Fractional Flow Reserve (FFR) Estimation from OCT-Based CFD Simulations: Role of Side Branches
Abstract
:1. Introduction
2. Material and Methods
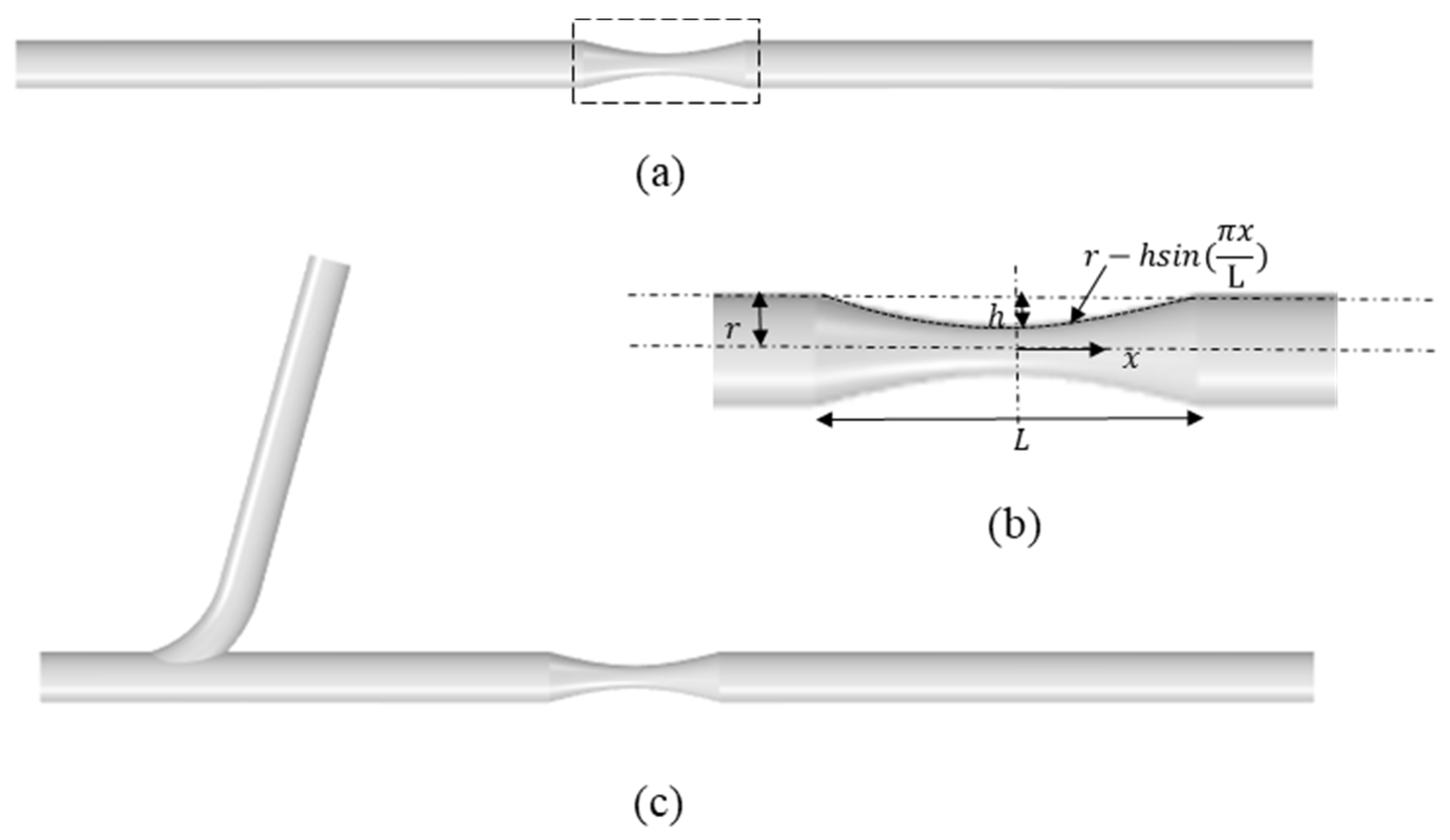
2.1. Idealized Stenosed Coronary Artery Models
2.2. IVOCT Imaging
2.3. Clinical FFR Measurement
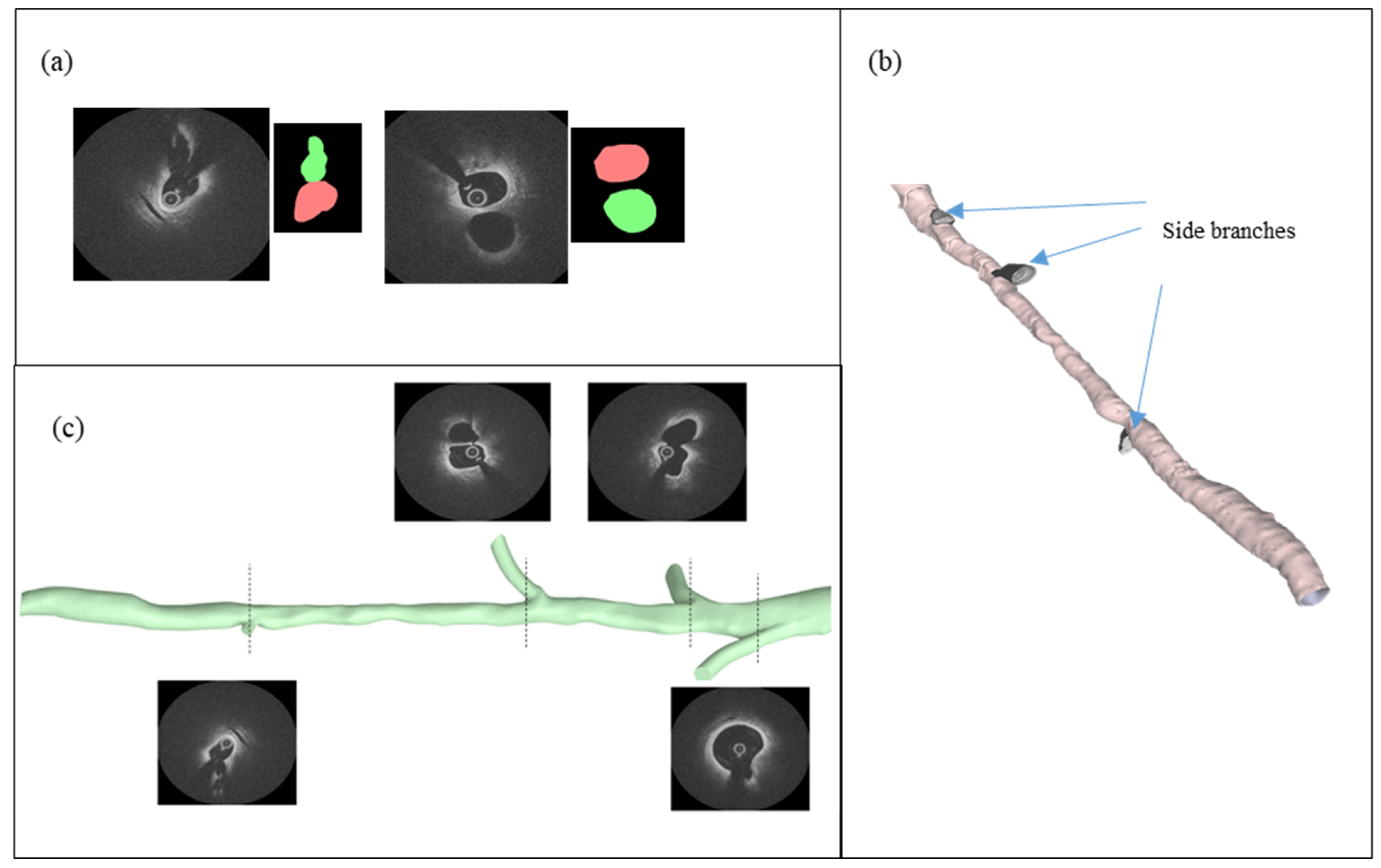
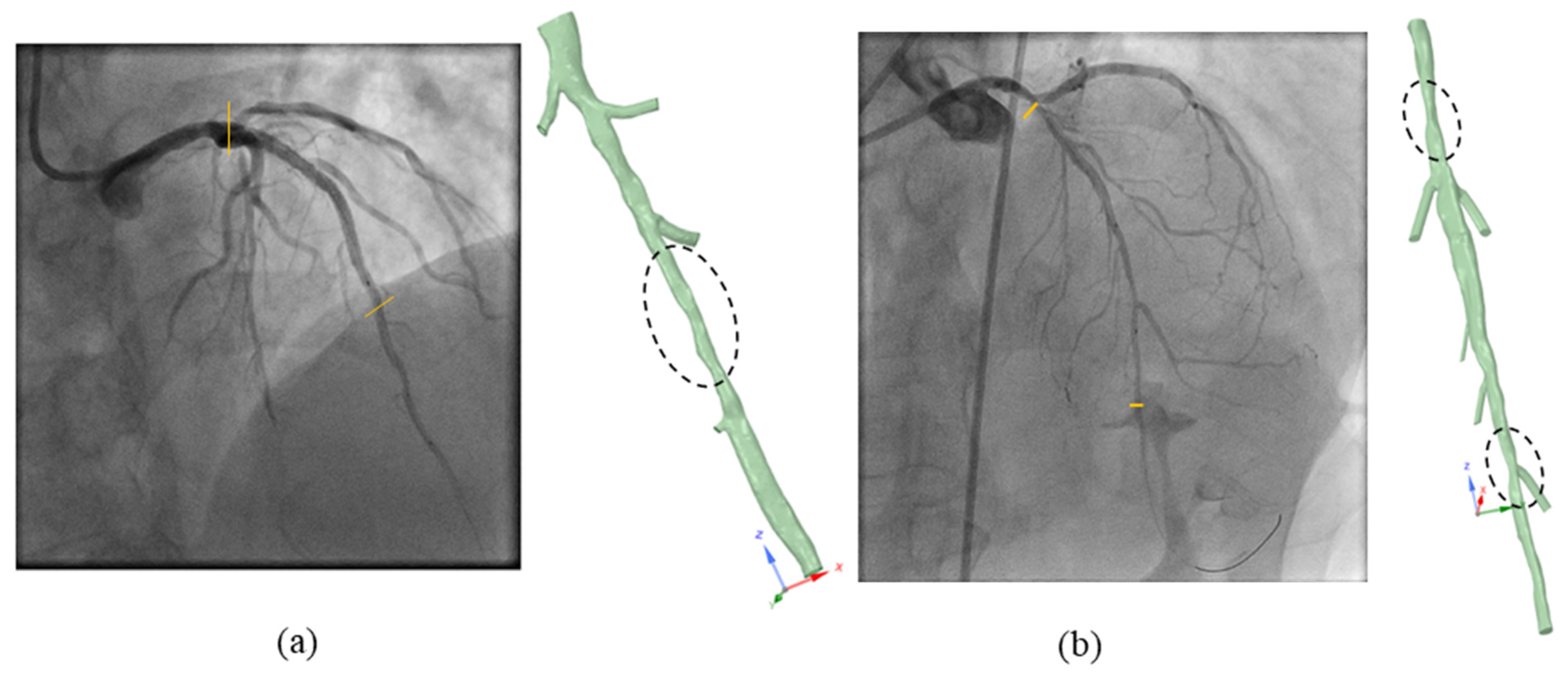
2.4. Vessel Segmentation and Reconstruction
2.5. Blood Flow Modeling
2.6. Boundary Conditions
3. Results and Discussion
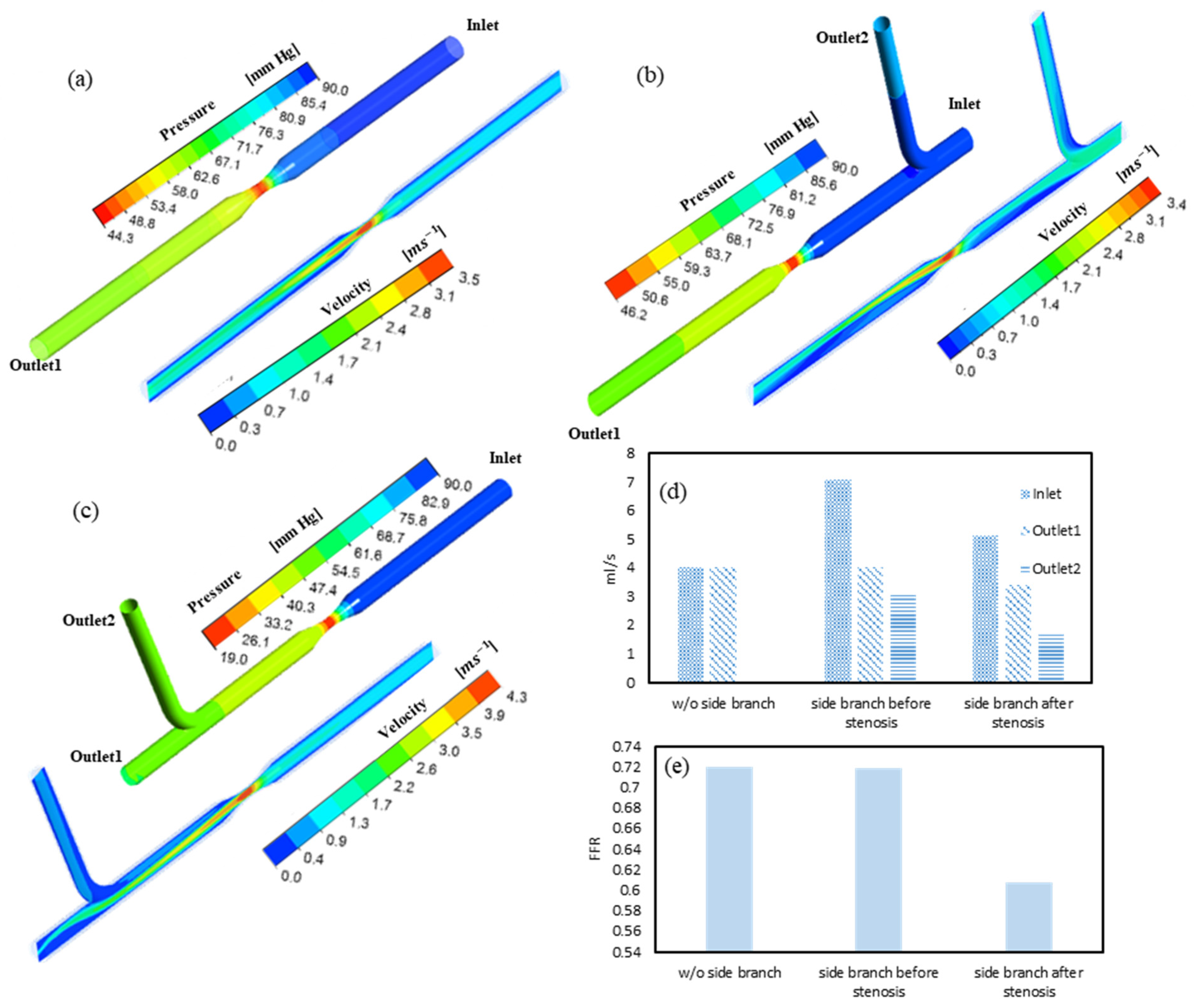
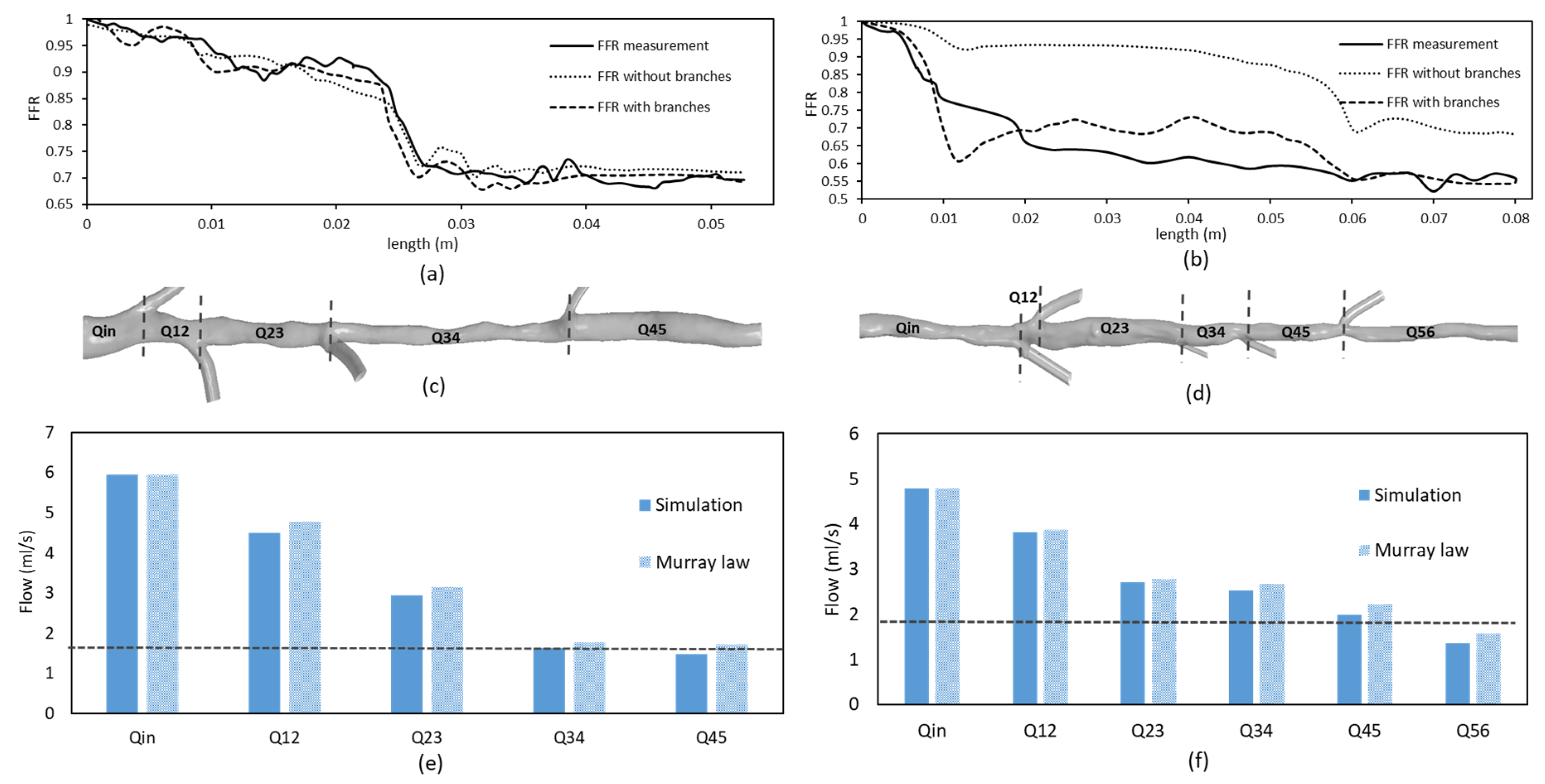
3.1. FFR and Flow Distribution in Idealized Artery Models
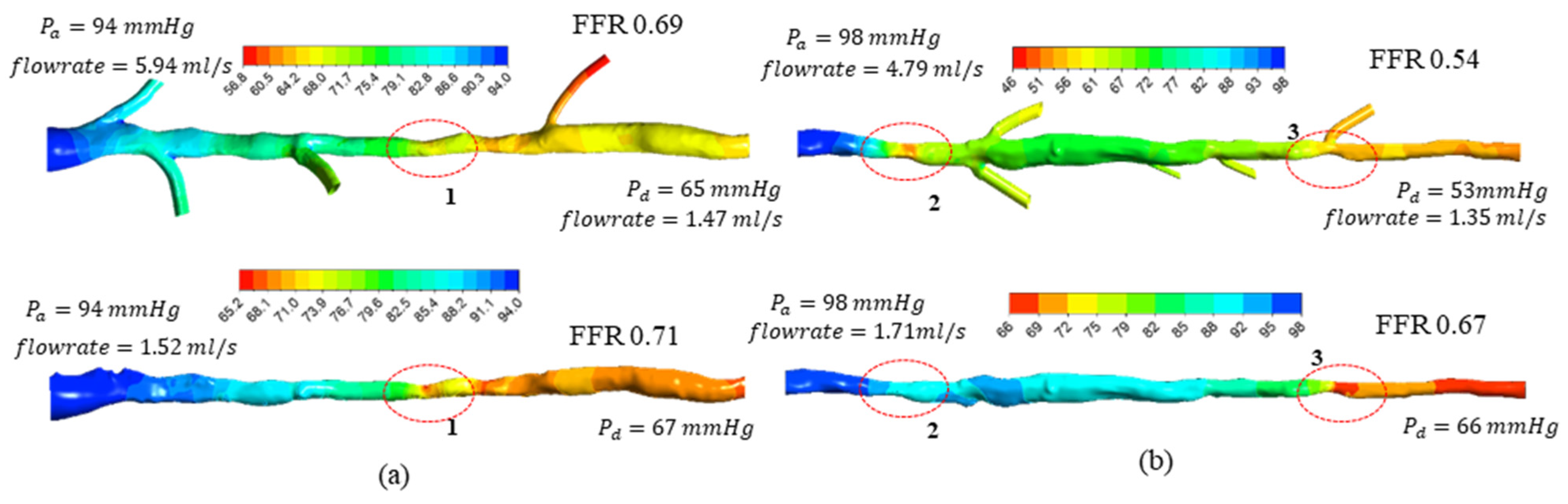
3.2. FFR Analysis in OCT-Reconstructed Vessel Models
3.3. Implications on FFR Computation Using OCT Imaging
4. Limitations
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pijls, N.H.J.; De Bruyne, B. Coronary pressure measurement and fractional flow reserve. Heart 1998, 80, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Xaplanteris, P.; Fournier, S.; Pijls, N.H.; Fearon, W.F.; Barbato, E.; Tonino, P.A.; Engstrøm, T.; Kääb, S.; Dambrink, J.-H.; Rioufol, G.; et al. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N. Engl. J. Med. 2018, 379, 250–259. [Google Scholar] [CrossRef]
- Dehmer, G.J.; Weaver, D.; Roe, M.T.; Milford-Beland, S.; Fitzgerald, S.; Hermann, A.; Messenger, J.; Moussa, I.; Garratt, K.; Rumsfeld, J.; et al. A Contemporary View of Diagnostic Cardiac Catheterization and Percutaneous Coronary Intervention in the United States. J. Am. Coll. Cardiol. 2012, 60, 2017–2031. [Google Scholar] [CrossRef] [Green Version]
