Effect of Moderate-Intensity Endurance Exercise on Inflammatory Cytokines in Leukocytes of Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Treadmill Adaptation for Dog Safety
2.3. Endurance Exercise Program
2.4. Hematology and Serum Biochemistry Parameter Analysis
2.5. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.6. Statistical Analyses
3. Results
3.1. Effect of Exercise on Hematological and Serum Biochemistry Parameters
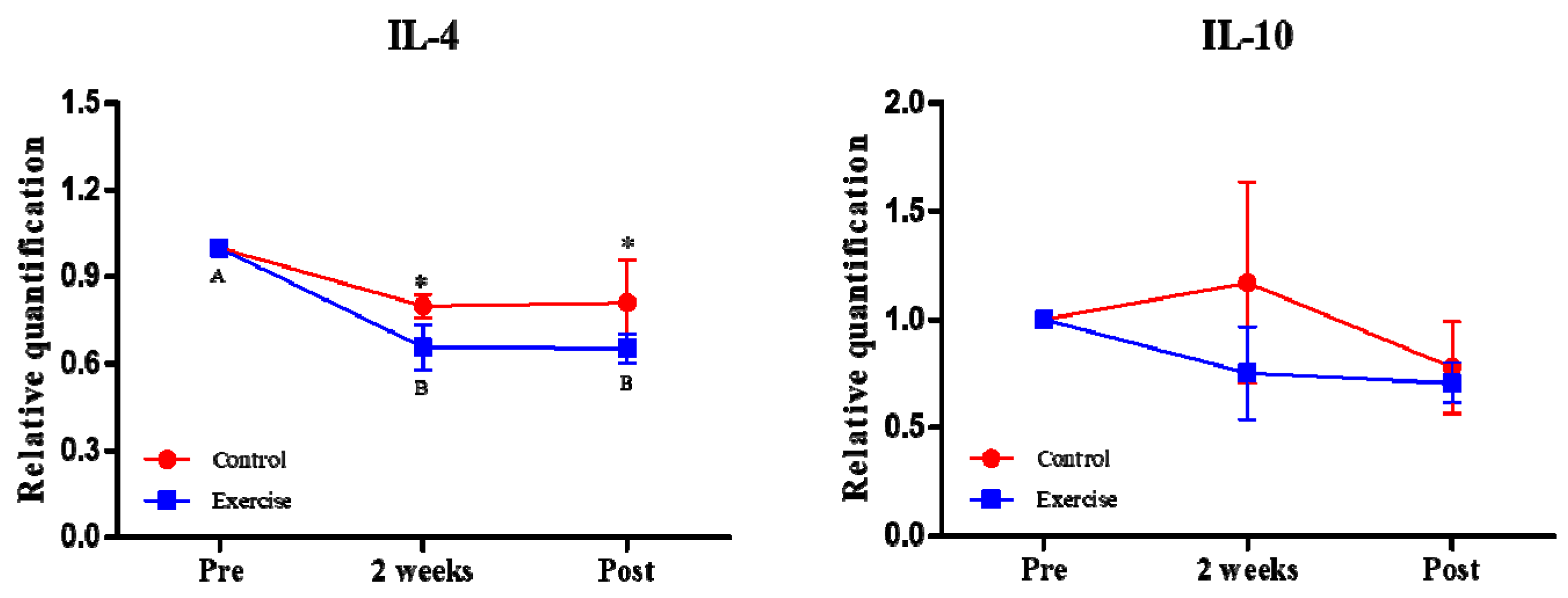
3.2. Effect of Exercise on the Expression of Immune-Related Cytokine Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Data Availability
References
- MacKinnon, M. ‘Sick as a dog’: Zooarchaeological evidence for pet dog health and welfare in the Roman world. World Archaeol. 2010, 42, 290–309. [Google Scholar] [CrossRef]
- Franklin, A.J.H. “Be[a]ware of the Dog”: A Post-Humanist Approach to Housing. Hous. Theory Soc. 2006, 23, 137–156. [Google Scholar] [CrossRef]
- Overall, K.L. Clinical Behavioral Medicine for Small Animals; Mosby-Year Book, Inc.: Maryland Heights, MO, USA, 1997. [Google Scholar]
- Westgarth, C.; Christley, R.M.; Marvin, G.; Perkins, E. I Walk My Dog Because It Makes Me Happy: A Qualitative Study to Understand Why Dogs Motivate Walking and Improved Health. Int. J. Environ. Res. Public Health 2017, 14, 936. [Google Scholar] [CrossRef] [PubMed]
- Medicine, A.C.o.S. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Radin, L.; Belic, M.; Bottegaro, N.B.; Hrastic, H.; Torti, M.; Vucetic, V.; Stanin, D.; Vrbanac, Z. Heart rate deflection point during incremental test in competitive agility border collies. Vet. Res. Commun. 2015, 39, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Rovira, S.; Munoz, A.; Riber, C.; Benito, M. Heart rate, electrocardiographic parameters and arrhythmias during agility exercises in trained dogs. Rev. Med. Vet. Toulouse 2010, 161, 307–313. [Google Scholar]
- Lee, H.S.; Oh, H.J.; Lee, S.H.; Kim, J.W.; Kim, J.-H. Comparison of physiological and hematological responses to treadmill exercise in younger and older adult dogs. Korean J. Sport Sci. 2019, 30, 677–688. [Google Scholar] [CrossRef]
- Piccione, G.; Casella, S.; Panzera, M.; Giannetto, C.; Fazio, F. Effect of Moderate Treadmill Exercise on Some Physiological Parameters in Untrained Beagle Dogs. Exp. Anim. Tokyo 2012, 61, 511–515. [Google Scholar] [CrossRef][Green Version]
- German, A.J.; Blackwell, E.; Evans, M.; Westgarth, C. Overweight dogs exercise less frequently and for shorter periods: Results of a large online survey of dog owners from the UK. J. Nutr. Sci. 2017, 6, e11. [Google Scholar] [CrossRef]
- Ostrowski, K.; Schjerling, P.; Pedersen, B.K. Physical activity and plasma interleukin-6 in humans—Effect of intensity of exercise. Eur. J. Appl. Physiol. 2000, 83, 512–515. [Google Scholar] [CrossRef]
