Mechanochemical Synthesis of the Catechol-Theophylline Cocrystal: Spectroscopic Characterization and Molecular Structure
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Mechanochemical Synthesis
2.2. Instruments
2.3. Theoretical Calculations
3. Results and Discussion
3.1. IR Spectroscopy
3.2. Powder X-ray Diffraction
3.3. Thermal Analysis
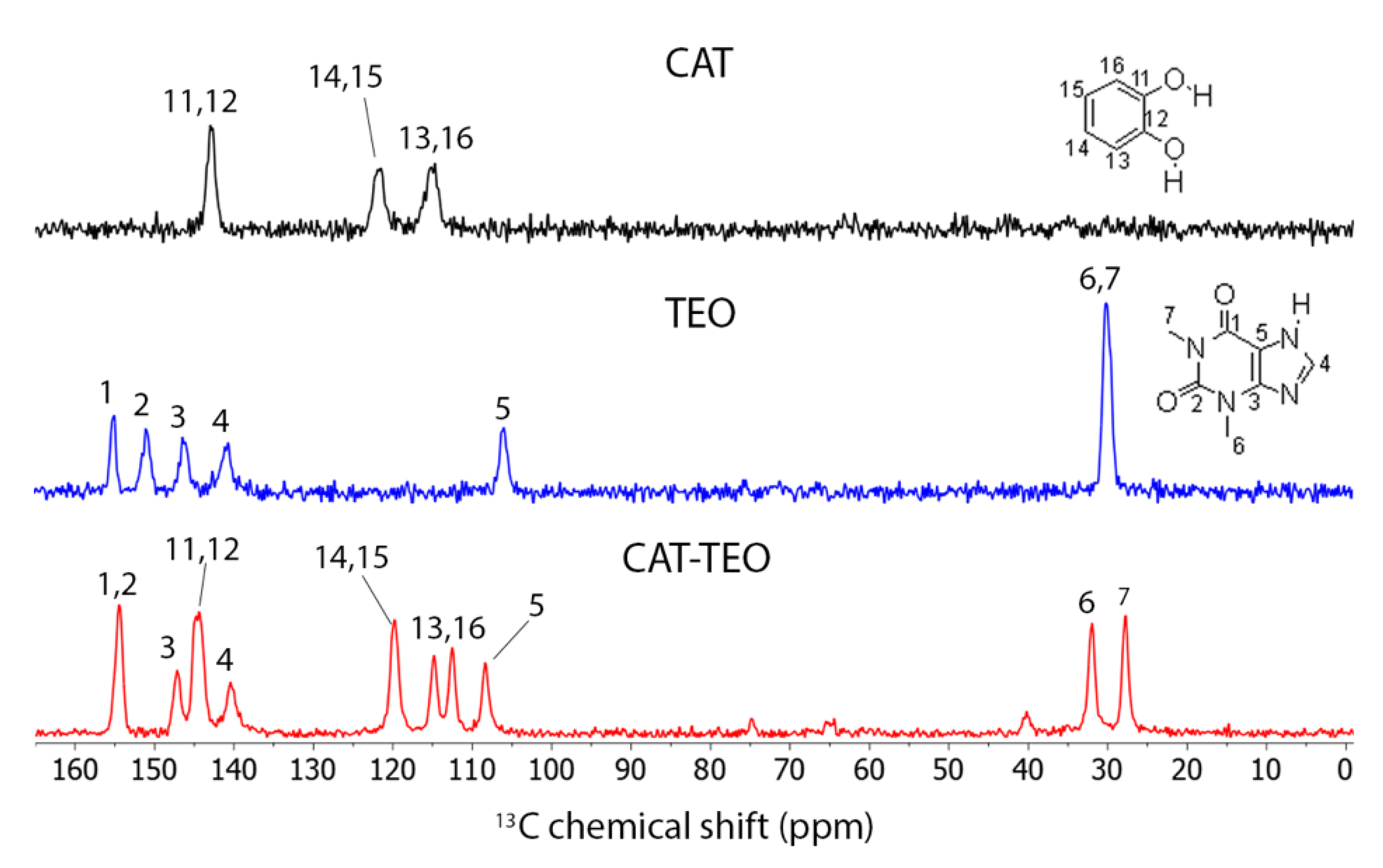
3.4. Solid-State 13C NMR
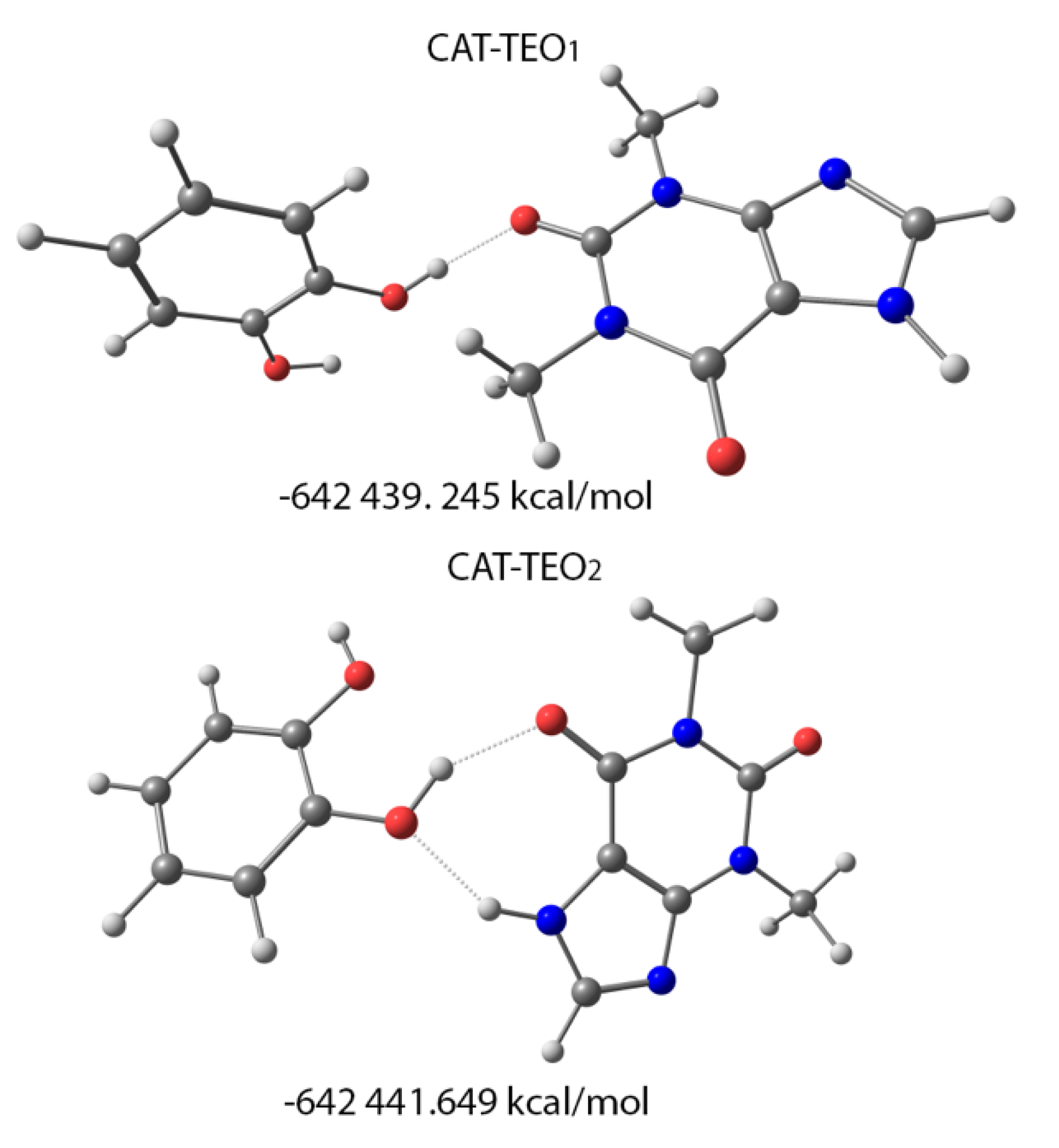
3.5. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, P.J. Theophylline. Am. J. Respir. Crit. Care. Med. 2013, 188, 901–906. [Google Scholar] [CrossRef]
- Dutta, A.; Roy, N.; Das, K.; Roy, D.; Ghosh, R.; Roy, M.N. Synthesis and Characterization of Host Guest Inclusion Complexes of Cyclodextrin Molecules with Theophylline by Diverse Methodologies. Emerg. Sci. J. 2020, 4, 52–72. [Google Scholar] [CrossRef]
- Francesconi, O.; Ienco, A.; Nativi, C.; Roelens, S. Effective Recognition of Caffeine by Diaminocarbazolic Receptors. ChemPlusChem 2020, 85, 1369–1373. [Google Scholar] [CrossRef]
- Jayram, P.; Sudheer, P. Pharmaceutical Co-crystals: A Systematic Review. Int. J. Pharm. Investig. 2020, 10, 246–252. [Google Scholar] [CrossRef]
- Bucar, D.K.; Henry, R.F.; Zhang, G.G.Z.; MacGillivray, L.R. Synthon Hierarchies in Crystal Forms Composed of Theophylline and Hydroxybenzoic Acids: Cocrystal Screening via Solution-Mediated Phase Transformation. Cryst. Growth Des. 2014, 14, 5318–5328. [Google Scholar] [CrossRef]
