Mechanochemical Synthesis of the Catechol-Theophylline Cocrystal: Spectroscopic Characterization and Molecular Structure
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Mechanochemical Synthesis
2.2. Instruments
2.3. Theoretical Calculations
3. Results and Discussion
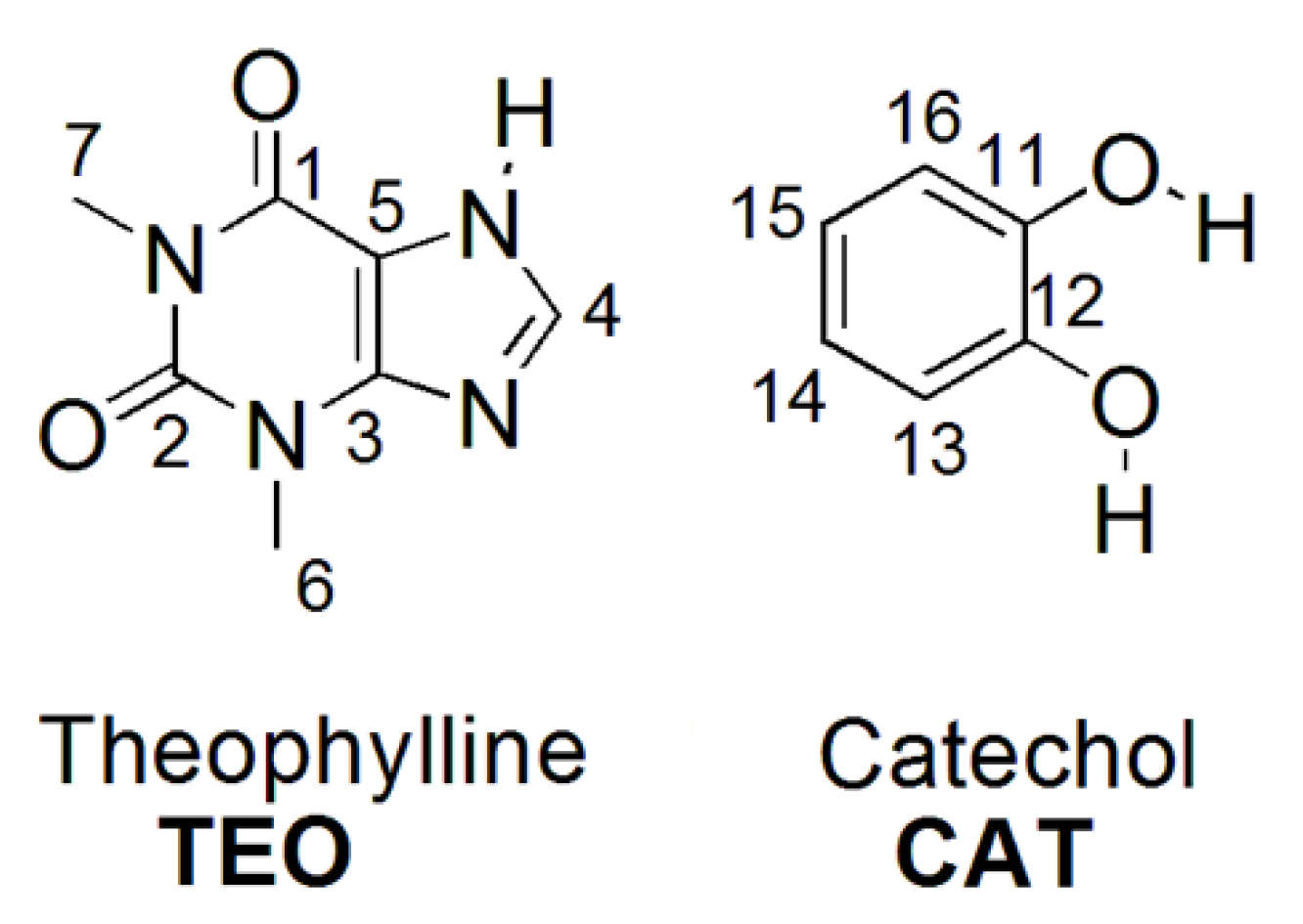
3.1. IR Spectroscopy
3.2. Powder X-ray Diffraction
3.3. Thermal Analysis
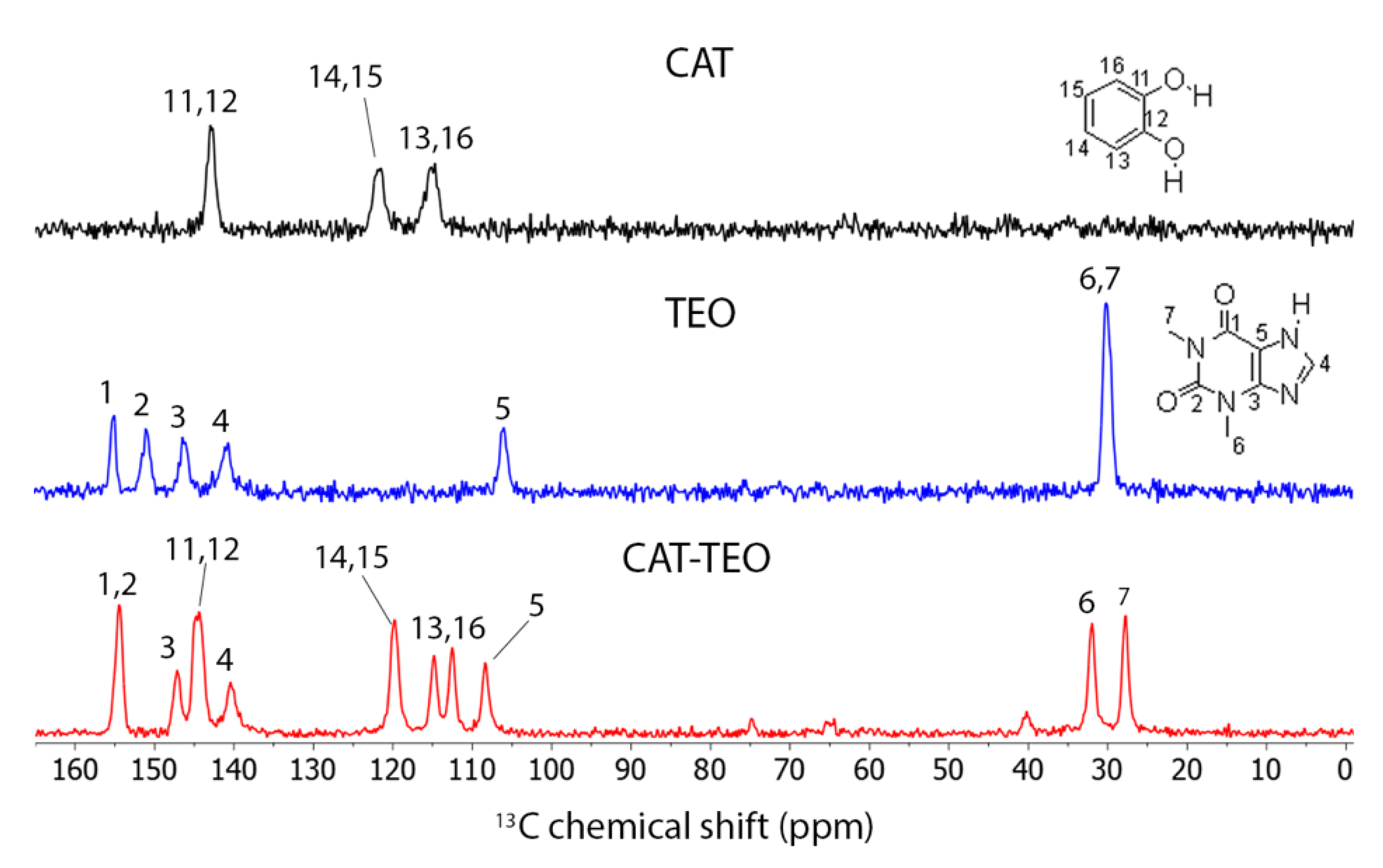
3.4. Solid-State 13C NMR
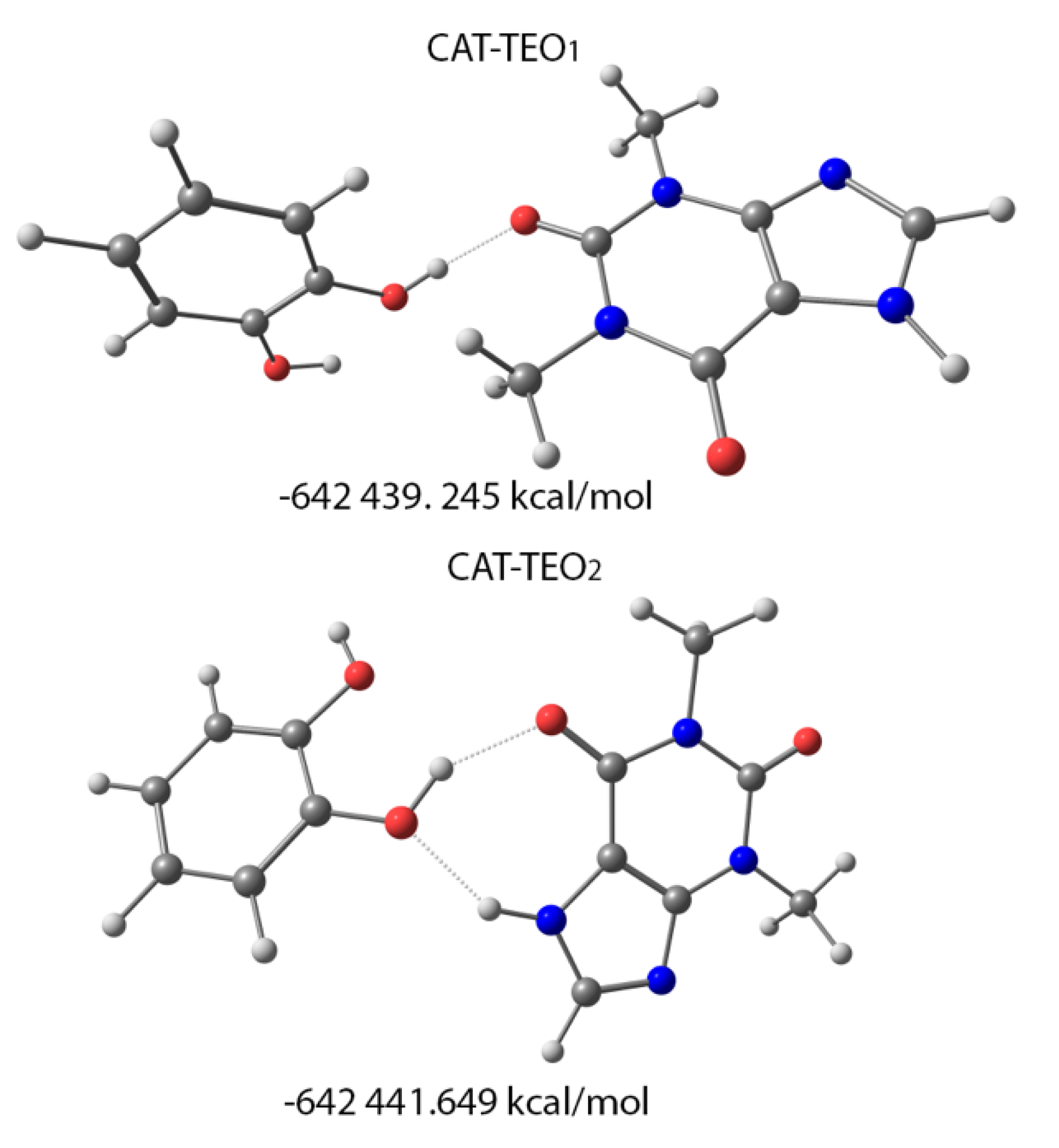
3.5. Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, P.J. Theophylline. Am. J. Respir. Crit. Care. Med. 2013, 188, 901–906. [Google Scholar] [CrossRef]
- Dutta, A.; Roy, N.; Das, K.; Roy, D.; Ghosh, R.; Roy, M.N. Synthesis and Characterization of Host Guest Inclusion Complexes of Cyclodextrin Molecules with Theophylline by Diverse Methodologies. Emerg. Sci. J. 2020, 4, 52–72. [Google Scholar] [CrossRef]
- Francesconi, O.; Ienco, A.; Nativi, C.; Roelens, S. Effective Recognition of Caffeine by Diaminocarbazolic Receptors. ChemPlusChem 2020, 85, 1369–1373. [Google Scholar] [CrossRef]
- Jayram, P.; Sudheer, P. Pharmaceutical Co-crystals: A Systematic Review. Int. J. Pharm. Investig. 2020, 10, 246–252. [Google Scholar] [CrossRef]
- Bucar, D.K.; Henry, R.F.; Zhang, G.G.Z.; MacGillivray, L.R. Synthon Hierarchies in Crystal Forms Composed of Theophylline and Hydroxybenzoic Acids: Cocrystal Screening via Solution-Mediated Phase Transformation. Cryst. Growth Des. 2014, 14, 5318–5328. [Google Scholar] [CrossRef]