- Duncan, G.E.; Anton, S.D.; Sydeman, S.; Newton, R.L.; Corsica, J.A.; Durning, P.E.; Ketterson, T.U.; Martin, A.D.; Limacher, M.C.; Perri, M.G. Prescribing exercise at varied levels of intensity and frequency—A randomized trial. Arch. Intern. Med. 2005, 165, 2362–2369. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Toft, A.D. Effects of exercise on lymphocytes and cytokines. Brit. J. Sport Med. 2000, 34, 246–251. [Google Scholar] [CrossRef]
- Hasegawa, H.; Mizoguchi, I.; Chiba, Y.; Ohashi, M.; Xu, M.L.; Yoshimoto, T. Expanding Diversity in Molecular Structures and Functions of the IL-6/IL-12 Heterodimeric Cytokine Family. Front. Immunol. 2016, 7, 479. [Google Scholar] [CrossRef]
- Hung, Y.-L.; Suzuki, K. The pattern recognition receptors and lipopolysaccharides (LPS)-induced systemic inflammation. Int. J. Res. Stud. Med. Health Sci. 2017, 2, 1–7. [Google Scholar]
- Suzuki, K. Cytokine Response to Exercise and Its Modulation. Antioxidants 2018, 7, 17. [Google Scholar] [CrossRef]
- Nemzek, J.A.; Agrodnia, M.D.; Hauptman, J.G. Breed-specific pro-inflammatory cytokine production as a predisposing factor for susceptibility to sepsis in the dog. J. Vet. Emerg. Crit. Care 2007, 17, 368–372. [Google Scholar] [CrossRef]
- Kingsnorth, A.J.G. Role of cytokines and their inhibitors in acute pancreatitis. Gut 1997, 40, 1. [Google Scholar] [CrossRef]
- Chen, Y.W.; Li, Y.T.; Chen, Y.C.; Li, Z.Y.; Hung, C.H. Exercise Training Attenuates Neuropathic Pain and Cytokine Expression After Chronic Constriction Injury of Rat Sciatic Nerve. Anesth. Analg. 2012, 114, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo-Calvo, D.; Davalos, A.; Montero, A.; Garcia-Gonzalez, A.; Tyshkovska, I.; Gonzalez-Medina, A.; Soares, S.M.A.; Martinez-Camblor, P.; Casas-Agustench, P.; Rabadan, M.; et al. Circulating inflammatory miRNA signature in response to different doses of aerobic exercise. J. Appl. Physiol. 2015, 119, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Wadley, A.J.; Chen, Y.W.; Lip, G.Y.; Fisher, J.P.; Aldred, S. Low volume-high intensity interval exercise elicits antioxidant and anti-inflammatory effects in humans. J. Sports Sci. 2016, 34, 1–9. [Google Scholar] [CrossRef]
- Koh, Y.; Park, K.S. Responses of inflammatory cytokines following moderate intensity walking exercise in overweight or obese individuals. J. Exerc. Rehabil. 2017, 13, 472–476. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Austin, M.D.; Brown, V.A. Immune response to a 30-minute walk. Med. Sci. Sport Exer. 2005, 37, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, I.; Hagman, R.; Johannisson, A.; Wang, L.; Karlstam, E.; Wernersson, S. Cytokines as Immunological Markers for Systemic Inflammation in Dogs with Pyometra. Reprod. Domest. Anim. 2012, 47, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Piantedosi, D.; Di Loria, A.; Guccione, J.; De Rosa, A.; Fabbri, S.; Cortese, L.; Carta, S.; Ciaramella, P. Serum biochemistry profile, inflammatory cytokines, adipokines and cardiovascular findings in obese dogs. Vet. J. 2016, 216, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ra, K.; Oh, H.J.; Kim, G.A.; Kang, S.K.; Ra, J.C.; Lee, B.C. High Frequency of Intravenous Injection of Human Adipose Stem Cell Conditioned Medium Improved Embryo Development of Mice in Advanced Maternal Age through Antioxidant Effects. Animals 2020, 10, 978. [Google Scholar] [CrossRef]
