Application of Sludge-Based Activated Carbons for the Effective Adsorption of Neonicotinoid Pesticides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Carbon Materials
2.3. Characterization of the Sludge
2.4. Characterization of the Activated Carbons
2.5. Batch Adsorption Studies
2.6. Analytical Procedure
3. Results and Discussion
3.1. Characterization of the Sludge
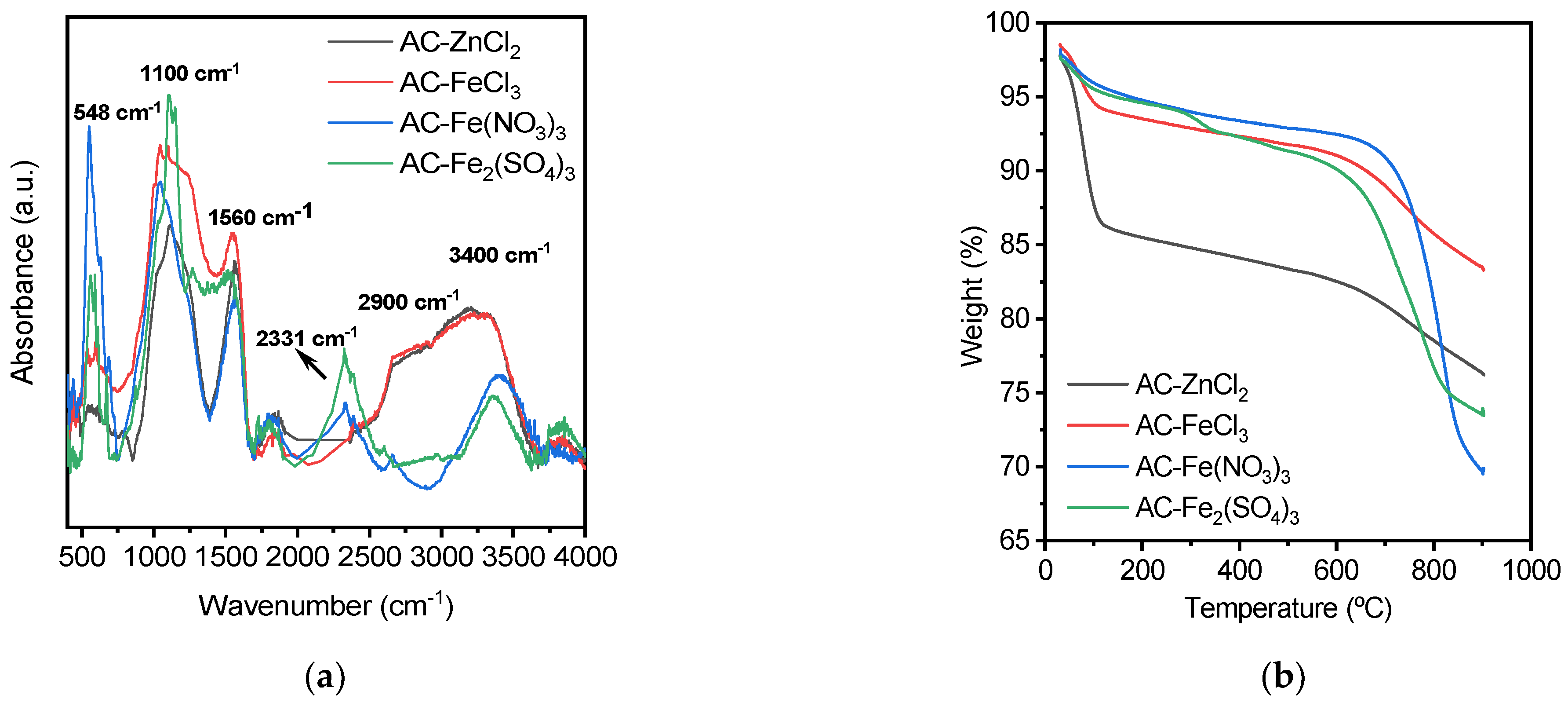
3.2. Characterization of the Activated Carbons
3.3. Pesticide Adsorption Studies
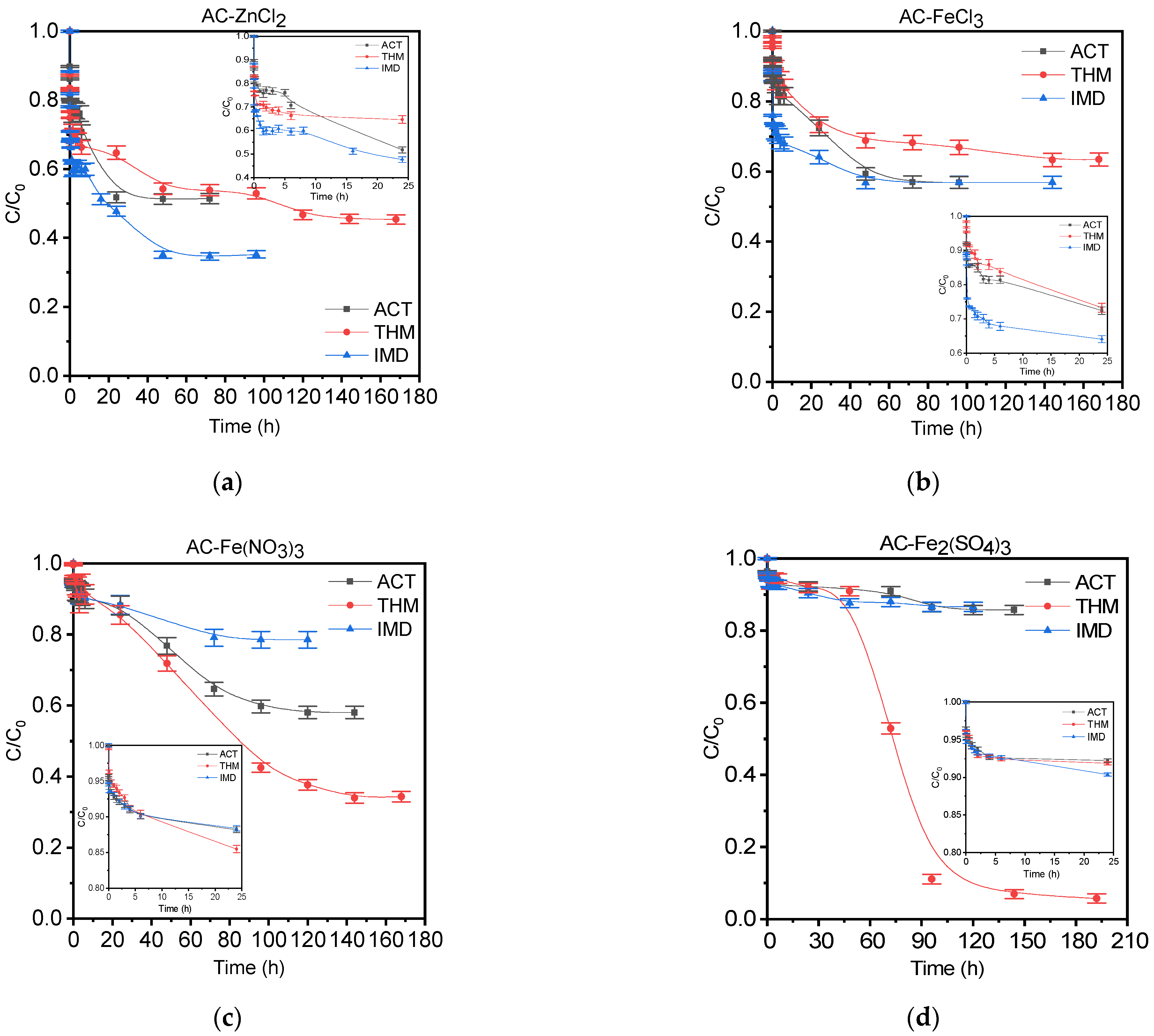
3.3.1. Kinetic Adsorption Studies
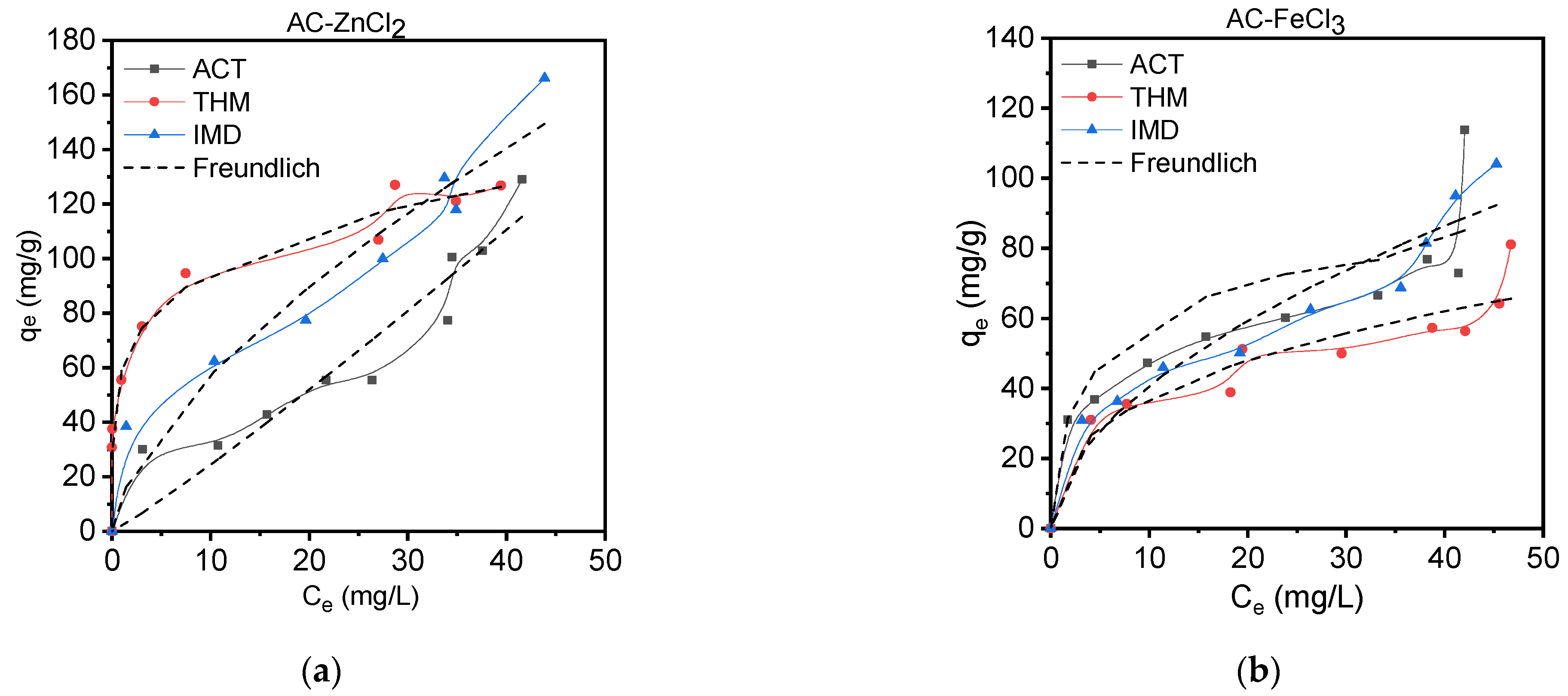
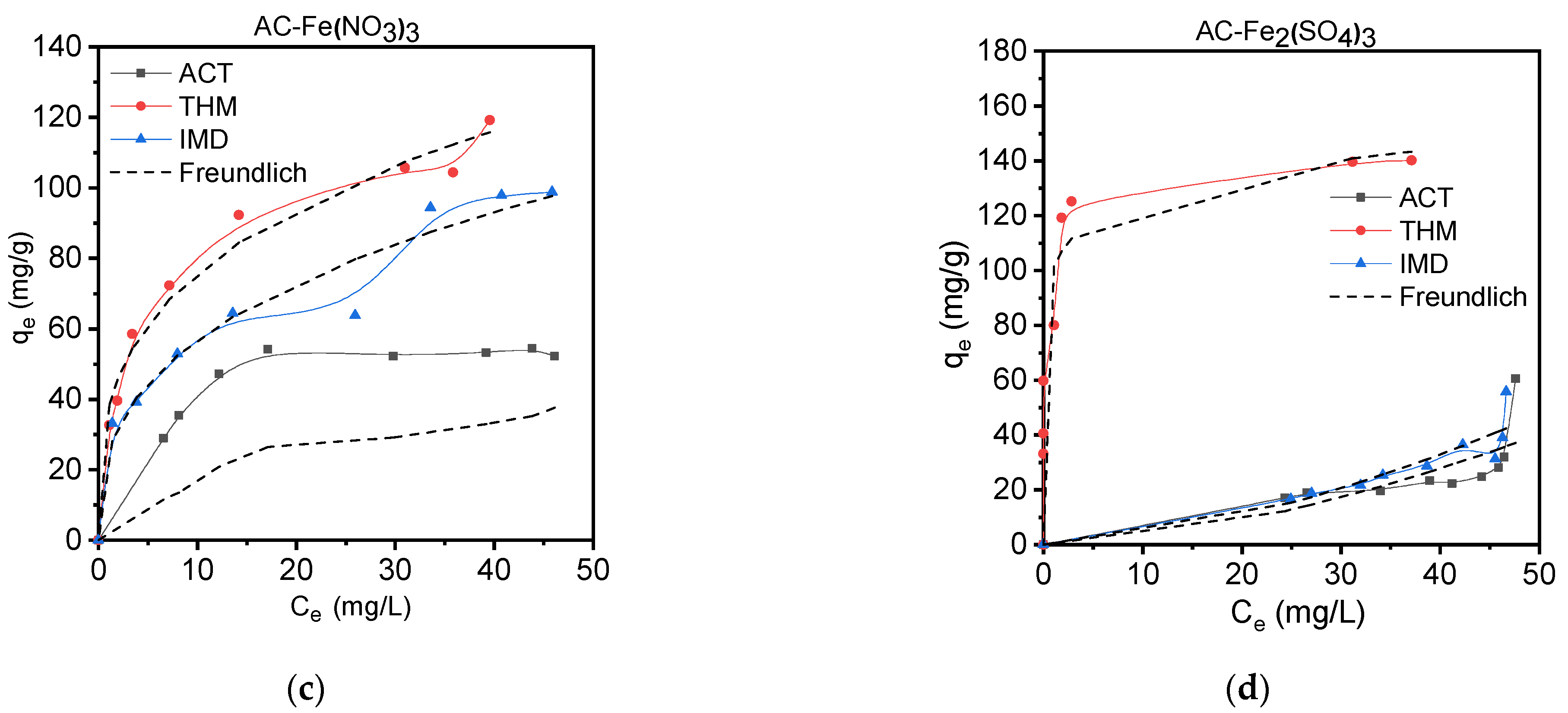
3.3.2. Isotherm Adsorption Studies
3.4. Comparison of Pesticide Adsorption Capacity with That of Other Adsorbents Derived from Biomass Sources
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Xu, G.; Yu, H.; Zhang, Z. Preparation of ferric-activated sludge-based adsorbent from biological sludge for tetracycline removal. Bioresour. Technol. 2016, 211, 566–573. [Google Scholar] [CrossRef]
- Xu, G.; Yang, X.; Spinosa, L. Development of sludge-based adsorbents: Preparation, characterization, utilization and its feasibility assessment. J. Environ. Manag. 2015, 151, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Yue, Q.Y.; Gao, B.Y.; Wang, X.J.; Qi, Y.F.; Zhao, Y.Q.; Li, Y.J. Preparation of sludge-based activated carbon made from paper mill sewage sludge by steam activation for dye wastewater treatment. Desalination 2011, 278, 179–185. [Google Scholar] [CrossRef]
- Samolada, M.C.; Zabaniotou, A.A. Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece. Waste Manag. 2014, 34, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Méndez, A.; Gascó, G.; Freitas, M.M.A.; Siebielec, G.; Stuczynski, T.; Figueiredo, J.L. Preparation of carbon-based adsorbents from pyrolysis and air activation of sewage sludges. Chem. Eng. J. 2005, 108, 169–177. [Google Scholar] [CrossRef]
- Ding, T.; Huang, T.; Wu, Z.; Li, W.; Guo, K.; Li, J. Adsorption-desorption behavior of carbendazim by sewage sludge-derived biochar and its possible mechanism. RSC Adv. 2019, 9, 35209–35216. [Google Scholar] [CrossRef]
- Liu, C.; Tang, Z.; Chen, Y.; Su, S.; Jiang, W. Characterization of mesoporous activated carbons prepared by pyrolysis of sewage sludge with pyrolusite. Bioresour. Technol. 2010, 101, 1097–1101. [Google Scholar] [CrossRef]