- Heiden, S.; Tröbs, L.; Wenzel, K.; Emmerling, F. Mechanochemical synthesis and structural characterisation of a theophylline-benzoic acid cocrystal (1:1). CrystEngComm 2012, 14, 5128–5129. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Manin, A.N.; Manin, N.G.; Kuzmina, L.G.; Churakov, A.V.; Perlovich, G.L. Pharmaceutical Cocrystals of Diflunisal and Diclofenac with Theophylline. Mol. Pharm. 2014, 11, 3707–3715. [Google Scholar] [CrossRef]
- Li, P.; Chu, Y.; Wang, L.; Wenslow, R.M., Jr.; Yu, K.; Zhang, H.; Denga, Z. Structure determination of the theophylline–nicotinamide cocrystal: A combined powder XRD, 1D solid-state NMR, and theoretical calculation study. CrystEngComm 2014, 16, 3141–3147. [Google Scholar] [CrossRef]
- Wang, L.; Luo, M.; Li, J.; Wang, J.; Zhang, H.; Deng, Z. Sweet Theophylline Cocrystal with Two Tautomers of Acesulfame. Cryst. Growth Des. 2015, 15, 2574–2578. [Google Scholar] [CrossRef]
- Gangavaram, S.; Raghavender, S.; Sanphui, P.; Pal, S.; Manjunatha, S.G.; Nambiar, S.; Nangia, A. Polymorphs and cocrystals of nalidixic acid. Cryst. Growth Des. 2012, 12, 4963–4971. [Google Scholar] [CrossRef]
- Bolla, G.; Sanphui, P.; Nangia, A. Solubility advantage of tenoxicam phenolic cocrystals compared to salts. Cryst. Growth Des. 2013, 13, 1988–2003. [Google Scholar] [CrossRef]
- Arabiani, M.R.; Bhunia, S.; Teja, P.K.; Lodagekar, A.; Chavan, R.B.; Shastri, N.R.; Reddi, C.M.; Shelat, P.; Dave, D. Brexpiprazole–catechol cocrystal: Structure elucidation, excipient compatibility and stability. CrystEngComm 2019, 21, 6703–6708. [Google Scholar] [CrossRef]