- Ihdayhid, A.R.; Norgaard, B.L.; Gaur, S.; Leipsic, J.; Nerlekar, N.; Osawa, K.; Miyoshi, T.; Jensen, J.M.; Kimura, T.; Shiomi, H.; et al. Prognostic Value and Risk Continuum of Noninvasive Fractional Flow Reserve Derived from Coronary CT Angiography. Radiology 2019, 292, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Huang, J.; Jia, D.; Chen, S.-L.; Raffel, O.C.; Ding, D.; Tian, F.; Kan, J.; Zhang, S.; Yan, F.; et al. Diagnostic accuracy of intracoronary optical coherence tomography-derived fractional flow reserve for assessment of coronary stenosis severity. EuroIntervention 2019, 15, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Koo, B.-K.; Erglis, A.; Doh, J.-H.; Daniels, D.V.; Jegere, S.; Kim, H.-S.; Dunning, A.; DeFrance, T.; Lansky, A.; Leipsic, J.; et al. Diagnosis of Ischemia-Causing Coronary Stenoses by Noninvasive Fractional Flow Reserve Computed from Coronary Computed Tomographic Angiograms. J. Am. Coll. Cardiol. 2011, 58, 1989–1997. [Google Scholar] [CrossRef] [Green Version]
- Min, J.K.; Leipsic, J.; Pencina, M.J.; Berman, D.S.; Koo, B.-K.; van Mieghem, C.; Erglis, A.; Lin, F.Y.; Dunning, A.M.; Apruzzese, P.; et al. Diagnostic Accuracy of Fractional Flow Reserve from Anatomic CT Angiography. JAMA 2012, 308, 1237. [Google Scholar] [CrossRef]
- Gosling, R.C.; Morris, P.; Soto, D.A.S.; Lawford, P.V.; Hose, D.R.; Gunn, J.P. Virtual Coronary Intervention. JACC Cardiovasc. Imaging 2019, 12, 865–872. [Google Scholar] [CrossRef]