- Ferasin, L.; Marcora, S. A pilot study to assess the feasibility of a submaximal exercise test to measure individual response to cardiac medication in dogs with acquired heart failure. Vet. Res. Commun. 2007, 31, 725–737. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, S.H.; Kim, J.W.; Lee, Y.S.; Lee, B.C.; Oh, H.J.; Kim, J.H. Development of Novel Continuous and Interval Exercise Programs by Applying the FITT-VP Principle in Dogs. Sci. World J. 2020, 2020, 3029591. [Google Scholar] [CrossRef]
- Cerqueira, J.A.; Restan, W.A.Z.; Fonseca, M.G.; Catananti, L.A.; de Almeida, M.L.M.; Feringer, W.H.; Pereira, G.T.; Carciofi, A.C.; Ferraz, G.D. Intense exercise and endurance-training program influence serum kinetics of muscle and cardiac biomarkers in dogs. Res. Vet. Sci. 2018, 121, 31–39. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, J.H.; Oh, H.J.; Kim, J.H.J.A. Effects of Interval Exercise Training on Serum Biochemistry and Bone Mineral Density in Dogs. Animals 2021, 11, 2528. [Google Scholar] [CrossRef]
- Lee, C.D.; Folsom, A.R.; Nieto, F.J.; Chambless, L.E.; Shahar, E.; Wolfe, D.A. White blood cell count and incidence of coronary heart disease and ischemic stroke, and mortality from cardiovascular disease in African-American and white men and women: The Atherosclerosis Risk in Communities Study. Circulation 2001, 103, 1357–1358. [Google Scholar]
- Lippi, G.; Bassi, A.; Guidi, G.; Zatti, M. Relation between regular aerobic physical exercise and inflammatory markers. Am. J. Cardiol. 2002, 90, 820. [Google Scholar] [CrossRef]
- Mccarthy, D.A.; Dale, M.M. The Leukocytosis of Exercise—A Review and Model. Sports Med. 1988, 6, 333–363. [Google Scholar] [CrossRef]
- Perna, F.M.; Schneiderman, N.; LaPerriere, A. Psychological stress, exercise and immunity. Int. J. Sports Med. 1997, 18, S78–S83. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Goetz, L.; Pervaiz, N.; Packer, N.; Guan, J. Freewheel training decreases pro- and increases anti-inflammatory cytokine expression in mouse intestinal lymphocytes. Brain Behav. Immun. 2010, 24, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Wang, J.S.; Fu, T.C.; Hsu, C.C.; Huang, Y.C. Hypoxic Exercise Training Elevates Erythrocyte Aggregation. Appl Sci. 2021, 11, 6038. [Google Scholar] [CrossRef]
- Wierzba, T.H.; Olek, R.A.; Fedeli, D.; Falcioni, G. Lymphocyte DNA Damage in Rats Challenged with a Single Bout of Strenuous Exercise. J. Physiol. Pharmacol. 2006, 57, 115–131. [Google Scholar] [PubMed]
- Brines, R.; Hoffman-Goetz, L.; Pedersen, B.K. Can you exercise to make your immune system fitter? Immunol. Today 1996, 17, 252–254. [Google Scholar] [CrossRef]
- Horn, P.L.; Pyne, D.B.; Hopkins, W.G.; Barnes, C.J. Lower white blood cell counts in elite athletes training for highly aerobic sports. Eur. J. Appl. Physiol. 2010, 110, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Shek, P.N.; Shephard, R.J. Physical exercise as a human model of limited inflammatory response. Can. J. Physiol. Pharm. 1998, 76, 589–597. [Google Scholar] [CrossRef]