- González-González, J.S.; Martínez-Santiago, A.M.M.; Martínez-Martínez, F.J.; Emparán-Legaspi, M.J.; Pineda-Contreras, A.; Flores-Alamo, M.; García-Ortega, H. Cocrystals of Isoniazid with Polyphenols: Mechanochemical Synthesis and Molecular Structure. Crystals 2020, 10, 569. [Google Scholar] [CrossRef]
- Rodrigues, M.; Baptista, B.; Almeida, J.L.; Cruz, S.M. Pharmaceutical cocrystallization techniques. Advances and challenges. Int. J. Pharm. 2018, 547, 404–420. [Google Scholar] [CrossRef]
- Izutsu, K.; Koide, T.; Takata, N.; Ikeda, Y.; Ono, M.; Inoue, M.; Fukami, T.; Yonemochi, E. Characterization and Quality Control of Pharmaceutical Cocrystals. Chem. Pharm. Bull. 2016, 64, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.L.; Stahly, G.P.; Park, A. The Salt-Cocrystal Continuum: The Influence of Crystal Structure on Ionization State. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef]
- Chan, K.L.A.; Fleming, O.S.; Kazarian, S.G.; Vassou, D.; Chryssikos, G.D.; Gionis, V. Polymorphism and devitrification of nifedipine under controlled humidity: A combined FT-Raman, IR and Raman microscopic investigation. J. Raman Spectrosc. 2004, 35, 353–359. [Google Scholar] [CrossRef]
- González-González, J.S.; Zúñiga-Lemus, O.; Hernández-Galindo, M.C. Hydrated Solid Forms of Theophylline and Caffeine Obtained by Mechanochemistry. IOSR J. Pharm. 2017, 7, 28–30. [Google Scholar] [CrossRef]
- Grdadolnik, J. ATR-FTIR Spectroscopy: Its Advantages and Limitations. Acta Chim. Slov. 2002, 49, 631–642. [Google Scholar]