- Heiden, S.; Tröbs, L.; Wenzel, K.; Emmerling, F. Mechanochemical synthesis and structural characterisation of a theophylline-benzoic acid cocrystal (1:1). CrystEngComm 2012, 14, 5128–5129. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Manin, A.N.; Manin, N.G.; Kuzmina, L.G.; Churakov, A.V.; Perlovich, G.L. Pharmaceutical Cocrystals of Diflunisal and Diclofenac with Theophylline. Mol. Pharm. 2014, 11, 3707–3715. [Google Scholar] [CrossRef]
- Li, P.; Chu, Y.; Wang, L.; Wenslow, R.M., Jr.; Yu, K.; Zhang, H.; Denga, Z. Structure determination of the theophylline–nicotinamide cocrystal: A combined powder XRD, 1D solid-state NMR, and theoretical calculation study. CrystEngComm 2014, 16, 3141–3147. [Google Scholar] [CrossRef]
- Wang, L.; Luo, M.; Li, J.; Wang, J.; Zhang, H.; Deng, Z. Sweet Theophylline Cocrystal with Two Tautomers of Acesulfame. Cryst. Growth Des. 2015, 15, 2574–2578. [Google Scholar] [CrossRef]
- Gangavaram, S.; Raghavender, S.; Sanphui, P.; Pal, S.; Manjunatha, S.G.; Nambiar, S.; Nangia, A. Polymorphs and cocrystals of nalidixic acid. Cryst. Growth Des. 2012, 12, 4963–4971. [Google Scholar] [CrossRef]
- Bolla, G.; Sanphui, P.; Nangia, A. Solubility advantage of tenoxicam phenolic cocrystals compared to salts. Cryst. Growth Des. 2013, 13, 1988–2003. [Google Scholar] [CrossRef]
- Arabiani, M.R.; Bhunia, S.; Teja, P.K.; Lodagekar, A.; Chavan, R.B.; Shastri, N.R.; Reddi, C.M.; Shelat, P.; Dave, D. Brexpiprazole–catechol cocrystal: Structure elucidation, excipient compatibility and stability. CrystEngComm 2019, 21, 6703–6708. [Google Scholar] [CrossRef]
- González-González, J.S.; Martínez-Santiago, A.M.M.; Martínez-Martínez, F.J.; Emparán-Legaspi, M.J.; Pineda-Contreras, A.; Flores-Alamo, M.; García-Ortega, H. Cocrystals of Isoniazid with Polyphenols: Mechanochemical Synthesis and Molecular Structure. Crystals 2020, 10, 569. [Google Scholar] [CrossRef]