- Björklund, K.; Li, L.Y. Adsorption of organic stormwater pollutants onto activated carbon from sewage sludge. J. Environ. Manag. 2017, 197, 490–497. [Google Scholar] [CrossRef]
- Khoshbouy, R.; Takahashi, F.; Yoshikawa, K. Preparation of high surface area sludge-based activated hydrochar via hydrothermal carbonization and application in the removal of basic dye. Environ. Res. 2019, 175, 457–467. [Google Scholar] [CrossRef]
- Jaria, G.; Silva, C.P.; Ferreira, C.I.A.; Otero, M.; Calisto, V. Sludge from paper mill effluent treatment as raw material to produce carbon adsorbents: An alternative waste management strategy. J. Environ. Manag. 2017, 188, 203–211. [Google Scholar] [CrossRef]
- Wong, S.; Ngadi, N.; Inuwa, I.M.; Hassan, O. Recent advances in applications of activated carbon from biowaste for wastewater treatment: A short review. J. Clean. Prod. 2018, 175, 361–375. [Google Scholar] [CrossRef]
- Singh, S.; Singh, H. Development of economical adsorbent from bio-waste precursor: Waste as a resource for waste water treatment. J. Gujarat Res. Soc. 2019, 21, 391–411. [Google Scholar]
- Devi, P.; Saroha, A.K. Utilization of sludge based adsorbents for the removal of various pollutants: A review. Sci. Total Environ. 2017, 578, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Sandoval, S.J.; Pastrana-Martínez, L.M.; Ocampo-Pérez, R.; Morales-Torres, S.; Berber-Mendoza, M.S.; Carrasco-Marín, F. Synthesis and characterization of carbon xerogel/graphene hybrids as adsorbents for metronidazole pharmaceutical removal: Effect of operating parameters. Sep. Purif. Technol. 2020, 237, 116341. [Google Scholar] [CrossRef]
- Vella, K. Commission Implementing Decision (EU) 2018/840 of 5 June 2018 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council and Repealing Comm. Off. J. Eur. Union 2018, 141, 9–12. [Google Scholar]
- Pietrzak, D.; Kania, J.; Malina, G.; Kmiecik, E.; Wątor, K. Pesticides from the EU First and Second Watch Lists in the Water Environment. Clean Soil Air Water 2019, 47, 1800376. [Google Scholar] [CrossRef]
- Serrano, E.; Munoz, M.; de Pedro, Z.M.; Casas, J.A. Fast oxidation of the neonicotinoid pesticides listed in the EU Decision 2018/840 from aqueous solutions. Sep. Purif. Technol. 2020, 235, 116168. [Google Scholar] [CrossRef]
