Investigation of THz Absorptive Signatures in Opioids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methods by Combining Modeling and Experiment
2.2. Sample Preparation
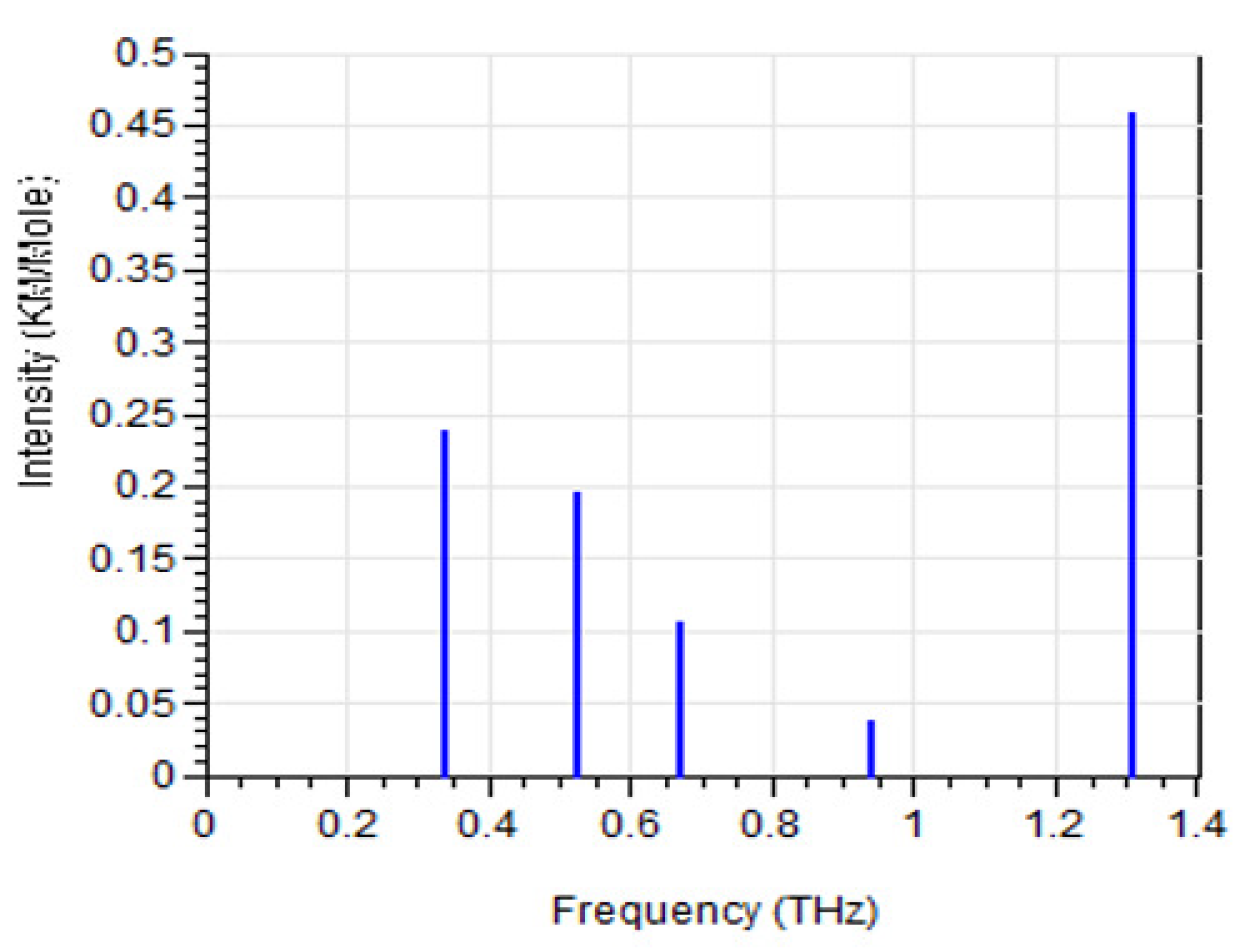
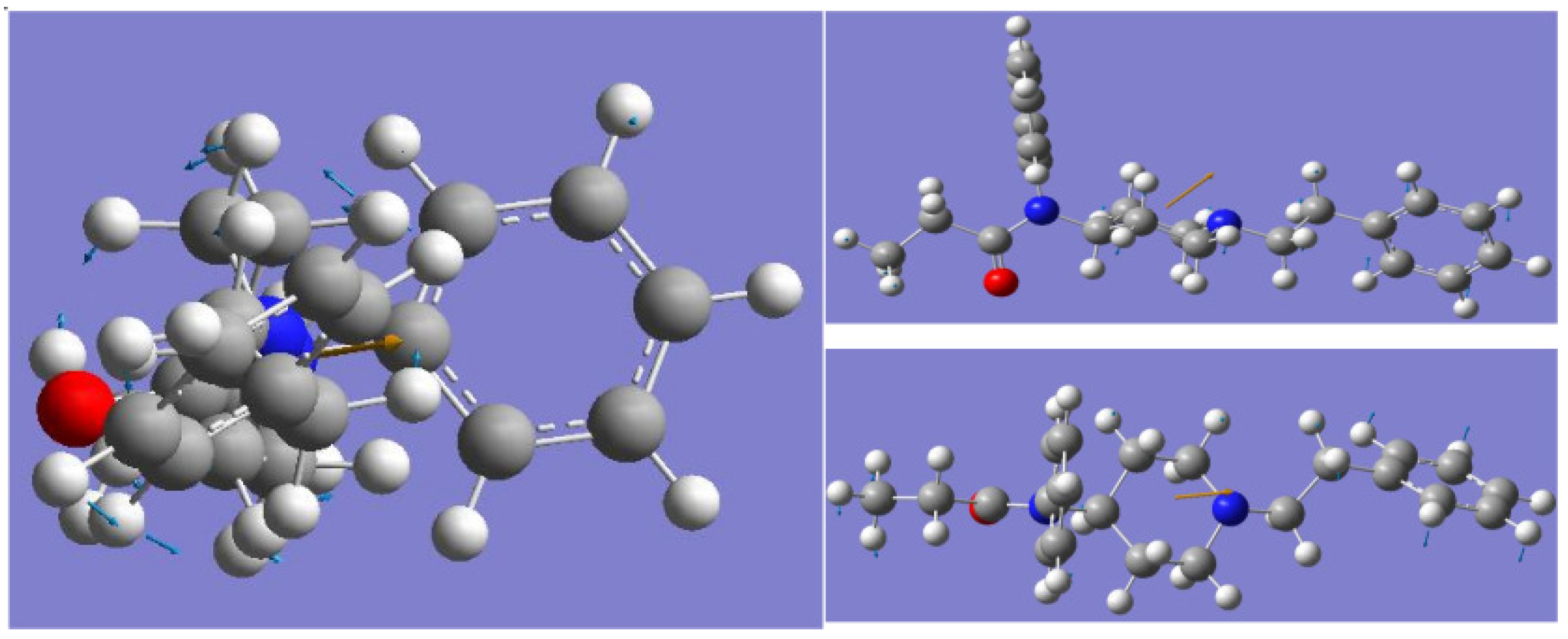
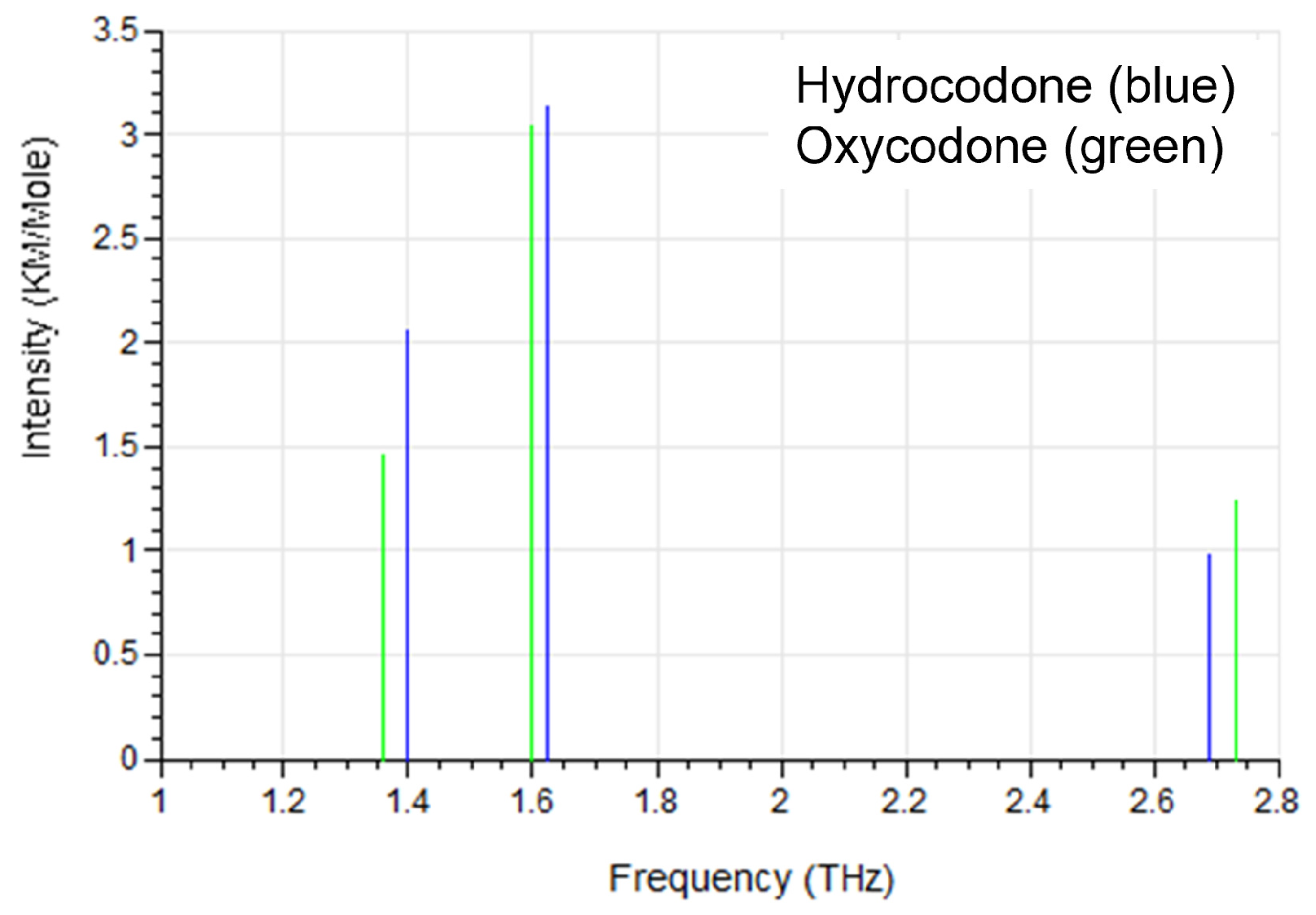
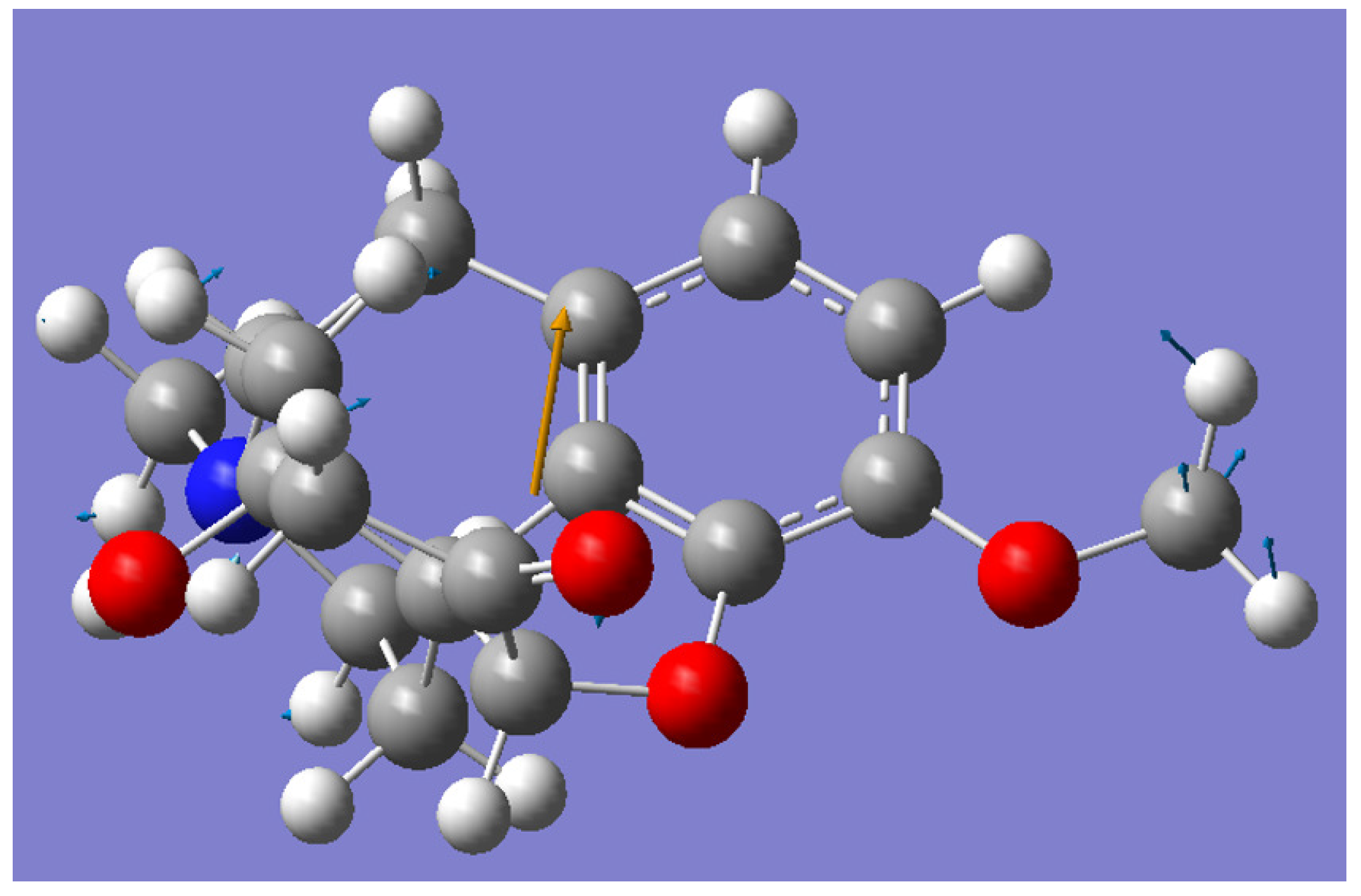
3. Molecular Dynamics (MD) Simulation Results
4. Experimental Results
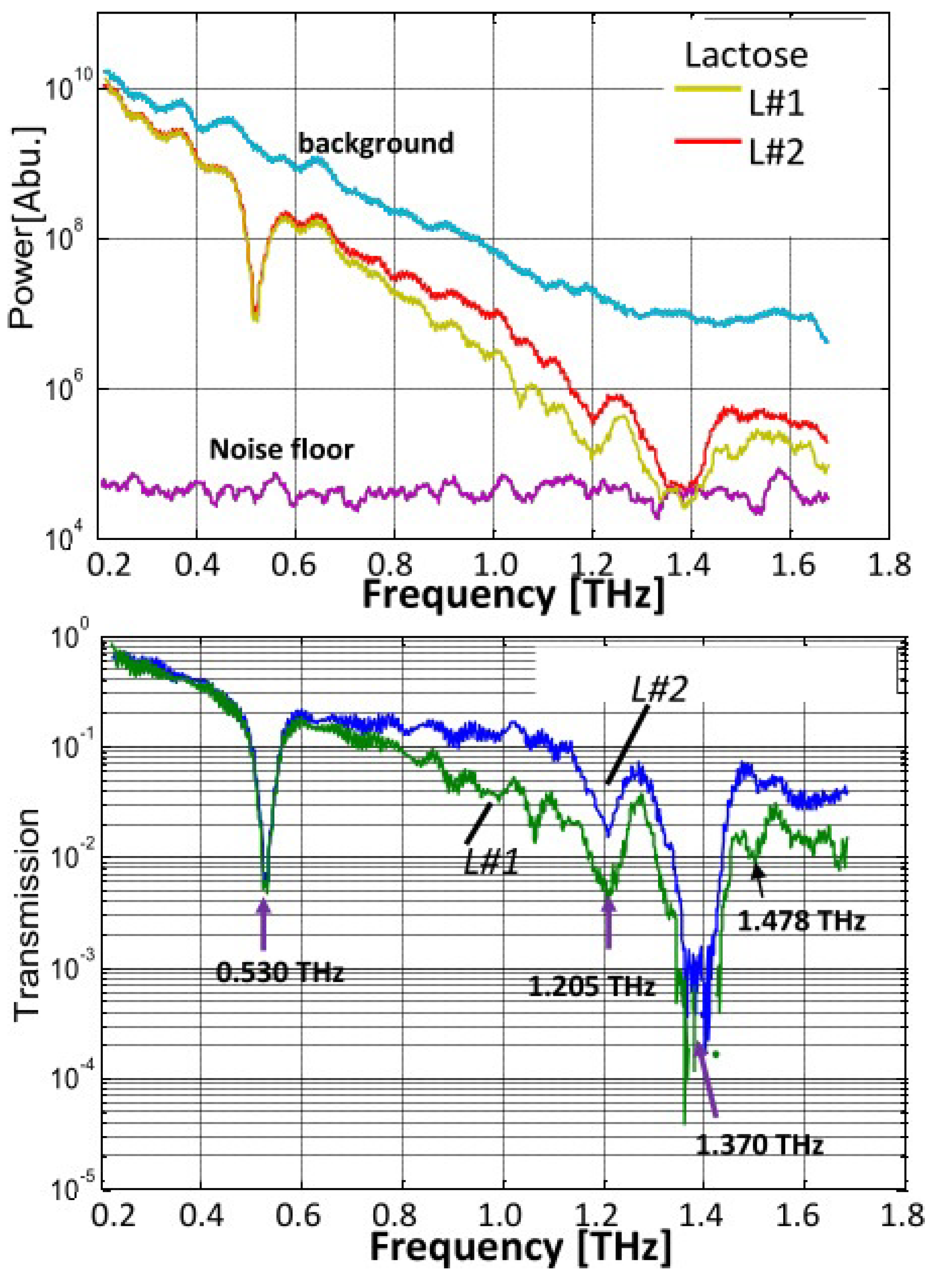
4.1. Control Samples
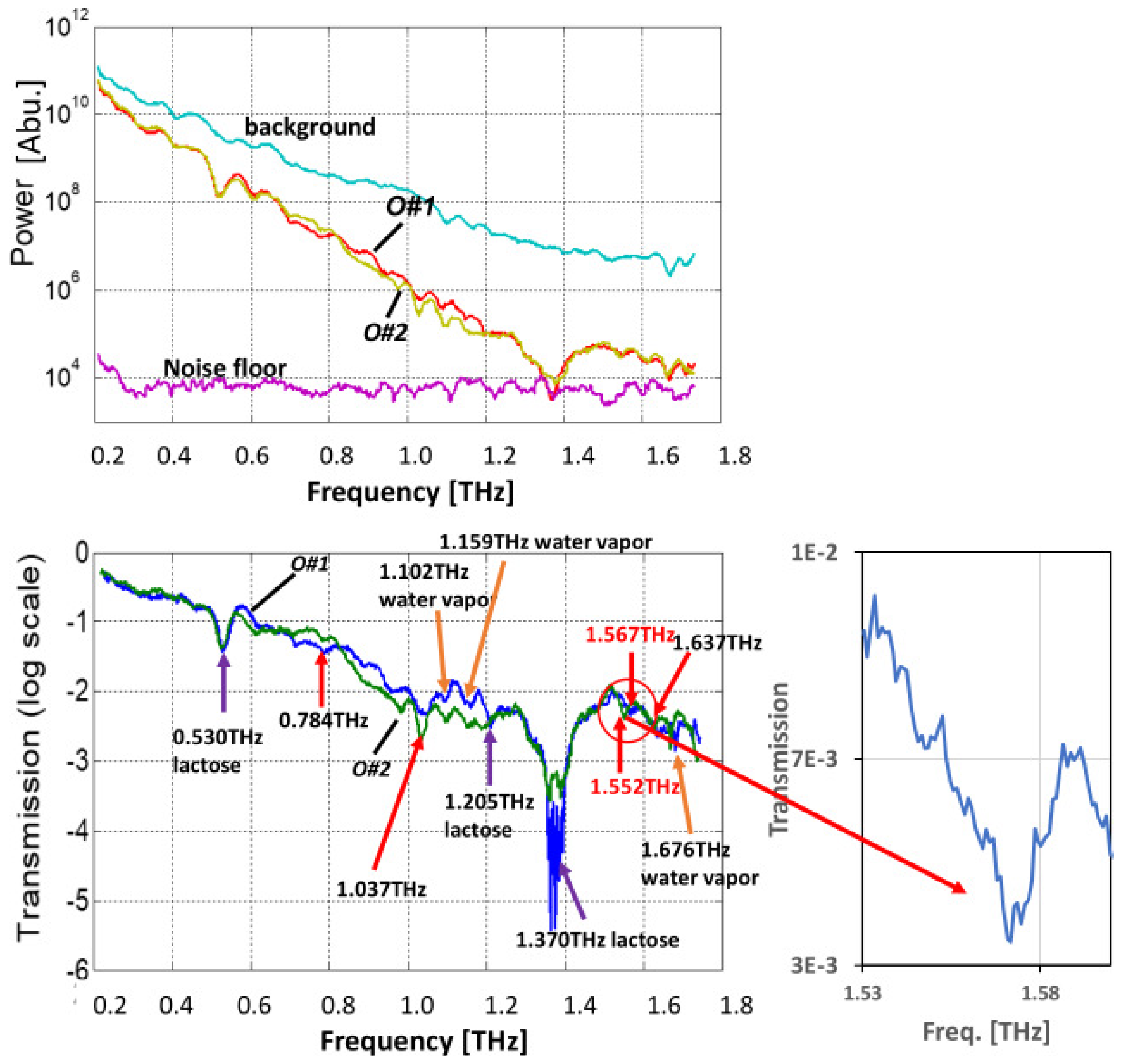
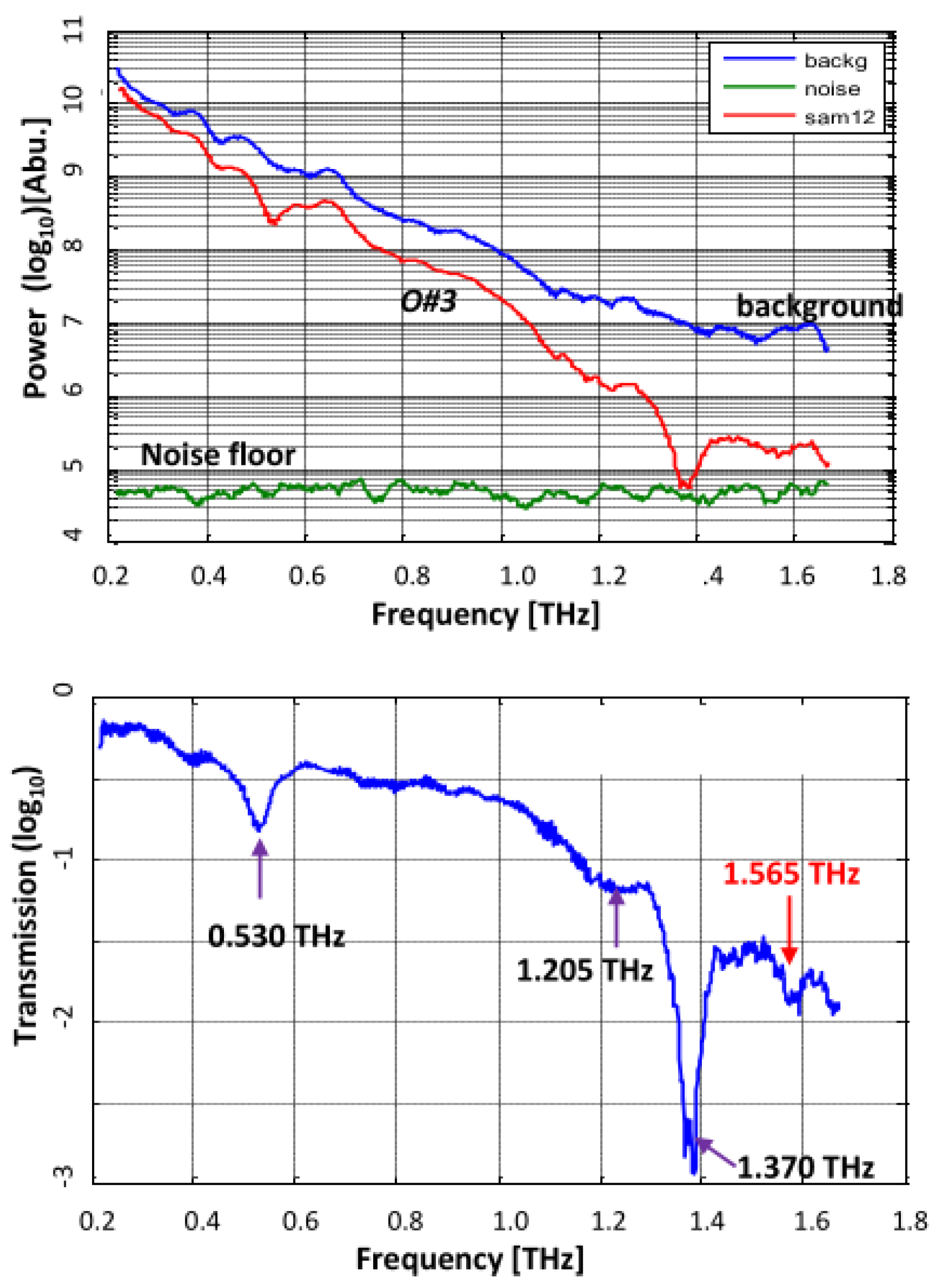
4.2. Oxycodone Samples