- Rodrigues, M.; Baptista, B.; Almeida, J.L.; Cruz, S.M. Pharmaceutical cocrystallization techniques. Advances and challenges. Int. J. Pharm. 2018, 547, 404–420. [Google Scholar] [CrossRef]
- Izutsu, K.; Koide, T.; Takata, N.; Ikeda, Y.; Ono, M.; Inoue, M.; Fukami, T.; Yonemochi, E. Characterization and Quality Control of Pharmaceutical Cocrystals. Chem. Pharm. Bull. 2016, 64, 1421–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, S.L.; Stahly, G.P.; Park, A. The Salt-Cocrystal Continuum: The Influence of Crystal Structure on Ionization State. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.L.A.; Fleming, O.S.; Kazarian, S.G.; Vassou, D.; Chryssikos, G.D.; Gionis, V. Polymorphism and devitrification of nifedipine under controlled humidity: A combined FT-Raman, IR and Raman microscopic investigation. J. Raman Spectrosc. 2004, 35, 353–359. [Google Scholar] [CrossRef]
- González-González, J.S.; Zúñiga-Lemus, O.; Hernández-Galindo, M.C. Hydrated Solid Forms of Theophylline and Caffeine Obtained by Mechanochemistry. IOSR J. Pharm. 2017, 7, 28–30. [Google Scholar] [CrossRef]
- Grdadolnik, J. ATR-FTIR Spectroscopy: Its Advantages and Limitations. Acta Chim. Slov. 2002, 49, 631–642. [Google Scholar]
- Mukherjee, A.; Tothadi, S.; Chakraborty, S.; Ganguly, S.; Desiraju, G.R. Synthon identification in co-crystals and polymorphs with IR spectroscopy. Primary amides as a case study. CrystEngComm 2013, 15, 4640–4654. [Google Scholar] [CrossRef]