- Sankaran, S.; Kim, H.J.; Choi, G.; Taylor, C.A. Uncertainty quantification in coronary blood flow simulations: Impact of geometry, boundary conditions and blood viscosity. J. Biomech. 2016, 49, 2540–2547. [Google Scholar] [CrossRef]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.-M.; Chowdhary, S.; et al. Consensus Standards for Acquisition, Measurement, and Reporting of Intravascular Optical Coherence Tomography Studies. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef] [Green Version]
- Wijns, W.; Shite, J.; Jones, M.R.; Lee, S.W.-L.; Price, M.J.; Fabbiocchi, F.; Barbato, E.; Akasaka, T.; Bezerra, H.; Holmes, D. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur. Heart J. 2015, 36, 3346–3355. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.; Kim, J.-S.; Lim, J.; Kim, G.; Lee, S.; Lee, J.S.; Shin, D.-H.; Kim, B.-K.; Ko, Y.-G.; Choi, D.; et al. Assessing Computational Fractional Flow Reserve from Optical Coherence Tomography in Patients With Intermediate Coronary Stenosis in the Left Anterior Descending Artery. Circ. Cardiovasc. Interv. 2016, 9, e003613. [Google Scholar] [CrossRef]
- Seike, F.; Uetani, T.; Nishimura, K.; Kawakami, H.; Higashi, H.; Aono, J.; Nagai, T.; Inoue, K.; Suzuki, J.; Kawakami, H.; et al. Intracoronary Optical Coherence Tomography-Derived Virtual Fractional Flow Reserve for the Assessment of Coronary Artery Disease. Am. J. Cardiol. 2017, 120, 1772–1779. [Google Scholar] [CrossRef]