- Mukherjee, A.; Tothadi, S.; Chakraborty, S.; Ganguly, S.; Desiraju, G.R. Synthon identification in co-crystals and polymorphs with IR spectroscopy. Primary amides as a case study. CrystEngComm 2013, 15, 4640–4654. [Google Scholar] [CrossRef]
- Vogt, F.G.; Clawson, J.S.; Strohmeier, M.; Edwards, A.J.; Pham, T.N.; Watson, S.A. Solid-state NMR analysis of organic cocrystals and complexes. Cryst. Growth Des. 2009, 9, 921–937. [Google Scholar] [CrossRef]
- Li, M.; Xu, W.; Su, Y. Solid-state NMR spectroscopy in pharmaceutical sciences. Trends Anal. Chem. 2021, 135, 116152. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- González-González, J.S.; Martínez-Martínez, F.J.; García-Báez, E.V.; Cruz, A.; Morín-Sánchez, L.M.; Rojas-Lima, S.; Padilla-Martínez, I.I. Molecular complexes of diethyl N, N′-1, 3-phenyldioxalamate and resorcinols: Conformational switching through intramolecular three-centered hydrogen-bonding. Cryst. Growth Des. 2014, 14, 628–642. [Google Scholar] [CrossRef]
- Saucedo-Balderas, M.M.; Delgado-Alfaro, R.A.; Martínez-Martínez, F.J.; Ortegón-Reyna, D.; Bernabé-Pineda, M.; Zúñiga-Lemus, O.; González-González, J.S. Synthesis, Molecular Structure of Diethyl Phenylenebis (Methylene) Dicarbamates and FTIR Spectroscopy Molecular Recognition Study with Benzenediols. J. Braz. Chem. Soc. 2015, 26, 396–402. [Google Scholar] [CrossRef]
- Seton, L.; Khamar, D.; Bradshaw, I.J.; Hutcheon, G.A. Solid state forms of theophylline: Presenting a new anhydrous polymorph. Cryst. Growth Des. 2010, 10, 3879–3886. [Google Scholar] [CrossRef]
- 1,2-Dihydroxybenzene. Available online: https://www.sigmaaldrich.com/catalog/product/sial/135011?lang=es®ion=MX&cm_sp=Insite-_-caSrpResults_srpRecs_srpModel_catechol-_-srpRecs3-1 (accessed on 27 February 2021).