- Jung, C.; Oh, J.; Yoon, Y. Removal of acetaminophen and naproxen by combined coagulation and adsorption using biochar: Influence of combined sewer overflow components. Environ. Sci. Pollut. Res. 2015, 22, 10058–10069. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Catalytic ozonation for the removal of organic contaminants in water on alumina. Appl. Catal. B Environ. 2015, 165, 408–418. [Google Scholar] [CrossRef]
- Akkari, M.; Aranda, P.; Belver, C.; Bedia, J.; Ben Haj Amara, A.; Ruiz-Hitzky, E. Reprint of ZnO/Sepiolite heterostructured materials for solar photocatalytic degradation of pharmaceuticals in wastewater. Appl. Clay Sci. 2018, 160, 3–8. [Google Scholar] [CrossRef]
- Serra-Pérez, E.; Ferronato, C.; Giroir-Fendler, A.; Álvarez-Torrellas, S.; Ovejero, G.; García, J. Highly Efficient Ru Supported on Carbon Nanosphere Nanoparticles for Ciprofloxacin Removal: Effects of Operating Parameters, Degradation Pathways, and Kinetic Study. Ind. Eng. Chem. Res. 2020, 59, 15515–15530. [Google Scholar] [CrossRef]
- Molina, C.B.; Sanz-Santos, E.; Boukhemkhem, A.; Bedia, J.; Belver, C.; Rodriguez, J.J. Removal of emerging pollutants in aqueous phase by heterogeneous Fenton and photo-Fenton with Fe2O3-TiO2-clay heterostructures. Environ. Sci. Pollut. Res. 2020, 27, 38434–38445. [Google Scholar] [CrossRef]
- Hernández-Abreu, A.B.; Álvarez-Torrellas, S.; Águeda, V.I.; Larriba, M.; Delgado, J.A.; Calvo, P.A.; García, J. New insights from modelling and estimation of mass transfer parameters in fixed-bed adsorption of Bisphenol A onto carbon materials. J. Contam. Hydrol. 2020, 228, 103566. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Munoz, M.; Gläsel, J.; de Pedro, Z.M.; Domínguez, C.M.; García, J.; Etzold, B.J.M.; Casas, J.A. Highly efficient removal of pharmaceuticals from water by well-defined carbide-derived carbons. Chem. Eng. J. 2018, 347, 595–606. [Google Scholar] [CrossRef]
- Spaltro, A.; Pila, M.; Simonetti, S.; Álvarez-Torrellas, S.; García Rodríguez, J.; Ruiz, D.; Díaz Compañy, A.; Juan, A.; Allegretti, P. Adsorption and removal of phenoxy acetic herbicides from water by using commercial activated carbons: Experimental and computational studies. J. Contam. Hydrol. 2018, 218, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Gong, X. Modification and Utilization of Sewage Sludge-Based Activated Carbon as Metal Adsorbents. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2013; p. 152. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. 2540 B. Total Solids Dried at 103–105 °C & 5220 B. Open Reflux Method. Stand. Method Exam. Water Wastewater 2017, 23, 185–587. [Google Scholar]