- Suzuki, K.; Totsuka, M.; Nakaji, S.; Yamada, M.; Kudoh, S.; Liu, Q.; Sugawara, K.; Yamaya, K.; Sato, K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J. Appl. Physiol. 1999, 87, 1360–1367. [Google Scholar] [CrossRef]
- Schnelle, A.N.; Barger, A.M. Neutropenia in dogs and cats: Causes and consequences. Vet. Clin. N. Am. Small Anim. Pr. 2012, 42, 111–122. [Google Scholar] [CrossRef]
- Barreda, D.R.; Hanington, P.C.; Belosevic, M. Regulation of myeloid development and function by colony stimulating factors. Dev. Comp. Immunol. 2004, 28, 509–554. [Google Scholar] [CrossRef]
- Parisotto, R.; Pyne, D.; Martin, D.; Gore, C.; Fallon, K.; Fricker, P.; Hahn, A. Neutropenia in elite male cyclists. Clin. J. Sport Med. 2003, 13, 303–305. [Google Scholar] [CrossRef]
- Abramson, J.; Wheeler, J. The Neutrophil: The Natural Immune System; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The Multifaceted Functions of Neutrophils. Annu Rev. Pathol. Mech. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Maeda, K.; Malykhin, A.; Teague-Weber, B.N.; Sun, X.H.; Farris, A.D.; Coggeshall, K.M. Interleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus-prone B6.Sle1.Yaa animals. Blood 2009, 113, 4534–4540. [Google Scholar] [CrossRef]
- Baldridge, M.T.; King, K.Y.; Boles, N.C.; Weksberg, D.C.; Goodell, M.A. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature 2010, 465, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Pronk, C.J.H.; Veiby, O.P.; Bryder, D.; Jacobsen, S.E.W. Tumor necrosis factor restricts hematopoietic stem cell activity in mice: Involvement of two distinct receptors. J. Exp. Med. 2011, 208, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Mossadegh-Keller, N.; Sarrazin, S.; Kandalla, P.K.; Espinosa, L.; Stanley, E.R.; Nutt, S.; Moore, J.; Sieweke, M.H. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nat. Cell Biol. 2013, 497, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Manz, M.G.; Boettcher, S. Emergency granulopoiesis. Nat. Rev. Immunol. 2014, 14, 302–314. [Google Scholar] [CrossRef]
- Cowland, J.B.; Borregaard, N. Granulopoiesis and granules of human neutrophils. Immunol. Rev. 2016, 273, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, K.; Shock, E.J.; Rochelle, P.; Kearns, C.F.; Guirnalda, P.D.; McKeever, K.H. Plasma beta-endorphin, cortisol and immune responses to acute exercise are altered by age and exercise training in horses. Equine Vet. J. Suppl 2006, 267–273. [Google Scholar] [CrossRef]
- Watson, H.G.; Meiklejohn, D.J. Leucopenia in professional football players. Brit. J. Haematol. 2001, 112, 826–827. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Della Gatta, P.; Suzuki, K.; Nieman, D.C. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar]