- Theophylline. Available online: https://www.sigmaaldrich.com/catalog/product/sigma/t1633?lang=es®ion=MX (accessed on 27 February 2021).
- Finkelstein-Shapiro, D.; Davidowski, S.K.; Lee, P.B.; Guo, C.; Holland, G.P.; Rajh, T.; Kimberly, A.; Gray, K.A.; Yarger, J.L.; Calatayud, M. Direct evidence of chelated geometry of catechol on TiO2 by a combined solid-state NMR and DFT study. J. Phys. Chem. C 2016, 120, 23625–23630. [Google Scholar] [CrossRef]
- Du, Y.; Fang, X.; Zhang, Q.; Zhang, H.L.; Hong, Z. Spectroscopic investigation on cocrystal formation between adenine and fumaric acid based on infrared and Raman techniques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 580–585. [Google Scholar] [CrossRef]
- Bruni, G.; Maietta, M.; Berbenni, V.; Mustarelli, P.; Ferrara, C.; Freccero, M.; Grande, V.; Maggi, L.; Milanese, C.; Girella, A.; et al. Mechanochemical Synthesis of Bumetanide-4-Aminobenzoic Acid Molecular Cocrystals: A Facile and Green Approach to Drug Optimization. J. Phys. Chem. B 2014, 118, 9180–9190. [Google Scholar] [CrossRef]
- Srivastava, K.; Shimpi, M.R.; Srivastava, A.; Tandon, P.; Sinha, K.; Velaga, S.P. Vibrational analysis and chemical activity of paracetamol–oxalic acid cocrystal based on monomer and dimer calculations: DFT and AIM approach. RSC Adv. 2016, 6, 10024–10037. [Google Scholar] [CrossRef]
- Majerz, I. Directionality of Inter- and Intramolecular OHO Hydrogen Bonds: DFT Study Followed by AIM and NBO Analysis. J. Phys. Chem. A 2012, 116, 7992–8000. [Google Scholar] [CrossRef]