- Lee, K.E.; Lee, S.H.; Shin, E.-S.; Shim, E.B. A vessel length-based method to compute coronary fractional flow reserve from optical coherence tomography images. Biomed. Eng. Online 2017, 16, 83. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, Y.; Nakamoto, M.; Nakamura, M.; Hoshi, T.; Yamamoto, E.; Imai, S.; Kawase, Y.; Okubo, M.; Shiomi, H.; Kondo, T.; et al. On-site evaluation of CT-based fractional flow reserve using simple boundary conditions for computational fluid dynamics. Int. J. Cardiovasc. Imaging 2020, 36, 337–346. [Google Scholar] [CrossRef]
- Morris, P.D.; Soto, D.A.S.; Feher, J.F.; Rafiroiu, D.; Lungu, A.; Varma, S.; Lawford, P.V.; Hose, D.R.; Gunn, J.P. Fast Virtual Fractional Flow Reserve Based upon Steady-State Computational Fluid Dynamics Analysis. JACC Basic Transl. Sci. 2017, 2, 434–446. [Google Scholar] [CrossRef]
- Chien, S.; Usami, S.; Taylor, H.M.; Lundberg, J.L.; Gregersen, M.I. Effects of hematocrit and plasma proteins on human blood rheology at low shear rates. J. Appl. Physiol. 1966, 21, 81–87. [Google Scholar] [CrossRef]
- Seo, T.; Schachter, L.G.; Barakat, A.I. Computational Study of Fluid Mechanical Disturbance Induced by Endovascular Stents. Ann. Biomed. Eng. 2005, 33, 444–456. [Google Scholar] [CrossRef]
- ANSYS, Inc. ANSYS CFX-Solver Theory Guide, Release 16.2; ANSYS, Inc.: Canonsburg, PA, USA, 2015. [Google Scholar]
- Olufsen, M.S. Structured tree outflow condition for blood flow in larger systemic arteries. Am. J. Physiol. Circ. Physiol. 1999, 276, H257–H268. [Google Scholar] [CrossRef]
- Lo, E.W.C.; Menezes, L.J.; Torii, R. Impact of Inflow Boundary Conditions on the Calculation of CT-Based FFR. Fluids 2019, 4, 60. [Google Scholar] [CrossRef] [Green Version]