- Vogt, F.G.; Clawson, J.S.; Strohmeier, M.; Edwards, A.J.; Pham, T.N.; Watson, S.A. Solid-state NMR analysis of organic cocrystals and complexes. Cryst. Growth Des. 2009, 9, 921–937. [Google Scholar] [CrossRef]
- Li, M.; Xu, W.; Su, Y. Solid-state NMR spectroscopy in pharmaceutical sciences. Trends Anal. Chem. 2021, 135, 116152. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- González-González, J.S.; Martínez-Martínez, F.J.; García-Báez, E.V.; Cruz, A.; Morín-Sánchez, L.M.; Rojas-Lima, S.; Padilla-Martínez, I.I. Molecular complexes of diethyl N, N′-1, 3-phenyldioxalamate and resorcinols: Conformational switching through intramolecular three-centered hydrogen-bonding. Cryst. Growth Des. 2014, 14, 628–642. [Google Scholar] [CrossRef]
- Saucedo-Balderas, M.M.; Delgado-Alfaro, R.A.; Martínez-Martínez, F.J.; Ortegón-Reyna, D.; Bernabé-Pineda, M.; Zúñiga-Lemus, O.; González-González, J.S. Synthesis, Molecular Structure of Diethyl Phenylenebis (Methylene) Dicarbamates and FTIR Spectroscopy Molecular Recognition Study with Benzenediols. J. Braz. Chem. Soc. 2015, 26, 396–402. [Google Scholar] [CrossRef]
- Seton, L.; Khamar, D.; Bradshaw, I.J.; Hutcheon, G.A. Solid state forms of theophylline: Presenting a new anhydrous polymorph. Cryst. Growth Des. 2010, 10, 3879–3886. [Google Scholar] [CrossRef]
- 1,2-Dihydroxybenzene. Available online: https://www.sigmaaldrich.com/catalog/product/sial/135011?lang=es®ion=MX&cm_sp=Insite-_-caSrpResults_srpRecs_srpModel_catechol-_-srpRecs3-1 (accessed on 27 February 2021).
- Theophylline. Available online: https://www.sigmaaldrich.com/catalog/product/sigma/t1633?lang=es®ion=MX (accessed on 27 February 2021).
