Human Laryngeal Mucus from the Vocal Folds: Rheological Characterization by Particle Tracking Microrheology and Oscillatory Shear Rheology
Abstract
1. Introduction
- Human laryngeal mucus from the vocal folds is viscoelastic and reveals gel characteristics, as mucus of another origin.
- Variations of the viscoelasticity of the investigated mucus samples can be correlated with the demographic data.
- Bulk rheology of human laryngeal mucus can be assessed by PTM with microspheres of appropriate diameter. The measurement results gained by PTM are comparable with OSR, the second applied measurement technique.
2. Materials and Methods
2.1. Mucus Samples
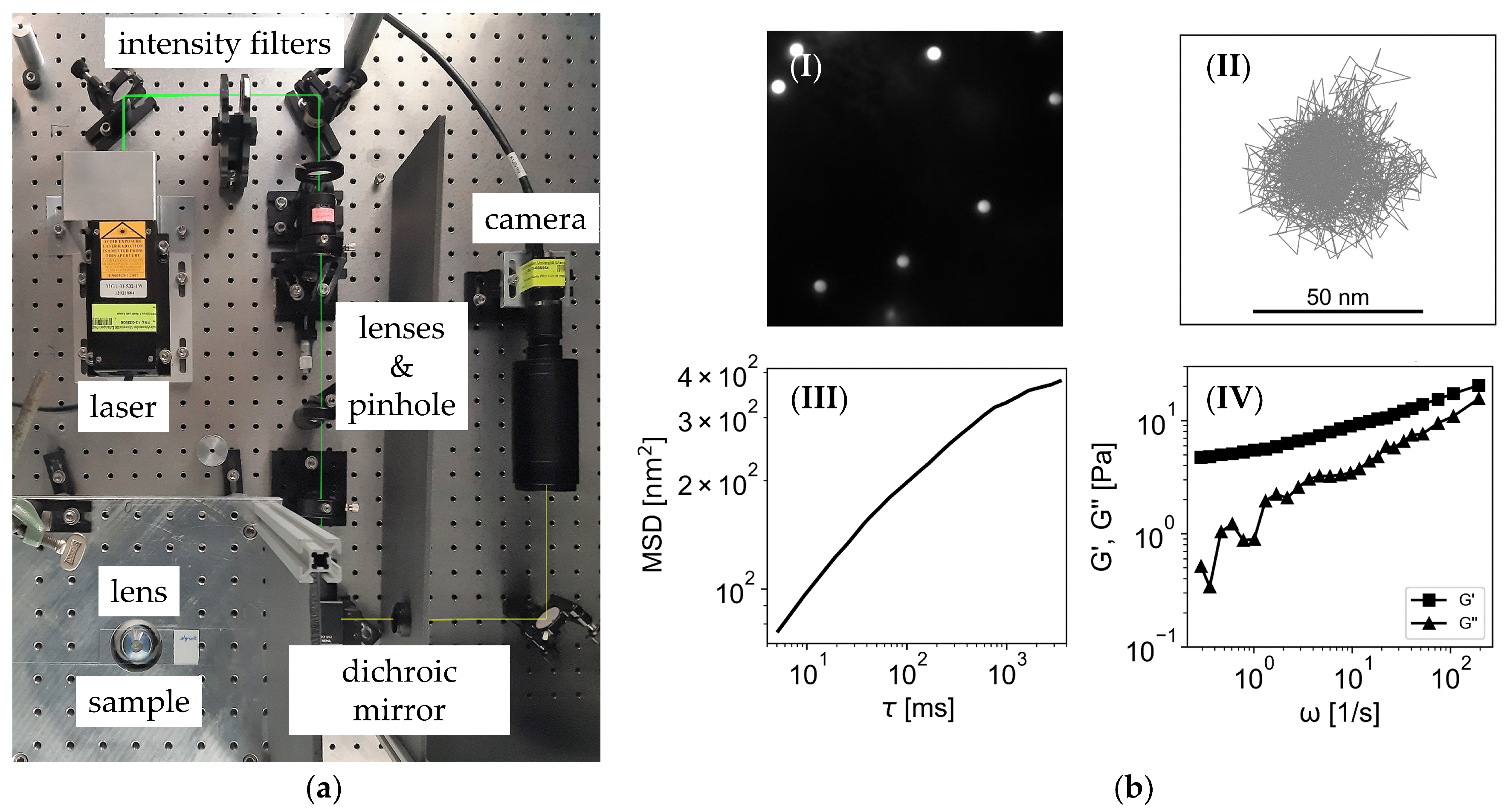
2.2. Particle Tracking Microrheology
2.3. Oscillatory Shear Rheology
2.4. Statistical Analysis
3. Results and Discussion
3.1. Mucus Samples
3.2. Viscoelastic Properties
3.2.1. Particle Tracking Microrheology
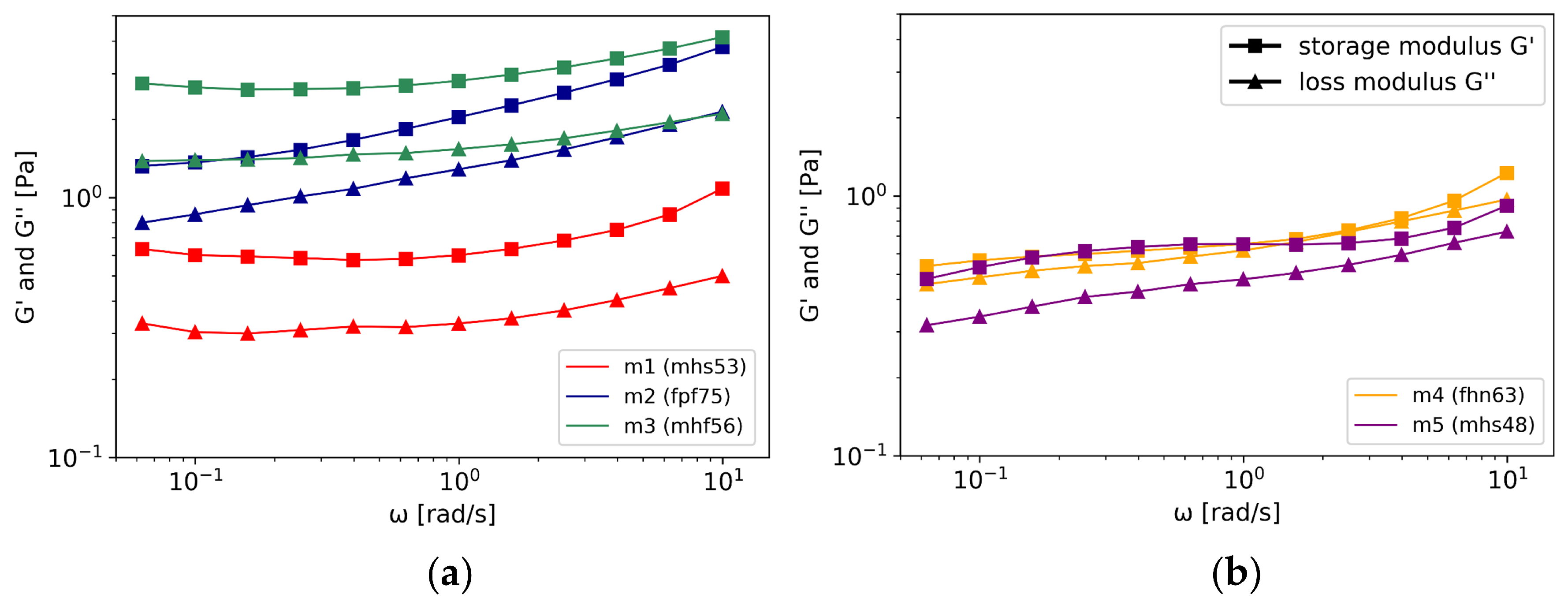
3.2.2. Oscillatory Shear Rheology
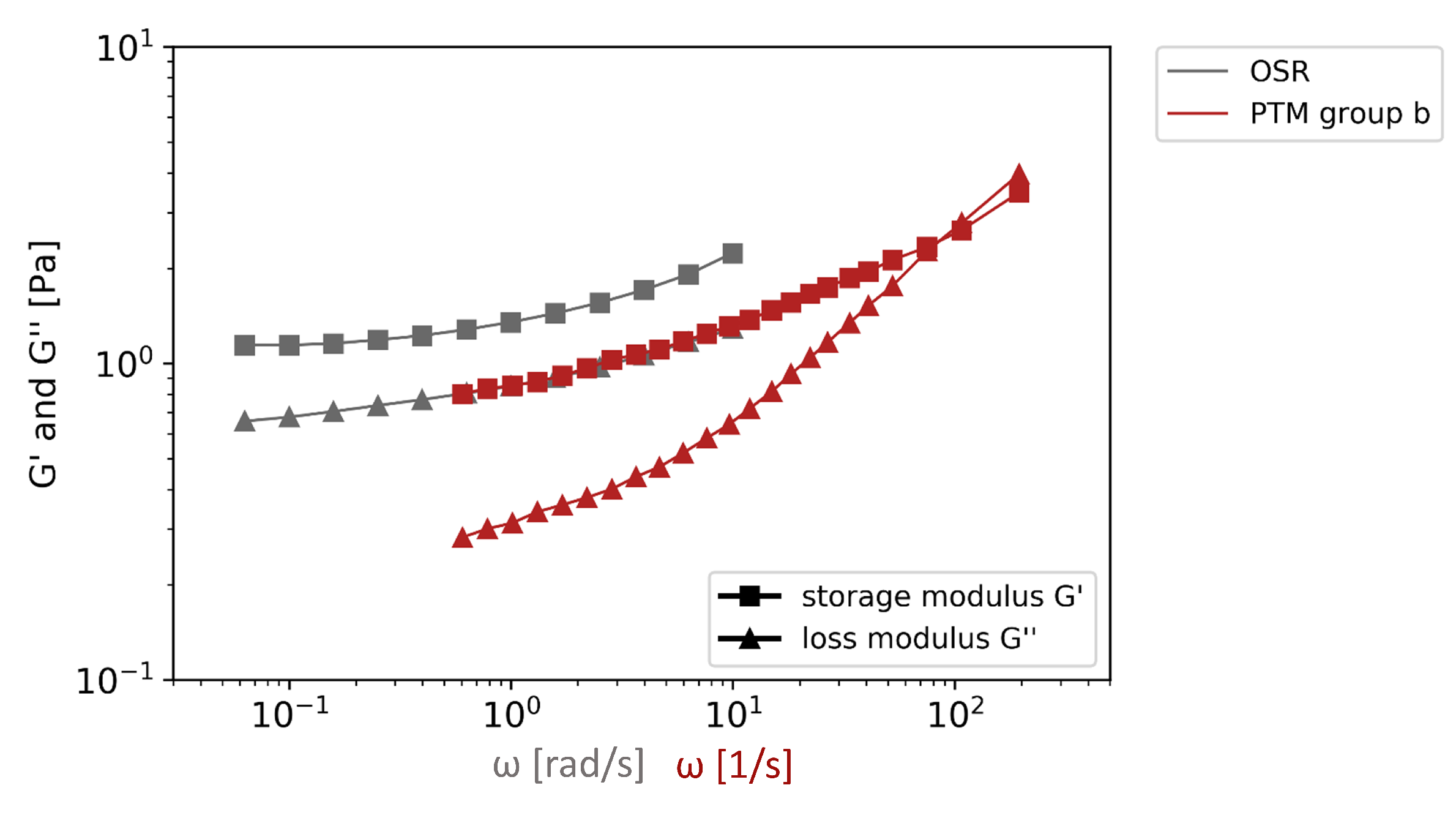
3.2.3. Comparison of Particle Tracking Microrheology and Oscillatory Shear Rheology
4. Shortcomings
5. Conclusions
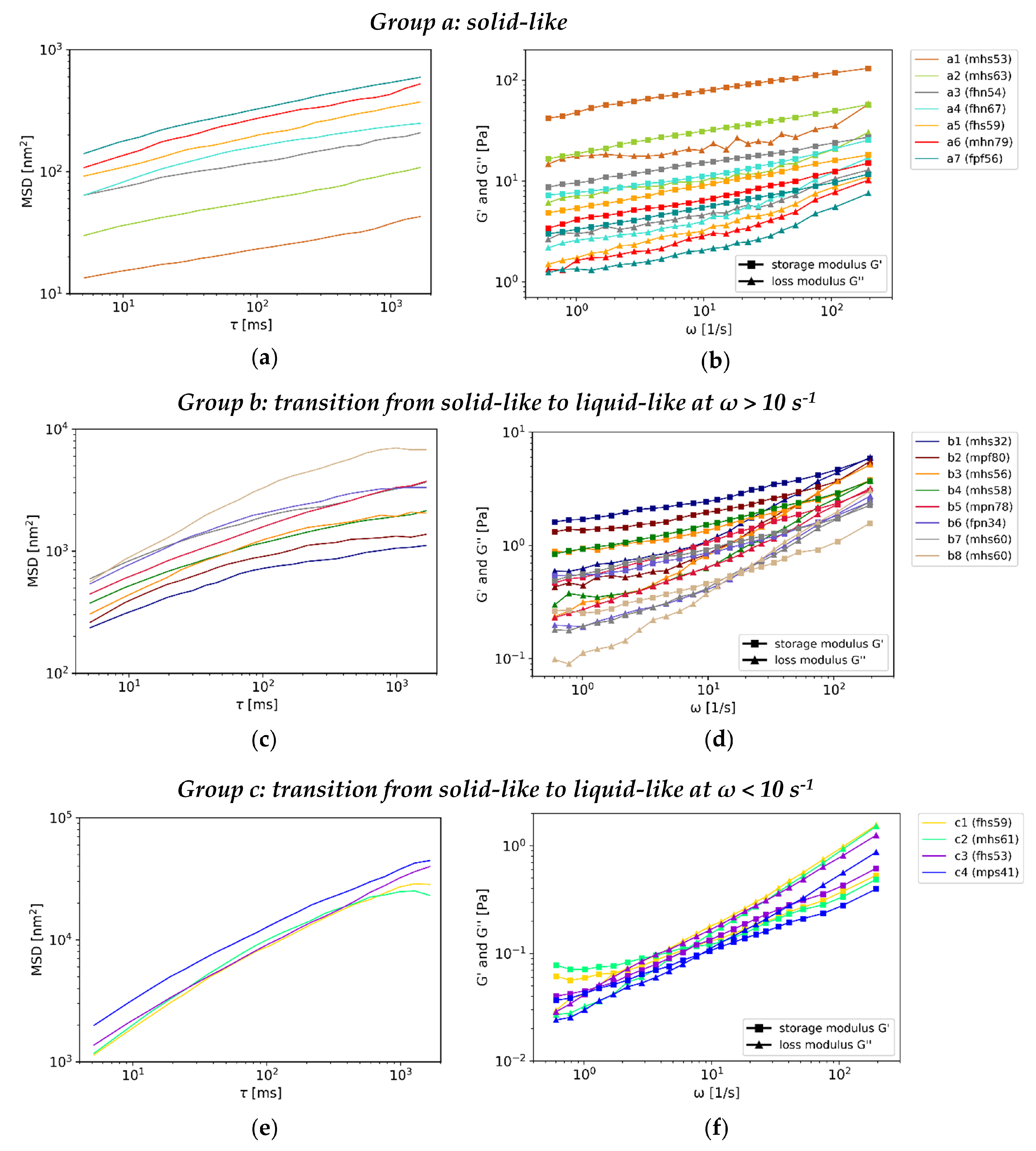
- All investigated human laryngeal mucus samples presented as viscoelastic solids, at rest with varying rigidity, independent of the measuring method.
- PTM led to a preliminary subdivision of the samples into three groups with different rigidity. Seven of the 19 investigated samples (group a) revealed consistent solid-like character, which is typical for gels. Their absolute storage and loss modulus were in the range of native human airway mucus investigated before [14,15,16]. For the other samples (group b, group c), a transition from solid-like to liquid-like character was found. The transition happened for group b samples at high frequencies, characterizing the samples predominantly as viscoelastic-solids. For group c mucus samples, the crossover was found at low frequencies, characterizing the samples predominantly as viscoelastic-liquids. We assume that this is caused by a looser mucin network, as a consequence of a varying hydration level of the samples. The hydration affects the concentration of the main gel-building component of mucus, the mucins [18,20], and according network-building factors as pH, salt and surfactant concentration [21].