- Duanmu, Z.; Yin, M.; Fan, X.; Yang, X.; Luo, X. A patient-specific lumped-parameter model of coronary circulation. Sci. Rep. 2018, 8, 874. [Google Scholar] [CrossRef]
- Ottesen, J.T.; Olufsen, M.S.; Larsen, J.K. Applied Mathematical Models in Human Physiology; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2004. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, D.; Kahn, A.M.; Burns, J.C.; Sankaran, S.; Shadden, S.C.; Marsden, A.L. Image-based modeling of hemodynamics in coronary artery aneurysms caused by Kawasaki disease. Biomech. Model. Mechanobiol. 2012, 11, 915–932. [Google Scholar] [CrossRef]
- Wilson, R.F.; Wyche, K.; Christensen, B.V.; Zimmer, S.; Laxson, D.D. Effects of adenosine on human coronary arterial circulation. Circulation 1990, 82, 1595–1606. [Google Scholar] [CrossRef] [Green Version]
- Wellnhofer, E.; Osman, J.; Kertzscher, U.; Affeld, K.; Fleck, E.; Goubergrits, L. Flow simulation studies in coronary arteries—Impact of side-branches. Atherosclerosis 2010, 213, 475–481. [Google Scholar] [CrossRef]
- Li, Y.; Gutiérrez-Chico, J.L.; Holm, N.R.; Yang, W.; Hebsgaard, L.; Christiansen, E.H.; Mæng, M.; Lassen, J.F.; Yan, F.; Reiber, J.H.; et al. Impact of Side Branch Modeling on Computation of Endothelial Shear Stress in Coronary Artery Disease. J. Am. Coll. Cardiol. 2015, 66, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.A.; Fonte, T.A.; Min, J.K. Computational Fluid Dynamics Applied to Cardiac Computed Tomography for Noninvasive Quantification of Fractional Flow Reserve. J. Am. Coll. Cardiol. 2013, 61, 2233–2241. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.D. The Physiological Principle of Minimum Work: I. The Vascular System and the Cost of Blood Volume. Proc. Natl. Acad. Sci. USA 1926, 12, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, A.; Togawa, T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am. J. Physiol. Circ. Physiol. 1980, 239, H14–H21. [Google Scholar] [CrossRef] [Green Version]
- Zarins, C.K.; Zatina, M.A.; Giddens, D.P.; Ku, D.N.; Glagov, S. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J. Vasc. Surg. 1987, 5, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Glagov, S.; Weisenberg, E.; Zarins, C.K.; Stankunavicius, R.; Kolettis, G.J. Compensatory Enlargement of Human Atherosclerotic Coronary Arteries. N. Engl. J. Med. 1987, 316, 1371–1375. [Google Scholar] [CrossRef]
- Gosling, R.C.; Sturdy, J.; Morris, P.D.; Fossan, F.E.; Hellevik, L.R.; Lawford, P.; Hose, D.R.; Gunn, J. Effect of side branch flow upon physiological indices in coronary artery disease. J. Biomech. 2020, 103, 109698. [Google Scholar] [CrossRef]
- Cao, Y.; Jin, Q.; Chen, Y.; Yin, Q.; Qin, X.; Li, J.; Zhu, R.; Zhao, W. Automatic Side Branch Ostium Detection and Main Vascular Segmentation in Intravascular Optical Coherence Tomography Images. IEEE J. Biomed. Health Inform. 2018, 22, 1531–1539. [Google Scholar] [CrossRef]
- Morris, P.D.; van de Vosse, F.; Lawford, P.V.; Hose, D.R.; Gunn, J.P. “Virtual” (Computed) Fractional Flow Reserve. JACC Cardiovasc. Interv. 