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walther, M.; Fischer, B.M.; Jepsen, P.D. Noncovalent intermolecular forces in polycrystalline andamorphous saccharides in the far infrared. Chem. Phys. 2003, 288, 261–268. [Google Scholar] [CrossRef]
- Allis, D.G.; Fedor, A.M.; Korter, T.M.; Bjarnason, J.E.; Brown, E.R. Assignment of the lowest-lying THz absorption signatures in biotin and lactose monohydrate by solid-state density functional theory. Chem. Phys. Lett. 2007, 440, 203–209. [Google Scholar] [CrossRef]
- Brown, E.R.; Zhang, W.-D.; Viveros, L.K.; Mendoza, E.A.; Kuznetsova, Y.; Brueck, S.R.J.; Burris, K.P.; Millwood, R.J.; Stewart, C.N. High-Resolution THz Spectroscopy of Biomolecules and Bioparticles: Concentration Methods. In Detection of Explosives and CBRN Using THz; NATO Science for Peace and Security Series B: Physics and Biophysics; Pereira, M.F., Ed.; Springer: New York, NY, USA, 2014; Chapter 2. [Google Scholar]
- Zhang, W.-D.; Bykhovski, A.; Deibel, J.; Brown, E.R. Experimental and Theoretical Study of Strong Low-Terahertz Absorption of Thymine Monohydrate. Int. J. Infrared Millim. Terahertz Waves 2017, 38, 1521–1529. [Google Scholar] [CrossRef]