- Velázquez, A.; Bolaños, E.; Pliego, Y.S. Optimización de la producción de carbón activado a partir de bambú. Rev. Mex. Ing. Química 2010, 9, 359–366. [Google Scholar]
- Zou, J.; Dai, Y.; Wang, X.; Ren, Z.; Tian, C.; Pan, K.; Li, S.; Abuobeidah, M.; Fu, H. Structure and adsorption properties of sewage sludge-derived carbon with removal of inorganic impurities and high porosity. Bioresour. Technol. 2013, 142, 209–217. [Google Scholar] [CrossRef]
- Pradhananga, R.; Adhikari, L.; Shrestha, R.; Adhikari, M.; Rajbhandari, R.; Ariga, K.; Shrestha, L. Wool carpet dye adsorption on nanoporous carbon materials derived from agro-product. C J. Carbon Res. 2017, 3, 12. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.S.; Wu, J. Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 2010, 89, 510–514. [Google Scholar] [CrossRef]
- Rojas-Morales, J.L.; Gutiérrez-González, E.C.; de Jesús Colina-Andrade, G. Obtención y caracterización de carbón activado obtenido de lodos de plantas de tratamiento de agua residual de una industria avícola. Ing. Investig. Tecnol. 2016, 17, 453–462. [Google Scholar] [CrossRef]
- Liu, L.; Lin, Y.; Liu, Y.; Zhu, H.; He, Q. Removal of Methylene Blue from aqueous solutions by sewage sludge based granular activated carbon: Adsorption equilibrium, kinetics, and thermodynamics. J. Chem. Eng. Data 2013, 58, 2248–2253. [Google Scholar] [CrossRef]
- Bagreev, A.; Bandosz, T.J.; Locke, D.C. Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage sludge-derived fertilizer. Carbon 2001, 39, 1971–1979. [Google Scholar] [CrossRef]
- Smith, K.M.; Fowler, G.D.; Pullket, S.; Graham, N.J.D. Sewage sludge-based adsorbents: A review of their production, properties and use in water treatment applications. Water Res. 2009, 43, 2569–2594. [Google Scholar] [CrossRef] [PubMed]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. Review on activated carbons by chemical activation with FeCl3. C J. Carbon Res. 2020, 6, 21. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Zhang, Y.; Liu, S.; Wang, C.; Chen, W.; Liu, C.; Chen, Z.; Zhang, Y. ZnCl2 modified biochar derived from aerobic granular sludge for developed microporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2020, 297, 122381. [Google Scholar] [CrossRef]