- A correspondence between the groups and the demographic data of the patients belonging to the samples was not found for gender, age and larynx status but was found regarding the smoking behavior. The mucus samples with the lowest rigidity (group c) were only assigned to current smokers.
- OSR revealed consistent gel characteristics for four of the five investigated mucus samples. The rigidity of the gels varied. The absolute viscoelastic properties were in the range of PTM and could be assigned to group b mucus samples.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| = 5.14 ms | = 1662 ms | = 0.6 s | = 195 s | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | MSD [nm] | MSD [nm] | G′ [Pa] | G″ [Pa] | tan | G′ [Pa] | G″ [Pa] | tan | ||
| a1 | 13.48 | 0.26 | 42.85 | 0.21 | 42.07 | 14.76 | 0.35 | 131.30 | 57.72 | 0.44 |
| a2 | 30.01 | 0.31 | 108.30 | 0.22 | 16.60 | 6.10 | 0.37 | 57.43 | 30.39 | 0.53 |
| a3 | 64.30 | 0.28 | 208.42 | 0.19 | 8.70 | 2.64 | 0.30 | 27.28 | 12.90 | 0.47 |
| a4 | 64.23 | 0.37 | 249.01 | 0.15 | 7.31 | 1.76 | 0.24 | 25.47 | 16.96 | 0.67 |
| a5 | 92.15 | 0.35 | 372.90 | 0.19 | 4.86 | 1.50 | 0.31 | 18.16 | 11.07 | 0.61 |
| a6 | 108.18 | 0.38 | 524.41 | 0.24 | 3.42 | 1.32 | 0.39 | 15.04 | 10.21 | 0.68 |
| a7 | 140.96 | 0.36 | 594.19 | 0.25 | 3.00 | 1.24 | 0.41 | 11.71 | 7.54 | 0.64 |
| b1 | 235.54 | 0.51 | 1112.81 | 0.22 | 1.62 | 0.59 | 0.37 | 5.86 | 5.97 | 1.02 |
| b2 | 260.46 | 0.52 | 1372.63 | 0.20 | 1.32 | 0.43 | 0.33 | 5.16 | 5.53 | 1.07 |
| b3 | 305.89 | 0.60 | 2049.39 | 0.17 | 0.89 | 0.24 | 0.27 | 3.77 | 5.17 | 1.37 |
| b4 | 375.42 | 0.50 | 2141.22 | 0.22 | 0.84 | 0.30 | 0.35 | 3.70 | 3.73 | 1.01 |
| b5 | 445.83 | 0.51 | 3732.58 | 0.29 | 0.47 | 0.23 | 0.49 | 3.07 | 3.18 | 1.04 |
| b6 | 538.98 | 0.54 | 3321.64 | 0.22 | 0.54 | 0.20 | 0.36 | 2.43 | 2.73 | 1.12 |
| b7 | 593.90 | 0.52 | 3675.53 | 0.22 | 0.49 | 0.18 | 0.37 | 2.26 | 2.43 | 1.07 |
| b8 | 561.99 | 0.70 | 6782.61 | 0.23 | 0.26 | 0.10 | 0.37 | 1.57 | 3.04 | 1.94 |
| c1 | 1141.26 | 0.79 | 28450.66 | 0.28 | 0.06 | 0.03 | 0.48 | 0.53 | 1.56 | 2.93 |
| c2 | 1175.78 | 0.80 | 23114.57 | 0.21 | 0.08 | 0.03 | 0.35 | 0.49 | 1.52 | 3.11 |
| c3 | 1373.96 | 0.71 | 39911.90 | 0.40 | 0.04 | 0.03 | 0.72 | 0.62 | 1.25 | 2.03 |
| c4 | 1991.76 | 0.73 | 44669.08 | 0.37 | 0.04 | 0.02 | 0.66 | 0.40 | 0.87 | 2.20 |
References
- Ruben, R.J. Redefining the Survival of the Fittest: Communication Disorders in the 21st Century. Laryngoscope 2000, 110, 241–245. [Google Scholar] [CrossRef]
- Bodaghi, D.; Xue, Q.; Zheng, X.; Thomson, S. Effect of Subglottic Stenosis on Vocal Fold Vibration and Voice Production Using Fluid–Structure–Acoustics Interaction Simulation. Appl. Sci. 2021, 11, 1221. [Google Scholar] [CrossRef]
- Stevens, K.N. Acoustic Phonetics. J. Acoust. Soc. Am. 2001, 109, 17–18. [Google Scholar] [CrossRef]
- Leydon, C.; Sivasankar, M.; Falciglia, D.L.; Atkins, C.; Fisher, K.V. Vocal Fold Surface Hydration: A Review. J. Voice 2009, 23, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Turner, B. The biology of mucus: Composition, synthesis and organization. Ad. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef]