2015, 8, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- Min, J.K.; Taylor, C.A.; Achenbach, S.; Koo, B.K.; Leipsic, J.; Nørgaard, B.; Pijls, N.J.; De Bruyne, B. Noninvasive Fractional Flow Reserve Derived from Coronary CT Angiography. JACC Cardiovasc. Imaging 2015, 8, 1209–1222. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, C.G.; Hideo-Kajita, A.; Bulant, C.A.; Dsc, G.D.M.; Mariani, J.; Pinton, F.A.; Falcão, B.A.A.; Esteves-Filho, A.; Franken, M.; Feijóo, R.A.; et al. Coronary fractional flow reserve derived from intravascular ultrasound imaging: Validation of a new computational method of fusion between anatomy and physiology. Catheter. Cardiovasc. Interv. 2019, 93, 266–274. [Google Scholar] [CrossRef]
- Kishi, S.; Giannopoulos, A.A.; Tang, A.; Kato, N.; Chatzizisis, Y.S.; Dennie, C.; Horiuchi, Y.; Tanabe, K.; Lima, J.A.C.; Rybicki, F.J.; et al. Fractional Flow Reserve Estimated at Coronary CT Angiography in Intermediate Lesions: Comparison of Diagnostic Accuracy of Different Methods to Determine Coronary Flow Distribution. Radiology 2018, 287, 76–84. [Google Scholar] [CrossRef]
- Govindaraju, K.; Viswanathan, G.N.; Badruddin, I.A.; Kamangar, S.; Ahmed, N.S.; Al-Rashed, A.A. A parametric study of the effect of arterial wall curvature on non-invasive assessment of stenosis severity: Computational foluid dynamics study. Curr. Sci. 2016, 111, 483–491. [Google Scholar] [CrossRef]
- Malota, Z.; Glowacki, J.; Sadowski, W.; Kostur, M. Numerical analysis of the impact of flow rate, heart rate, vessel geometry, and degree of stenosis on coronary hemodynamic indices. BMC Cardiovasc. Disord. 2018, 18, 132. [Google Scholar] [CrossRef]