- Kawase, K.; Ogawa, Y.; Watanabe, Y.; Inoue, H. Non-destructive terahertz imaging of illicit drugs using spectral fingerprints. Opt. Express 2003, 11, 2549–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.-H.; Shen, J.-L.; Li, N.; Zhang, Y.; Zhang, C.-L.; Liang, L.-S.; Xu, X.-Y. Detection and identification of illicit drugs using Terahertz imaging. J. Appl. Phys. 2006, 100, 103104. [Google Scholar]
- Davies, A.G.; Burnett, A.D.; Fan, W.-H.; Linfield, E.H.; Cunningham, J.E. Terahertz spectroscopy of explosives and drugs. Mater. Today 2008, 11, 18–26. [Google Scholar] [CrossRef]
- Burnett, A.-D.; Fan, W.-H.; Upadhya, P.C.; Cunningham, J.E.; Hargreavesm, M.D.; Munshi, T.; Edwards, H.G.M.; Linfield, E.H.; Davies, A.G. Broadband terahertz time-domain spectroscopy of drugs-of-abuse and the use of principal component analysis. Analyst 2009, 134, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, A.J.; Taday, P.F.; Newnham, D.A.; Pepper, M.; Gordon, K.C.; Rades, T. Terahertz pulsed spectroscopy and imaging in the pharmaceutical setting—A review. J. Pharm. Phaymacol. 2007, 59, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-T.; Li, J.-W.; Du, C.-Y.; Sun, Z.