |
| Group B | 19.53 ± 1.24 | 170.47 ± 4.17 | 72.00 ± 7.01 | 6.20 ± 0.56 | 9 | 6 |
| Program | Intensity of Intervention | Time of Intervention |
|---|---|---|
| I | sensory intensity + 0.2 mA | 30 min |
| II | sensory intensity | 30 min |
| III | sensory intensity + 0.2 mA | 20 min |
| IV | sensory intensity | 20 min |
| V | sham stimulation | 0 min |
| Indicators | Program | Moderate Physical Fatigue | Severe Physical Fatigue | ||||
|---|---|---|---|---|---|---|---|
| T | p | Rate (%) | T | p | Rate (%) | ||
| RMSSD | I | −1.405 | 0.182 | 12.1 | −6.394 | 0.000 | 32.6 |
| II | −5.050 | 0.000 | 25.4 | −3.407 | 0.004 | 24.6 | |
| III | −3.959 | 0.001 | 18.3 | −3.362 | 0.005 | 26.1 | |
| IV | −3.483 | 0.004 | 17.5 | −3.706 | 0.002 | 14.9 | |
| V | −0.785 | 0.446 | 7.7 | −1.082 | 0.298 | 7.6 | |
| SDNN | I | −3.617 | 0.003 | 14.8 | −4.907 | 0.000 | 40.2 |
| II | −5.689 | 0.000 | 27.9 | −5.245 | 0.000 | 32.1 | |
| III | −3.175 | 0.007 | 17.2 | −3.607 | 0.003 | 33.1 | |
| IV | −3.284 | 0.005 | 19.0 | −3.948 | 0.001 | 30.7 | |
| V | −1.705 | 0.110 | 9.6 | −1.873 | 0.082 | 15.2 | |
| LF | I | 2.035 | 0.061 | −8.1 | 4.751 | 0.000 | −22.5 |
| II | 5.401 | 0.000 | −19.8 | 5.120 | 0.001 | −23.5 | |
| III | 2.347 | 0.034 | −10.4 | 4.013 | 0.001 | −14.7 | |
| IV | 4.527 | 0.000 | −20.9 | 2.033 | 0.061 | −10.3 | |
| V | 1.103 | 0.289 | −4.5 | 1.289 | 0.218 | −4.5 | |
| HF | I | −2.393 | 0.031 | 12.3 | −5.529 | 0.000 | 32.6 |
| II | −3.652 | 0.003 | 20.6 | −5.283 | 0.000 | 27.2 | |
| III | −4.046 | 0.001 | 16.2 | −3.270 | 0.006 | 18.1 | |
| IV | −3.340 | 0.005 | 15.9 | −2.933 | 0.011 | 16.4 | |
| V | −0.721 | 0.483 | 3.5 | −0.994 | 0.337 | 5.7 | |
| HbO2 | I | −3.255 | 0.006 | −59.0 | −11.499 | 0.000 | −127.2 |
| II | −6.492 | 0.000 | −109.2 | −4.218 | 0.001 | −85.6 | |
| III | −2.245 | 0.041 | −54.8 | −4.035 | 0.001 | −68.5 | |
| IV | −4.392 | 0.001 | −70.7 | −4.136 | 0.001 | −84.2 | |
| V | −1.615 | 0.129 | −32.8 | −1.966 | 0.069 | −30.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Fang, G.; Zhao, J.; Liu, J. Study on the Effect of Different Transcranial Pulse Current Stimulation Intervention Programs for Eliminating Physical Fatigue. Appl. Sci. 2022, 12, 5609. https://doi.org/10.3390/app12115609
Wu Q, Fang G, Zhao J, Liu J. Study on the Effect of Different Transcranial Pulse Current Stimulation Intervention Programs for Eliminating Physical Fatigue. Applied Sciences. 2022; 12(11):5609. https://doi.org/10.3390/app12115609
Chicago/Turabian StyleWu, Qingchang, Guoliang Fang, Jiexiu Zhao, and Jian Liu. 2022. "Study on the Effect of Different Transcranial Pulse Current Stimulation Intervention Programs for Eliminating Physical Fatigue" Applied Sciences 12, no. 11: 5609. https://doi.org/10.3390/app12115609
APA StyleWu, Q., Fang, G., Zhao, J., & Liu, J. (2022). Study on the Effect of Different Transcranial Pulse Current Stimulation Intervention Programs for Eliminating Physical Fatigue. Applied Sciences, 12(11), 5609. https://doi.org/10.3390/app12115609