- Sumalinog, D.A.G.; Capareda, S.C.; de Luna, M.D.G. Evaluation of the effectiveness and mechanisms of acetaminophen and Methylene Blue dye adsorption on activated biochar derived from municipal solid wastes. J. Environ. Manag. 2018, 210, 255–262. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 846, 3973–3993. [Google Scholar] [CrossRef]
- Martins Moreira, W.; Viotti, P.V.; Gurgel Adeodato Vieira, M.; dos Santos Gaudêncio Baptista, C.M.; Neves Olsen Scaliante, M.H.; Gimenes, M.L. Hydrothermal synthesis of biobased carbonaceous composite from a blend of Kraft black liquor and tannin and its application to aspirin and paracetamol removal. Colloid. Surf. A 2021, 608, 125597. [Google Scholar] [CrossRef]
- Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E. Adsorption Processes for Water Treatment and Purification; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Álvarez-Torrellas, S.; Peres, J.A.; Gil-Álvarez, V.; Ovejero, G.; García, J. Effective adsorption of non-biodegradable pharmaceuticals from hospital wastewater with different carbon materials. Chem. Eng. J. 2017, 320, 319–329. [Google Scholar] [CrossRef]
- Saleh, T.A.; Danmaliki, G.I. Adsorptive desulfurization of dibenzothiophene from fuels by rubber tyres-derived carbons: Kinetics and isotherms evaluation. Process Saf. Environ. 2016, 102, 9–19. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydin, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, T.; Yuan, Z.; Zhang, D.; Sun, Z.; Huang, Y.X.; Chen, W.; Tian, D.; Deng, H.; Zhou, Y. Fabrication of cotton textile waste-based magnetic activated carbon using FeCl3 activation by the Box-Behnken design: Optimization and characteristics. RSC Adv. 2018, 8, 38081–38090. [Google Scholar] [CrossRef]
- Ayawei, N.; Seimokumo, S.A.; Wankasi, D.; Dikio, E.D. Synthesis, Characterization and Application of Mg/Al Layered Double Hydroxide for the Degradation of Congo Red in Aqueous Solution. Open J. Phys. Chem. 2015, 5, 56–70. [Google Scholar] [CrossRef]
- Urbain, K.Y.; Fodjo, E.K.; Ardjouma, D.; Serge, B.Y.; Aimé, E.S.; Irié Marc, G.B.; Albert, T. Removal of Imidacloprid Using Activated Carbon Produced from Ricinodendron Heudelotii Shells. Bull. Chem. Soc. Ethiop. 2017, 31, 397–409. [Google Scholar] [CrossRef]