- Tamassia, N.; Bianchetto-Aguilera, F.; Arruda-Silva, F.; Gardiman, E.; Gasperini, S.; Calzetti, F.; Cassatella, M.A. Cytokine production by human neutrophils: Revisiting the “dark side of the moon”. Eur. J. Clin. Investig. 2018, e12952. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.J.; Kim, S.T.; Costa, G.L.; Zhang, X.; Fathman, C.G.; Glimcher, L.H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000, 100, 655–669. [Google Scholar] [CrossRef]
- Gruenbacher, G.; Gander, H.; Rahm, A.; Nussbaumer, W.; Romani, N.; Thurnher, M. CD56+ human blood dendritic cells effectively promote TH1-type γδ T-cell responses. Blood J. Am. Soc. Hematol. 2009, 114, 4422–4431. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef]
- Fischer, C.P.; Hiscock, N.J.; Penkowa, M.; Basu, S.; Vessby, B.; Kallner, A.; Sjoberg, L.B.; Pedersen, B.K. Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. J. Physiol. 2004, 558, 633–645. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Harris, T.B.; Ferrucci, L.; Tracy, R.P.; Corti, M.C.; Wacholder, S.; Ettinger, W.H.; Heimovitz, H.; Cohen, H.J.; Wallace, R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med. 1999, 106, 506–512. [Google Scholar] [CrossRef]
- Bruunsgaard, H.; Ladelund, S.; Pedersen, A.N.; Schroll, M.; Jorgensen, T.; Pedersen, B.K. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin. Exp. Immunol. 2003, 132, 24–31. [Google Scholar] [CrossRef]
- Ferrucci, L.; Corsi, A.; Lauretani, F.; Bandinelli, S.; Bartali, B.; Taub, D.D.; Guralnik, J.M.; Longo, D.L. The origins of age-related proinflammatory state. Blood 2005, 105, 2294–2299. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, N.M.; Swift, D.L.; Johnson, W.D.; Dixit, V.D.; Earnest, C.P.; Blair, S.N.; Church, T.S. Effect of Different Doses of Aerobic Exercise on Total White Blood Cell (WBC) and WBC Subfraction Number in Postmenopausal Women: Results from DREW. PLoS ONE 2012, 7, e31319. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Halson, S.L.; Khan, Q.; Drysdale, P.; Wallace, F.; Jeukendrup, A.E.; Drayson, M.T.; Gleeson, M. Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes. Exerc. Immunol.Rev. 2004, 10, 91–106. [Google Scholar] [PubMed]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro-and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 1999, 515, 287–291. [Google Scholar] [CrossRef]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Kometani, T. Urinary excretion of cytokines versus their plasma levels after endurance exercise. Exerc. Immunol. Rev. 2013, 19, 29–48. [Google Scholar]
- Lu, J.; Zhang, H.L.; Yin, Z.Z.; Tu, Y.; Li, Z.G.; Zhao, B.X.; Guo, J.Y. Moxibustion Attenuates Inflammatory Response to Chronic Exhaustive Exercise in Rats. Int J. Sports Med. 2012, 33, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Gokhale, R.; Chandrashekara, S.; Vasanthakumar, K.C. Cytokine response to strenuous exercise in athletes and non-athletes—An adaptive response. Cytokine 2007, 40, 123–127. [Google Scholar] [CrossRef]
- Keller, C.; Keller, P.; Giralt, M.; Hidalgo, J.; Pedersen, B.K. Exercise normalises overexpression of TNF-alpha in knockout mice. Biochem. Bioph. Res. Co. 2004, 321, 179–182. [Google Scholar] [CrossRef]