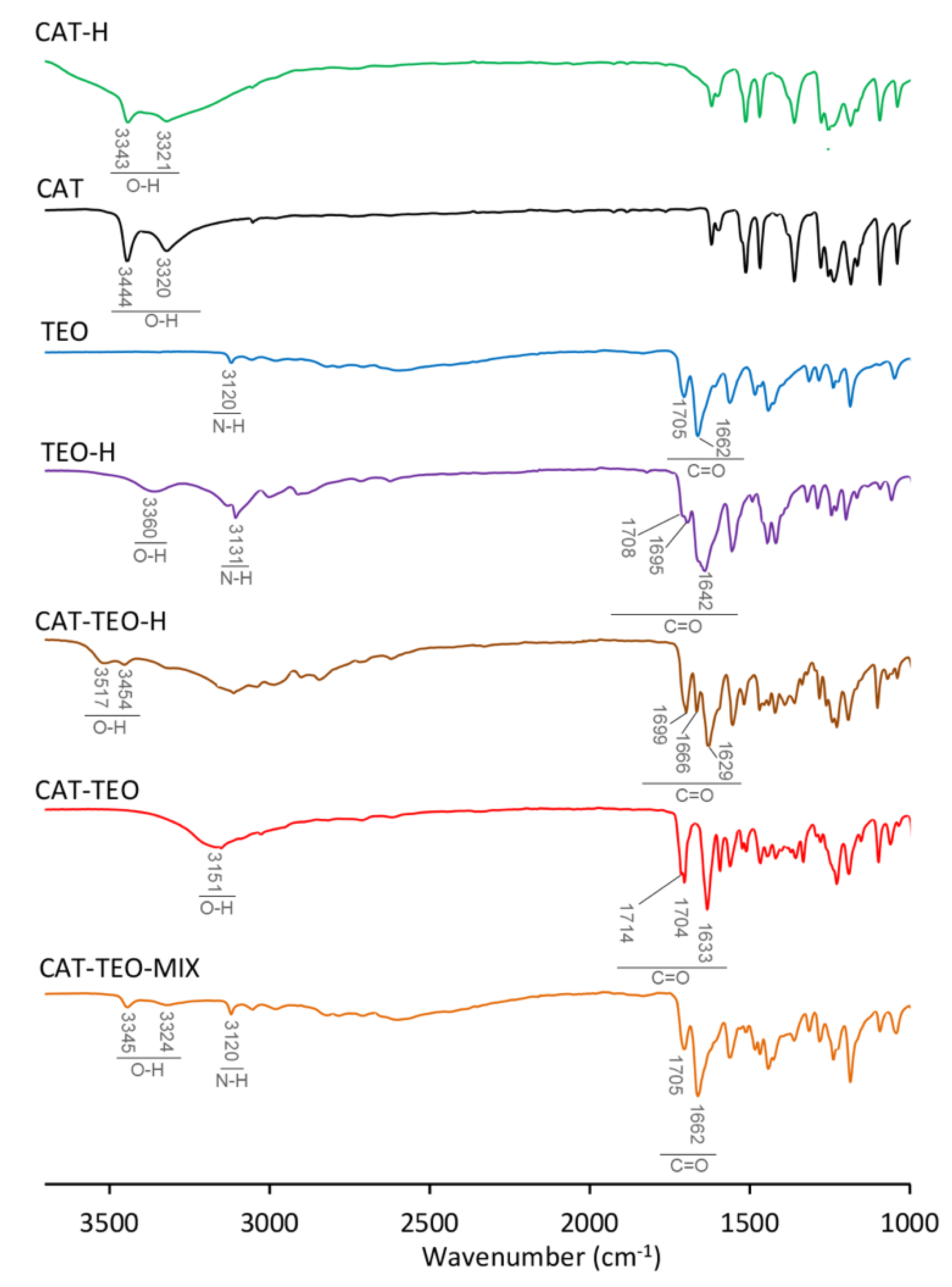


| νO-H | Δ(νO-H) | νC=O | Δ(νC=O) | νN-H | Δ(νN-H) | |
|---|---|---|---|---|---|---|
| CAT-H | 3443, 3321 | ----- | ----- | ----- | ----- | ----- |
| CAT | 3444, 3320 | ----- | ----- | ----- | ----- | ----- |
| TEO | ----- | ----- | 1705, 1662 | ----- | 3120 | ----- |
| TEO-H | 3360 | ----- | 1708, 1695, 1642 | 3, −10, −20 | 3131 | 11 |
| CAT-TEO-H | 3517, 3454 | 73, 314 | 1699, 1666, 1629 | −6, 4, −33 | N.O. | ----- |
| CAT-TEO | 3151 | −293, −169 | 1714, 1704, 1633 | 9, −1 *, −29 | N.O. | ----- |
| CAT-TEO-MIX | 3345, 3324 | ----- | 1705, 1662 | ----- | 3120 | ----- |
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | |
|---|---|---|---|---|---|---|---|
| TEO | 154.9 | 150.9 | 146.3 | 140.5 | 105.8 | 30.0 | 30.0 |
| CAT-TEO | 154.5 | 154.5 | 144.2 | 140.2 | 108.3 | 31.9 | 27.6 |
| C11 | C12 | C13 | C14 | C15 | C16 | ||
| CAT | 142.9 | 142.9 | 114.6 | 121.5 | 121.5 | 114.6 | |
| CAT-TEO | 144.7 | 144.7 | 114.7 112.4 | 119.8 | 119.8 | 114.7 112.4 | |
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | |
|---|---|---|---|---|---|---|---|
| CAT-TEO (δexp) | 154.5 | 154.5 | 144.2 | 140.2 | 108.3 | 31.9 | 27.6 |
| CAT-TEO1 (δcalc) σiso | 154.6 (45.3) | 155.3 (44.6) | 150.2 (49.7) | 138.2 (61.7) | 114.0 (85.9) | 38.9 (161.0) | 35.7 (164.1) |
| CAT-TEO2 (δcalc) σiso | 157.5 (42.4) | 151.9 (48.0) | 151.9 (48.0) | 139.7 (60.2) | 113.4 (86.5) | 37.2 (162.7) | 36.7 (163.1) |
| C11 | C12 | C13 | C14 | C15 | C16 | ||
| CAT-TEO (δexp) | 144.7 | 144.7 | 114.7 112.4 | 119.8 | 119.8 | 114.7 112.4 | |