- Ayache, S.; Ouaknine, M.; Dejonkere, P.; Prindere, P.; Giovanni, A. Experimental study of the effects of surface mucus viscosity on the glottic cycle. J. Voice 2004, 18, 107–115. [Google Scholar] [CrossRef]
- Döllinger, M.; Gröhn, F.; Berry, D.; Eysholdt, U.; Luegmaira, G. Preliminary results on the influence of engineered artificial mucus layer on phonation. J. Speech Lang. Hear. Res. 2014, 57, S637–S647. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, H.; Aikman, A.; Hines, K.; Deliyski, D. Vocal fold mucus aggregation in vocally normal speakers. Logop. Phoniatr. Vocol. 2008, 33, 136–142. [Google Scholar] [CrossRef]
- Bonilha, H.S.; White, L.; Kuckhahn, K.; Gerlach, T.T.; Deliyski, D.D. Vocal fold mucus aggregation in persons with voice disorders. J. Commun. Disord. 2012, 45, 304–311. [Google Scholar] [CrossRef]
- Lucena, M.M.; da Silva, F.d.S.; da Costa, A.D.; Guimarães, G.R.; Ruas, A.C.N.; Braga, F.P.B.; Braga, M.P.B.; Reis, J.G.C.; da Costa, D.C.S.; Palmeiro, M.R.; et al. Evaluation of Voice Disorders in Patients with Active Laryngeal Tuberculosis. PLoS ONE 2015, 10, e0126876. [Google Scholar] [CrossRef]
- Lourenço, B.M.; Costa, K.M.; da Silva Filho, M. Voice Disorder in Cystic Fibrosis Patients. PLoS ONE 2014, 9, e0096769. [Google Scholar] [CrossRef] [PubMed]
- Witt, R.E.; Regner, M.F.; Tao, C.; Rieves, A.L.; Zhuang, P.; Jiang, J.J. Effect of Dehydration on Phonation Threshold Flow in Excised Canine Larynges. Anna. Otol. Rhinol. Laryngol. 2009, 118, 154–159. [Google Scholar] [CrossRef]
- Vasquez, P.A.; Forest, M.G. Complex Fluids and Soft Structures in the Human Body. In Complex Fluids in Biological Systems: Experiment, Theory, and Computation; Spagnolie, S.E., Ed.; Springer: New York, NY, USA, 2015; pp. 53–110. [Google Scholar] [CrossRef]
- Schuster, B.S.; Suk, J.S.; Woodworth, G.F.; Hanes, J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials 2013, 34, 3439–3446. [Google Scholar] [CrossRef]
- Innes, A.L.; Carrington, S.D.; Thornton, D.J.; Kirkham, S.; Rousseau, K.; Dougherty, R.H.; Raymond, W.W.; Caughey, G.H.; Muller, S.J.; Fahy, J.V. Ex Vivo Sputum Analysis Reveals Impairment of Protease-dependent Mucus Degradation by Plasma Proteins in Acute Asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Huck, B.C.; Hartwig, O.; Biehl, A.; Schwarzkopf, K.; Wagner, C.; Loretz, B.; Murgia, X.; Lehr, C.M. Macro- and Microrheological Properties of Mucus Surrogates in Comparison to Native Intestinal and Pulmonary Mucus. Biomacromolecules 2019, 20, 3504–3512. [Google Scholar] [CrossRef]
- Murgia, X.; Pawelzyk, P.; Schaefer, U.F.; Wagner, C.; Willenbacher, N.; Lehr, C.M. Size-Limited Penetration of Nanoparticles into Porcine Respiratory Mucus after Aerosol Deposition. Biomacromolecules 2016, 17, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Meldrum, O.; Yakubov, G.; Bonilla, M.; Deshmukh, O.; McGuckin, M.; Gidley, M. Mucin gel assembly is controlled by a collective action of non-mucin proteins, disulfide bridges, Ca2+ -mediated links, and hydrogen bonding. Sci. Rep. 2018, 8, 5802. [Google Scholar] [CrossRef]