- Finkelstein-Shapiro, D.; Davidowski, S.K.; Lee, P.B.; Guo, C.; Holland, G.P.; Rajh, T.; Kimberly, A.; Gray, K.A.; Yarger, J.L.; Calatayud, M. Direct evidence of chelated geometry of catechol on TiO2 by a combined solid-state NMR and DFT study. J. Phys. Chem. C 2016, 120, 23625–23630. [Google Scholar] [CrossRef]
- Du, Y.; Fang, X.; Zhang, Q.; Zhang, H.L.; Hong, Z. Spectroscopic investigation on cocrystal formation between adenine and fumaric acid based on infrared and Raman techniques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 580–585. [Google Scholar] [CrossRef]
- Bruni, G.; Maietta, M.; Berbenni, V.; Mustarelli, P.; Ferrara, C.; Freccero, M.; Grande, V.; Maggi, L.; Milanese, C.; Girella, A.; et al. Mechanochemical Synthesis of Bumetanide-4-Aminobenzoic Acid Molecular Cocrystals: A Facile and Green Approach to Drug Optimization. J. Phys. Chem. B 2014, 118, 9180–9190. [Google Scholar] [CrossRef]
- Srivastava, K.; Shimpi, M.R.; Srivastava, A.; Tandon, P.; Sinha, K.; Velaga, S.P. Vibrational analysis and chemical activity of paracetamol–oxalic acid cocrystal based on monomer and dimer calculations: DFT and AIM approach. RSC Adv. 2016, 6, 10024–10037. [Google Scholar] [CrossRef]
- Majerz, I. Directionality of Inter- and Intramolecular OHO Hydrogen Bonds: DFT Study Followed by AIM and NBO Analysis. J. Phys. Chem. A 2012, 116, 7992–8000. [Google Scholar] [CrossRef]
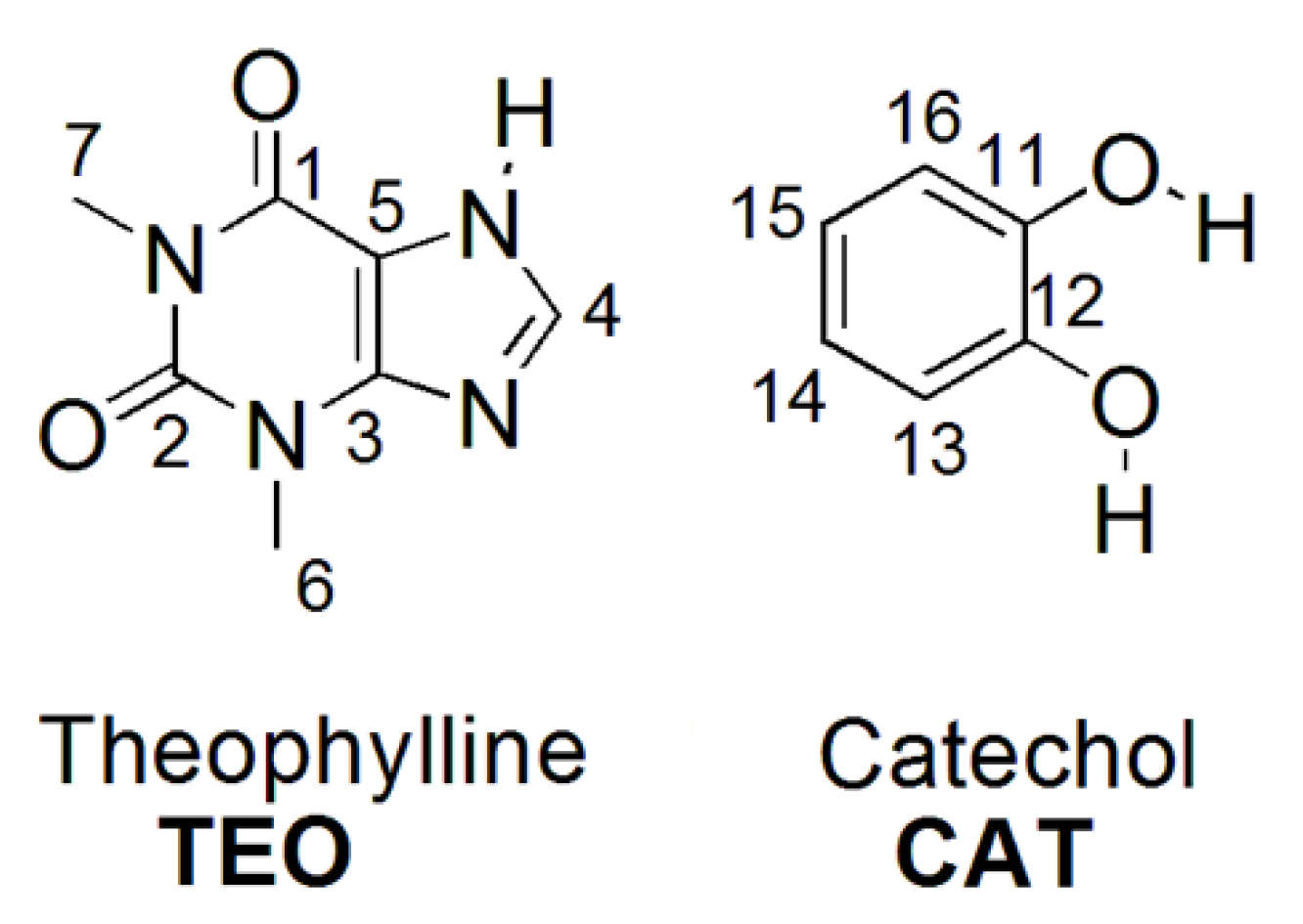
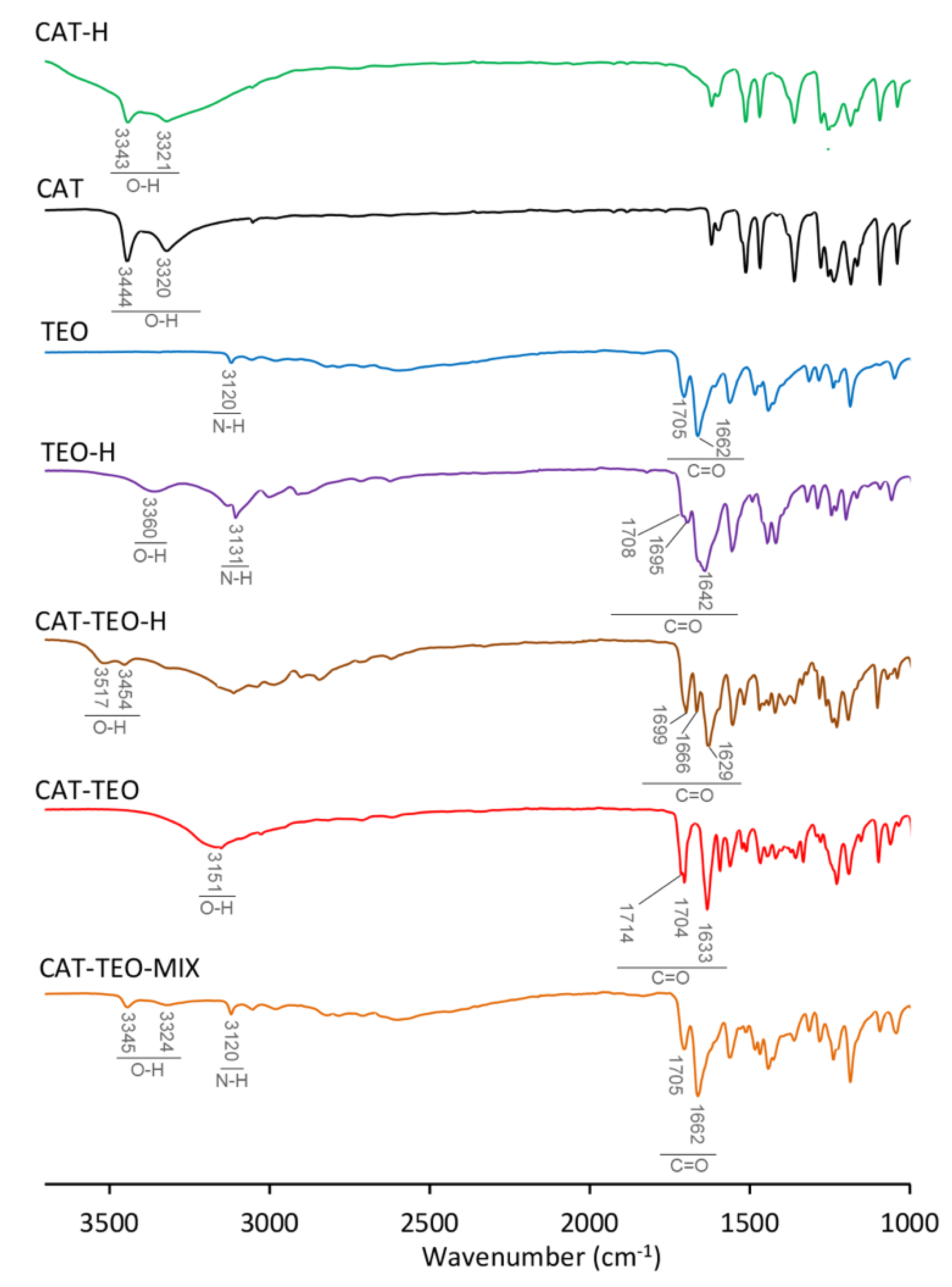


| νO-H | Δ(νO-H) | νC=O | Δ(νC=O) | νN-H | Δ(νN-H) | |