- Kalmykova, M.; Poyda, A.; Ilyin, V. An approach to point-to-point reconstruction of 3D structure of coronary arteries from 2D X-ray angiography, based on epipolar constraints. Procedia Comput. Sci. 2018, 136, 380–389. [Google Scholar] [CrossRef]
- Tu, S.; Hao, P.; Koning, G.; Wei, X.; Song, X.; Chen, A.; Reiber, J.H. In vivo assessment of optimal viewing angles from X-ray coronary angiography. EuroIntervention 2011, 7, 112–120. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamage, P.T.; Dong, P.; Lee, J.; Gharaibeh, Y.; Zimin, V.N.; Bezerra, H.G.; Wilson, D.L.; Gu, L. Fractional Flow Reserve (FFR) Estimation from OCT-Based CFD Simulations: Role of Side Branches. Appl. Sci. 2022, 12, 5573. https://doi.org/10.3390/app12115573
Gamage PT, Dong P, Lee J, Gharaibeh Y, Zimin VN, Bezerra HG, Wilson DL, Gu L. Fractional Flow Reserve (FFR) Estimation from OCT-Based CFD Simulations: Role of Side Branches. Applied Sciences. 2022; 12(11):5573. https://doi.org/10.3390/app12115573
Chicago/Turabian StyleGamage, Peshala T., Pengfei Dong, Juhwan Lee, Yazan Gharaibeh, Vladislav N. Zimin, Hiram G. Bezerra, David L. Wilson, and Linxia Gu. 2022. "Fractional Flow Reserve (FFR) Estimation from OCT-Based CFD Simulations: Role of Side Branches" Applied Sciences 12, no. 11: 5573. https://doi.org/10.3390/app12115573
APA StyleGamage, P. T., Dong, P., Lee, J., Gharaibeh, Y., Zimin, V. N., Bezerra, H. G., Wilson, D. L., & Gu, L. (2022). Fractional Flow Reserve (FFR) Estimation from OCT-Based CFD Simulations: Role of Side Branches. Applied Sciences, 12(11), 5573. https://doi.org/10.3390/app12115573