- Paolucci, E.M.; Loukov, D.; Bowdish, D.M.E.; Heisz, J.J. Exercise reduces depression and inflammation but intensity matters. Biol. Psychol. 2018, 133, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ibfelt, T.; Petersen, E.W.; Bruunsgaard, H.; Sandmand, M.; Pedersen, B.K. Exercise-induced change in type 1 cytokine-producing CD8(+) T cells is related to a decrease in memory T cells. J. Appl. Physiol. 2002, 93, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.; Nackley, A.G.; Satterfield, K.; Maixner, W.; Diatchenko, L.; Flood, P.M. β2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA-and NF-κB-independent mechanisms. Cell Signal. 2007, 19, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Moates, J.M.; Lacy, D.B.; Goldstein, R.E.; Cherrington, A.D.; Wasserman, D.H. Metabolic Role of the Exercise-Induced Increment in Epinephrine in the Dog. Am. J. Physiol. 1988, 255, E428–E436. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; Toft, A.D.; Bruunsgaard, H.; Sandmand, M.; Halkjaer-Kristensen, J.; Pedersen, B.K. Strenuous exercise decreases the percentage of type 1 T cells in the circulation. J. Appl. Physiol. 2001, 91, 1708–1712. [Google Scholar] [CrossRef]
- Marshall, G.D.; Agarwal, S.K. Stress, immune regulation, and immunity: Applications for asthma. Allergy Asthma Proc. 2000, 21, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Edwards, J.E. Type 1 type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001, 32, 76–102. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Pilaszewicz, O.; Baska, P.; Czopowicz, M.; Zmigrodzka, M.; Szarska, E.; Szczepaniak, J.; Nowak, Z.; Winnicka, A.; Cywinska, A. Anti-Inflammatory State in Arabian Horses Introduced to the Endurance Training. Animals 2019, 9, 616. [Google Scholar] [CrossRef]
- Volpin, G.; Cohen, M.; Assaf, M.; Meir, T.; Katz, R.; Pollack, S. Cytokine Levels (IL-4, IL-6, IL-8 and TGF beta) as Potential Biomarkers of Systemic Inflammatory Response in Trauma Patients. Int. Orthop. 2014, 38, 1303–1309. [Google Scholar] [CrossRef]
- Hart, P.H.; Vitti, G.F.; Burgess, D.R.; Whitty, G.A.; Piccoli, D.S.; Hamilton, J.A. Potential Antiinflammatory Effects of Interleukin-4—Suppression of Human Monocyte Tumor Necrosis Factor-Alpha, Interleukin-1, and Prostaglandin-E2. Proc. Natl. Acad. Sci. USA 1989, 86, 3803–3807. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Salehi, I.; Alahgholi-Hajibehzad, M.J. Moderate exercise enhances the production of interferon-γ and interleukin-12 in peripheral blood mononuclear cells. Immune Netw. 2017, 17, 186–191. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Ostrowski, K.; Schjerling, P. Exercise and cytokines with particular focus on muscle-derived IL-6. Exerc. Immunol. Rev. 2001, 7, 18–31. [Google Scholar] [PubMed]
- La Gerche, A.; Inder, W.J.; Roberts, T.J.; Brosnan, M.J.; Heidbuchel, H.; Prior, D.L. Relationship between Inflammatory Cytokines and Indices of Cardiac Dysfunction following Intense Endurance Exercise. PLoS ONE 2015, 10, e0130031. [Google Scholar] [CrossRef]