|---|---|---|---|---|---|---|
| CAT-H | 3443, 3321 | ----- | ----- | ----- | ----- | ----- |
| CAT | 3444, 3320 | ----- | ----- | ----- | ----- | ----- |
| TEO | ----- | ----- | 1705, 1662 | ----- | 3120 | ----- |
| TEO-H | 3360 | ----- | 1708, 1695, 1642 | 3, −10, −20 | 3131 | 11 |
| CAT-TEO-H | 3517, 3454 | 73, 314 | 1699, 1666, 1629 | −6, 4, −33 | N.O. | ----- |
| CAT-TEO | 3151 | −293, −169 | 1714, 1704, 1633 | 9, −1 *, −29 | N.O. | ----- |
| CAT-TEO-MIX | 3345, 3324 | ----- | 1705, 1662 | ----- | 3120 | ----- |
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | |
|---|---|---|---|---|---|---|---|
| TEO | 154.9 | 150.9 | 146.3 | 140.5 | 105.8 | 30.0 | 30.0 |
| CAT-TEO | 154.5 | 154.5 | 144.2 | 140.2 | 108.3 | 31.9 | 27.6 |
| C11 | C12 | C13 | C14 | C15 | C16 | ||
| CAT | 142.9 | 142.9 | 114.6 | 121.5 | 121.5 | 114.6 | |
| CAT-TEO | 144.7 | 144.7 | 114.7 112.4 | 119.8 | 119.8 | 114.7 112.4 | |
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | |
|---|---|---|---|---|---|---|---|
| CAT-TEO (δexp) | 154.5 | 154.5 | 144.2 | 140.2 | 108.3 | 31.9 | 27.6 |
| CAT-TEO1 (δcalc) σiso | 154.6 (45.3) | 155.3 (44.6) | 150.2 (49.7) | 138.2 (61.7) | 114.0 (85.9) | 38.9 (161.0) | 35.7 (164.1) |
| CAT-TEO2 (δcalc) σiso | 157.5 (42.4) | 151.9 (48.0) | 151.9 (48.0) | 139.7 (60.2) | 113.4 (86.5) | 37.2 (162.7) | 36.7 (163.1) |
| C11 | C12 | C13 | C14 | C15 | C16 | ||
| CAT-TEO (δexp) | 144.7 | 144.7 | 114.7 112.4 | 119.8 | 119.8 | 114.7 112.4 | |