-H. Fuzzy recognition research on explosive and illegal drug based on terahertz spectroscopy detection. Guang Pu Xue Yu Guang Pu Fen Xi = Guang Pu 2010, 30, 401–405. [Google Scholar] [PubMed]
- Shen, Y.C. Terahertz pulsed spectroscopy and imaging for pharmaceutical applications: A review. Int. J. Pharm. 2011, 417, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Haaser, M.; Gordon, K.C.; Strachan, C.J.; Rades, T. Terahertz pulsed imaging as an advanced characterisation tool for film coatings—A review. Int. J. Pharm. 2013, 457, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Al Nabooda, M.O.; Shubair, R.M.; Rishani, N.R.; Aldabbagh, G. Terahertz spectroscopy and imaging for the detection and identification of Illicit drugs. In Proceedings of the 2017 Sensors Networks Smart and Emerging Technologies (SENSET), Beriut, Lebanon, 12–14 September 2017; pp. 1–4. [Google Scholar] [CrossRef]
- What Is fentanyl? Available online: https://www.drugabuse.gov/publications/drugfacts/fentanyl (accessed on 22 June 2021).
- The Drug Overdose Toll in 2020 and Near-Term Actions for Addressing It. Available online: https://www.commonwealthfund.org/blog/2021/drug-overdose-toll-2020-and-near-term-actions-addressing-it (accessed on 16 August 2021).