- Mohammad, S.G.; El-Sayed, M.M.H. Removal of Imidacloprid Pesticide Using Nanoporous Activated Carbons Produced via Pyrolysis of Peach Stone Agricultural Wastes. Chem. Eng. Commun. 2020, 1–12. [Google Scholar] [CrossRef]
- Mohammad, S.G.; Ahmed, S.M.; Amr, A.E.G.E.; Kamel, A.H. Porous Activated Carbon from Lignocellulosic Agricultural Waste for the Removal of Acetamiprid Pesticide from Aqueous Solutions. Molecules 2020, 25, 2339. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, X.; Xu, J.; Zhang, Q. Removal of the Pesticide Imidacloprid from Aqueous Solution by Biochar Derived from Peanut Shell. BioResources 2019, 13, 5656–5669. [Google Scholar]
- Ma, Y.; Qi, Y.; Yang, L.; Wu, L.; Li, P.; Gao, F.; Qi, X.; Zhang, Z. Adsorptive Removal of Imidacloprid by Potassium Hydroxide Activated Magnetic Sugarcane Bagasse Biochar: Adsorption Efficiency, Mechanism and Regeneration. J. Clean. Prod. 2021, 292, 126005. [Google Scholar] [CrossRef]

| Elemental Analysis (%) | |||||||||||||||||
| C | O | H | N | P | S | Cl | |||||||||||
| 47.89 | 6.84 | 6.62 | 3.38 | 1.18 | 0.49 | 1.13 | |||||||||||
| Metal Content (%) | |||||||||||||||||
| Na | Mg | Al | Si | K | Ca | Ti | Mn | Fe | Cu | Zn | Ni | ||||||
| 0.15 | 0.02 | 0.38 | 0.17 | 0.49 | 7.25 | 0.02 | 0.01 | 0.19 | 0.01 | 0.02 | 0.03 | ||||||
| Total solids (g/L) | 56.76 | ||||||||||||||||
| Volatile solids (g/L) | 46.24 | ||||||||||||||||
| Fixed solids (g/L) | 10.52 | ||||||||||||||||
| Chemical oxygen demand (COD) (g O2/L) | 55.36 | ||||||||||||||||
| Adsorbent | SBET (m2/g) | Sext (m2/g) | VTotal (cm3/g) | VMicro (cm3/g) | VMicro/VTotal |
|---|---|---|---|---|---|
| AC-ZnCl2 | 558 | 145 | 0.35 | 0.15 | 0.43 |
| AC-FeCl3 | 468 | 170 | 0.56 | 0.16 | 0.29 |
| AC-Fe(NO3)3 | 240 | 158 | 0.32 | 0.04 | 0.13 |
| AC-Fe2(SO4)3 | 233 | 203 | 0.48 | 0.01 | 0.02 |
| Adsorbent | C (wt.%) | H (wt.%) | N (wt.%) | S (wt.%) | pHIEP |
|---|---|---|---|---|---|
| AC-ZnCl2 | 55.60 | 2.55 | 4.00 | 1.82 | 2.14 |
| AC-FeCl3 | 59.77 | 2.36 | 3.36 | 1.35 | 5.40 |
| AC-Fe(NO3)3 | 33.51 | 0.99 | 2.05 | 0.66 | 6.81 |
| AC-Fe2(SO4)3 | 31.71 | 1.05 | 1.74 | 13.15 | 3.08 |
| Pseudo-First-Order Model | Pseudo-Second-Order Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Adsorbent | Pesticide | qexp (mg/g) | qteor (mg/g) | k1 (h−1) | R2 | qteor (mg/g) | k2 × 1−2 (g/mg∙h) | R2 |
| AC-ZnCl2 | ACT | 84.3 | 84.2 | 0.28 | 0.870 | 78.8 | 0.71 | 0.867 |
| THM | 96.9 | 96.9 | 1.39 | 0.804 | 96.7 | 3.50 | 0.840 | |