| CAT-TEO1 (δcalc) σiso | 148.8 (51.1) | 147 (52.9) | 116 (83.9) | 121.3 (78.6) | 122.9 (77.0) | 117.4 (82.4) | |
| CAT-TEO2 (δcalc) σiso | 146.6 (53.3) | 146.5 (53.4) | 116.7 (83.2) | 122.8 (77.1) | 122.9 (76.0) | 119.3 (80.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-González, J.S.; Jiménez-López, R.; Ortegón-Reyna, D.; Gonzalez-Carrillo, G.; Martínez-Martínez, F.J. Mechanochemical Synthesis of the Catechol-Theophylline Cocrystal: Spectroscopic Characterization and Molecular Structure. Appl. Sci. 2021, 11, 3810. https://doi.org/10.3390/app11093810
González-González JS, Jiménez-López R, Ortegón-Reyna D, Gonzalez-Carrillo G, Martínez-Martínez FJ. Mechanochemical Synthesis of the Catechol-Theophylline Cocrystal: Spectroscopic Characterization and Molecular Structure. Applied Sciences. 2021; 11(9):3810. https://doi.org/10.3390/app11093810
Chicago/Turabian StyleGonzález-González, Juan Saulo, Raquel Jiménez-López, David Ortegón-Reyna, Gabino Gonzalez-Carrillo, and Francisco Javier Martínez-Martínez. 2021. "Mechanochemical Synthesis of the Catechol-Theophylline Cocrystal: Spectroscopic Characterization and Molecular Structure" Applied Sciences 11, no. 9: 3810. https://doi.org/10.3390/app11093810
APA StyleGonzález-González, J. S., Jiménez-López, R., Ortegón-Reyna, D., Gonzalez-Carrillo, G., & Martínez-Martínez, F. J. (2021). Mechanochemical Synthesis of the Catechol-Theophylline Cocrystal: Spectroscopic Characterization and Molecular Structure. Applied Sciences, 11(9), 3810. https://doi.org/10.3390/app11093810