- Haddad, A.; Comanescu, M.; Green, O.; Kubic, T.; Lombardi, J. Detection and quantitation of trace fentanyl in heroin by surface enhanced Raman spectroscopy. Anal. Chem. 2018, 90, 12678–12685. [Google Scholar] [CrossRef] [PubMed]
- Oxycodone. Available online: https://www.drugs.com/monograph/oxycodone.html (accessed on 29 March 2021).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, Inc. Available online: https://gaussian.com (accessed on 3 November 2021).
- Percocet, Endo Pharmaceuticals Inc. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/040330s015,040341s013,040434s003lbl.pdf (accessed on 22 November 2006).
- Brown, E.R.; Bjarnason, J.E. On the strong and narrow absorption signature in lactose at 0.53 THz. Appl. Phys. Lett. 2007, 90, 061908. [Google Scholar] [CrossRef]
- Hou, L.; Shi, W.; Dong, C.-G.; Yang, L.; Wang, Y.-Z.; Wang, H.-Q.; Hang, Y.-H.; Xue, F. Probing trace lactose from aqueous solutions by terahertz time-domain spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 119044. [Google Scholar] [CrossRef]
- van Exter, M.; Fattinger, C.H.; Grischkowsky, D. Terahertz time-domain spectroscopy of water vapor. Opt. Lett. 1989, 14, 1128–1130. [Google Scholar] [CrossRef]
- Brown, E.R.; Zhang, W.-D. The Critical Effect of Hydration on the THz Signatures of Biomolecules and Bioparticles. In THz Diagnostics of CBRN Effects and Detection of Explosives and CBRN; NATO Science for Peace and Security Series B: Physics and Biophysics; Pereira, M.F., Ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Karaliūnas, M.; Venckevicius, R.; Kašalynas, I.; Puc, U.; Abina, A.; Jeglic, A.; Zidansek, A. Investigation of pharmaceutical drugs and caffeine-containing foods using Fourier and terahertz time-domain spectroscopy. In Terahertz Emitters, Receivers, and Applications VI; International Society for Optics and Photonics: San Diego, CA, USA, 2015; Volume 9585, p. 95850U. [Google Scholar]
- Chan, T.L.J.; Bjarnason, J.E.; Lee, A.W.M.; Celis, M.A.; Brown, E.R. Attenuation contrast between biomolecular and inorganic materials at terahertz frequencies. Appl. Phys. Lett. 2004, 85, 2523–2525. [Google Scholar] [CrossRef]
- Kaushik, M.; Ng, B.W.-H.; Fisher, B.M.; Abbott, D. Reduction of scattering effects in THz-TDS signals. IEEE Photonics Technol. Lett. 2012, 24, 155–156. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-Q.; Grischkowsky, D. Waveguide terahertz time-domain spectroscopy of nanometer water layers. Opt. Lett. 2004, 29, 1617–1619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-D.; Brown, E.R.; Viveros, L. Terahertz Conical Horn Waveguide Coupler for Spectroscopic Analysis of Biomaterials. In Proceedings of the 2013 IEEE SENSORS, Baltimore, MD, USA, 3–6 November 2013. [Google Scholar]
- Zhang, W.-D.; Mingardi, M.; Brown, E.R. High fill-factor interdigital electrodes for Terahertz spectroscopy. In Proceedings of the IRMMW 2016, Copenhagen, Denmark, 25–30 September 2016. [Google Scholar]
- Papari, G.P.; Koral, C.; Andreone, A. Encoded-enhancement of THZ metasurface figure of merit for label-free sensing. Sensors 2019, 19, 2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Bykhovski, A.; Brown, E.R. Investigation of THz Absorptive Signatures in Opioids. Appl. Sci. 2022, 12, 61. https://doi.org/10.3390/app12010061
Zhang W, Bykhovski A, Brown ER. Investigation of THz Absorptive Signatures in Opioids. Applied Sciences. 2022; 12(1):61. https://doi.org/10.3390/app12010061
Chicago/Turabian StyleZhang, Weidong, Alexei Bykhovski, and Elliott R. Brown. 2022. "Investigation of THz Absorptive Signatures in Opioids" Applied Sciences 12, no. 1: 61. https://doi.org/10.3390/app12010061
APA StyleZhang, W., Bykhovski, A., & Brown, E. R. (2022). Investigation of THz Absorptive Signatures in Opioids. Applied Sciences, 12(1), 61. https://doi.org/10.3390/app12010061