| IMD | 105.7 | 105.7 | 1.37 | 0.824 | 105.0 | 2.95 | 0.861 | |
| AC-FeCl3 | ACT | 70.1 | 70.0 | 0.17 | 0.929 | 65.0 | 0.39 | 0.931 |
| THM | 66.1 | 66.1 | 0.16 | 0.968 | 93.8 | 1.08 | 0.863 | |
| IMD | 75.9 | 64.3 | 0.02 | 0.943 | 59.0 | 70.2 | 0.963 | |
| AC-Fe(NO3)3 | ACT | 66.4 | 60.4 | 0.02 | 0.967 | 55.6 | 0.80 | 0.941 |
| THM | 135.0 | 124.5 | 0.02 | 0.984 | 111.9 | 0.02 | 0.964 | |
| IMD | 35.4 | 35.4 | 0.13 | 0.887 | 33.0 | 0.55 | 0.899 | |
| AC-Fe2(SO4)3 | ACT | 23.3 | 23.3 | 0.28 | 0.853 | 39.8 | 29.47 | 0.933 |
| THM | 194.8 | 156.1 | 0.01 | 0.912 | 138.7 | 0.01 | 0.904 | |
| IMD | 21.5 | 21.5 | 0.26 | 0.888 | 64.4 | 2.55 | 0.946 | |
| Langmuir | Freundlich | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Pesticide | qexp (mg/g) | qteor (mg/g) | qsat × 10−5 (mg/g) | b (L/mg) | R2 | qteor (mg/g) | KF (L/g) | nf | R2 |
| AC-ZnCl2 | ACT | 128.9 | 112.9 | 8.86 | 3.1 | 0.955 | 115.3 | 1.98 | 0.9 | 0.959 |
| THM | 126.8 | 149.5 | 8.99 | 4.2 | 0.875 | 126.3 | 59.16 | 4.9 | 0.978 | |
| IMD | 166.1 | 164.6 | 8.85 | 4.2 | 0.975 | 149.4 | 12.82 | 1.5 | 0.976 | |
| AC-FeCl3 | ACT | 113.9 | 95.9 | 8.85 | 2.6 | 0.889 | 85.1 | 16.24 | 2.3 | 0.922 |
| THM | 81.1 | 82.2 | 8.85 | 1.7 | 0.815 | 65.6 | 15.81 | 2.7 | 0.950 | |
| IMD | 104.1 | 103.6 | 8.85 | 2.6 | 0.960 | 92.3 | 11.71 | 1.9 | 0.969 | |
| AC-Fe(NO3)3 | ACT | 60.6 | 34.2 | 8.87 | 0.8 | 0.768 | 37.6 | 0.04 | 0.6 | 0.793 |
| THM | 140.2 | 160.5 | 8.99 | 4.8 | 0.679 | 143.3 | 100.95 | 10.3 | 0.931 | |
| IMD | 55.8 | 39.0 | 8.85 | 0.9 | 0.899 | 42.6 | 0.06 | 0.6 | 0.926 | |
| AC-Fe2(SO4)3 | ACT | 60.6 | 34.2 | 8.85 | 0.8 | 0.768 | 37.1 | 0.06 | 0.6 | 0.791 |
| THM | 140.2 | 160.5 | 8.99 | 4.8 | 0.678 | 143.3 | 100.95 | 10.3 | 0.931 | |
| IMD | 55.8 | 38.7 | 8.85 | 0.9 | 0.899 | 42.4 | 0.08 | 0.6 | 0.925 | |
| Sips | GAB | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Pesticide | qexp (mg/g) | qteor (mg/g) | qsat (mg/g) | b × 105 (L/mg) | n | R2 | qteor (mg/g) | qm × 10−5 (mg/g) | K1 × 106 (L/mg) | K2 × 106 (L/mg) | R2 |
| AC-ZnCl2 | ACT | 128.9 | 115.3 | 125,557 | 3.96 | 0.9 | 0.958 | 112.9 | 8.87 | 3.06 | 3.06 | 0.955 |
| THM | 126.8 | 124.9 | 1373 | 0.03 | 4.8 | 0.976 | 150.4 | 8.87 | 4.30 | 3.07 | 0.875 | |
| IMD | 166.1 | 149.3 | 50,683 | 0.29 | 1.5 | 0.972 | 164.6 | 8.87 | 4.23 | 3.07 | 0.972 | |
| AC-FeCl3 | ACT | 113.9 | 83.7 | 2538 | 0.52 | 2.4 | 0.866 | 95.9 | 8.87 | 2.57 | 3.06 | 0.894 |