- Felsburg, P.J. Overview of immune system development in the dog: Comparison with humans. Hum. Exp. Toxicol. 2002, 21, 487–492. [Google Scholar] [CrossRef] [PubMed]


| Parameters (Unit) | Dogs |
|---|---|
| No. of Dogs | 6 |
| Sex | Male |
| Age (month) | 31.8 ± 15.8 |
| Weight (kg) | 9.1 ± 1.3 |
| Protocol | Session | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| 1 | Time (min) | 5 | 5 | 5 | 5 | 5 |
| Slope (%) | 0 | 1 | 1 | 2 | 2 | |
| Speed (km/h) | 3.0 | 3.2 | 3.4 | 3.6 | 3.8 | |
| 2 | Time (min) | 5 | 5 | 5 | 5 | 5 |
| Slope (%) | 1 | 2 | 2 | 3 | 3 | |
| Speed (km/h) | 3.2 | 3.4 | 3.6 | 3.8 | 4.0 | |
| 3 | Time (min) | 5 | 5 | 5 | 5 | 5 |
| Slope (%) | 2 | 3 | 3 | 4 | 4 | |
| Speed (km/h) | 3.4 | 3.6 | 3.8 | 4.0 | 4.2 | |
| 4 | Time (min) | 5 | 5 | 5 | 5 | 5 |
| Slope (%) | 3 | 4 | 4 | 5 | 5 | |
| Speed (km/h) | 3.6 | 3.8 | 4.0 | 4.2 | 4.4 |
| Gene | Primer Sequence (5′ → 3′) | Accession Number |
|---|---|---|
| β-actin | F: GCGCAAGTACTCTGTGTGGA | NM_001195845.2 |
| R: ACATTTGCTGGAAGGTGGAC | ||
| IFN-γ | F: CGCAAGGCGATAAATGAACT | NM_001003174.1 |
| R: GACTCCTTTTCCGCTTCCTT | ||
| TNF-α | F: CCCCAAGTGACAAGCCAGTA | NM_001003244.4 |
| R: CTCAGCTTCGGGGTTTGCTA | ||
| IL-4 | F: ACTCACCAGCACCTTTGTCC | NM_001003159.1 |
| R: CTCGCTGTGAGGATGTTCAA | ||
| IL-1β | F: TTGTGCACGGGGATGAAAGT | NM_001037971.1 |
| R: TTGATGCCCAAGACCACAGG | ||
| IL-6 | F: GCAGGAGATTCCAAGGATGA | NM_001003301.1 |
| R: TTGTTTGCAGAGGTGAGTGG | ||
| IL-8 | F: TCAGAACTTCGATGCCAGTG | AF048717.1 |
| R: GGGCCACTGTCAATCACTCT | ||
| IL-10 | F: CCTGTCGGAGATGATCCAGT | NM_001003077.1 |
| R: GATGTCTGGGTCGTGGTTCT |
| Parameters | Group | Pre-Exercise | 2 Weeks of Exercise | Post-Exercise |
|---|---|---|---|---|
| WBC (k/μL) | Control Exercise | 7810.0 ± 1120.6 8650.0 ± 3085.7 A | 7313.3 ± 2043.4 4916.6 ± 1097.9 B | 7543.3 ± 976.5 6806.6 ± 450.8 C |
| AST (U/L) | Control Exercise | 37.6 ± 4.0 35.0 ± 2.6 A | 30.0 ± 1.0 31.0 ± 5.5 A | 30.6 ± 5.5 26.3 ± 2.0 B |
| ALP (U/L) | Control Exercise | 35.3 ± 8.6 40.0 ± 4.5 A | 34.8 ± 2.0 44.0 ± 2.6 A | 30.3 ± 5.1 49.6 ± 7.0 B |
| Glucose (mmol/L) | Control Exercise | 110.6 ± 7.0 110.3 ± 5.6 A | 110.3 ± 7.7 101.3 ± 7.5 B | 104.6 ± 6.6 108.0 ± 9.6 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.S.; Oh, H.J.; Ra, K.; Kim, J.-H. Effect of Moderate-Intensity Endurance Exercise on Inflammatory Cytokines in Leukocytes of Dogs. Appl. Sci. 2022, 12, 215. https://doi.org/10.3390/app12010215
Lee HS, Oh HJ, Ra K, Kim J-H. Effect of Moderate-Intensity Endurance Exercise on Inflammatory Cytokines in Leukocytes of Dogs. Applied Sciences. 2022; 12(1):215. https://doi.org/10.3390/app12010215
Chicago/Turabian StyleLee, Hae Sung, Hyun Ju Oh, Kihae Ra, and Jong-Hee Kim. 2022. "Effect of Moderate-Intensity Endurance Exercise on Inflammatory Cytokines in Leukocytes of Dogs" Applied Sciences 12, no. 1: 215. https://doi.org/10.3390/app12010215
APA StyleLee, H. S., Oh, H. J., Ra, K., & Kim, J.-H. (2022). Effect of Moderate-Intensity Endurance Exercise on Inflammatory Cytokines in Leukocytes of Dogs. Applied Sciences, 12(1), 215. https://doi.org/10.3390/app12010215