| CAT-TEO1 (δcalc) σiso | 148.8 (51.1) | 147 (52.9) | 116 (83.9) | 121.3 (78.6) | 122.9 (77.0) | 117.4 (82.4) | |
| CAT-TEO2 (δcalc) σiso | 146.6 (53.3) | 146.5 (53.4) | 116.7 (83.2) | 122.8 (77.1) | 122.9 (76.0) | 119.3 (80.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-González, J.S.; Jiménez-López, R.; Ortegón-Reyna, D.; Gonzalez-Carrillo, G.; Martínez-Martínez, F.J. Mechanochemical Synthesis of the Catechol-Theophylline Cocrystal: Spectroscopic Characterization and Molecular Structure. Appl. Sci. 2021, 11, 3810. https://doi.org/10.3390/app11093810
González-González JS, Jiménez-López R, Ortegón-Reyna D, Gonzalez-Carrillo G, Martínez-Martínez FJ. Mechanochemical Synthesis of the Catechol-Theophylline Cocrystal: Spectroscopic Characterization and Molecular Structure. Applied Sciences. 2021; 11(9):3810. https://doi.org/10.3390/app11093810
Chicago/Turabian StyleGonzález-González, Juan Saulo, Raquel Jiménez-López, David Ortegón-Reyna, Gabino Gonzalez-Carrillo, and Francisco Javier Martínez-Martínez. 2021. "Mechanochemical Synthesis of the Catechol-Theophylline Cocrystal: Spectroscopic Characterization and Molecular Structure" Applied Sciences 11, no. 9: 3810. https://doi.org/10.3390/app11093810
APA StyleGonzález-González, J. S., Jiménez-López, R., Ortegón-Reyna, D., Gonzalez-Carrillo, G., & Martínez-Martínez, F. J. (2021). Mechanochemical Synthesis of the Catechol-Theophylline Cocrystal: Spectroscopic Characterization and Molecular Structure. Applied Sciences, 11(9), 3810. https://doi.org/10.3390/app11093810