| THM | 81.1 | 65.9 | 1927 | 0.31 | 2.6 | 0.949 | 75.7 | 8.87 | 1.83 | 3.06 | 0.898 | |
| IMD | 104.1 | 91.8 | 1360 | 20.30 | 1.7 | 0.968 | 103.6 | 8.87 | 2.58 | 3.06 | 0.960 | |
| AC-Fe(NO3)3 | ACT | 60.5 | 37.2 | 243,432 | 9.57 | 0.6 | 0.791 | 34,.2 | 8.87 | 0.81 | 3.06 | 0.768 |
| THM | 140.2 | 159.5 | 1378 | 0.12 | 4.8 | 0.929 | 160.5 | 8.87 | 4.88 | 3.07 | 0.679 | |
| IMD | 55.8 | 42.7 | 285,175 | 11.70 | 0.5 | 0.925 | 39.0 | 8.87 | 0.94 | 3.06 | 0.899 | |
| AC-Fe2(SO4)3 | ACT | 60.5 | 37.9 | 3740 | 169.1 | 0.5 | 0.793 | 34.2 | 8.87 | 8.10 | 3.06 | 0.768 |
| THM | 140.2 | 139.1 | 139 | 108,617 | 0.4 | 0.950 | 160.5 | 8.87 | 4.88 | 3.07 | 0.678 | |
| IMD | 55.8 | 42.7 | 7231 | 104.4 | 0.5 | 0.926 | 38.7 | 886,713 | 0.93 | 3.06 | 0.899 | |
| Biomass Source | Pesticide | Adsorption Capacity (mg/g) | SBET (m2/g) | te (min) | Reference |
|---|---|---|---|---|---|
| Ricinodendron heudelotii shells | Imidacloprid | 43.48 | 1179 | 90 | [48] |
| Peach stone | Imidacloprid | 39.37 | 6 | 40 | [49] |
| Tangerine peels | Acetamiprid | 35.70 | 688 | 240 | [50] |
| Peanut shell | Imidacloprid | 8.68 | 535 | 240 | [51] |
| Sugarcane bagasse | Imidacloprid | 313.00 | 660 | 720 | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz-Santos, E.; Álvarez-Torrellas, S.; Ceballos, L.; Larriba, M.; Águeda, V.I.; García, J. Application of Sludge-Based Activated Carbons for the Effective Adsorption of Neonicotinoid Pesticides. Appl. Sci. 2021, 11, 3087. https://doi.org/10.3390/app11073087
Sanz-Santos E, Álvarez-Torrellas S, Ceballos L, Larriba M, Águeda VI, García J. Application of Sludge-Based Activated Carbons for the Effective Adsorption of Neonicotinoid Pesticides. Applied Sciences. 2021; 11(7):3087. https://doi.org/10.3390/app11073087
Chicago/Turabian StyleSanz-Santos, Eva, Silvia Álvarez-Torrellas, Lucía Ceballos, Marcos Larriba, V. Ismael Águeda, and Juan García. 2021. "Application of Sludge-Based Activated Carbons for the Effective Adsorption of Neonicotinoid Pesticides" Applied Sciences 11, no. 7: 3087. https://doi.org/10.3390/app11073087
APA StyleSanz-Santos, E., Álvarez-Torrellas, S., Ceballos, L., Larriba, M., Águeda, V. I., & García, J. (2021). Application of Sludge-Based Activated Carbons for the Effective Adsorption of Neonicotinoid Pesticides. Applied Sciences, 11(7), 3087. https://doi.org/10.3390/app11073087