- Hill, D.; Vasquez, P.; Mellnik, J.; McKinley, S.; Vose, A.; Mu, F.; Henderson, A.; Donaldson, S.; Alexis, N.; Boucher, R.; et al. A biophysical basis for mucus solids concentration as a candidate biomarker for airways disease. PLoS ONE 2014, 9, e0087681. [Google Scholar] [CrossRef]
- Wagner, C.; Turner, B.; Rubinstein, M.; McKinley, G.; Ribbeck, K. A Rheological Study of the Association and Dynamics of MUC5AC Gels. Biomacromolecules 2017, 18, 3654–3664, cited By 44. [Google Scholar] [CrossRef]
- Lai, S.; Wang, Y.Y.; Wirtz, D.; Hanes, J. Micro- and macrorheology of mucus. Adv. Drug Deliv. Rev. 2009, 61, 86–100. [Google Scholar] [CrossRef]
- Voynow, J.A.; Gendler, S.J.; Rose, M.C. Regulation of Mucin Genes in Chronic Inflammatory Airway Diseases. Am. J. Respir. Cell Mol. Biol. 2006, 34, 661–665. [Google Scholar] [CrossRef]
- Chen, E.Y.; Sun, A.; Chen, C.S.; Mintz, A.J.; Chin, W.C. Nicotine alters mucin rheological properties. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L149–L157. [Google Scholar] [CrossRef]
- Leal, J.; Smyth, H.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef]
- Eric, M.; Furst, T.M.S. Microrheology; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Suh, J.; Choy, K.L.; Lai, S.; Suk, J.; Tang, B.; Prabhu, S.; Hanes, J. PEGylation of nanoparticles improves their cytoplasmic transport. Int. J. Nanomed. 2007, 2, 735–741. [Google Scholar]
- Mason, T.G.; Gisler, T.; Kroy, K.; Frey, E.; Weitz, D.A. Rheology of F-actin solutions determined from thermally driven tracer motion. J. Rheol. 2000, 44, 917–928. [Google Scholar] [CrossRef]
- Shaw, M.T.; Macknight, W.J. Introduction to Polymer Viscoelasticity; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Philippe, A.M.; Cipelletti, L.; Larobina, D. Mucus as an Arrested Phase Separation Gel. Macromolecules 2017, 50, 8221–8230. [Google Scholar] [CrossRef]
- Fabry, B.; Maksym, G.; Butler, J.; Glogauer, M.; Navajas, D.; Fredberg, J. Scaling the microrheology of living cells. Phys. Rev. Lett. 2001, 87, 148102. [Google Scholar] [CrossRef] [PubMed]
- Fabry, B.; Maksym, G.; Butler, J.; Glogauer, M.; Navajas, D.; Taback, N.; Millet, E.; Fredberg, J. Time scale and other invariants of integrative mechanical behavior in living cells. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2003, 68, 041914. [Google Scholar] [CrossRef]
- Bergendal, B.; McAllister, A.; Stecksén-Blicks, C. Orofacial dysfunction in ectodermal dysplasias measured using the Nordic Orofacial Test-Screening protocol. Acta Odontol. Scand. 2009, 67, 377–381. [Google Scholar] [CrossRef]
- Fete, M.; Hermann, J.; Behrens, J.; Huttner, K.M. X-linked hypohidrotic ectodermal dysplasia (XLHED): Clinical and diagnostic insights from an international patient registry. Am. J. Med. Genet. Part A 2014, 164, 2437–2442. [Google Scholar] [CrossRef] [PubMed]

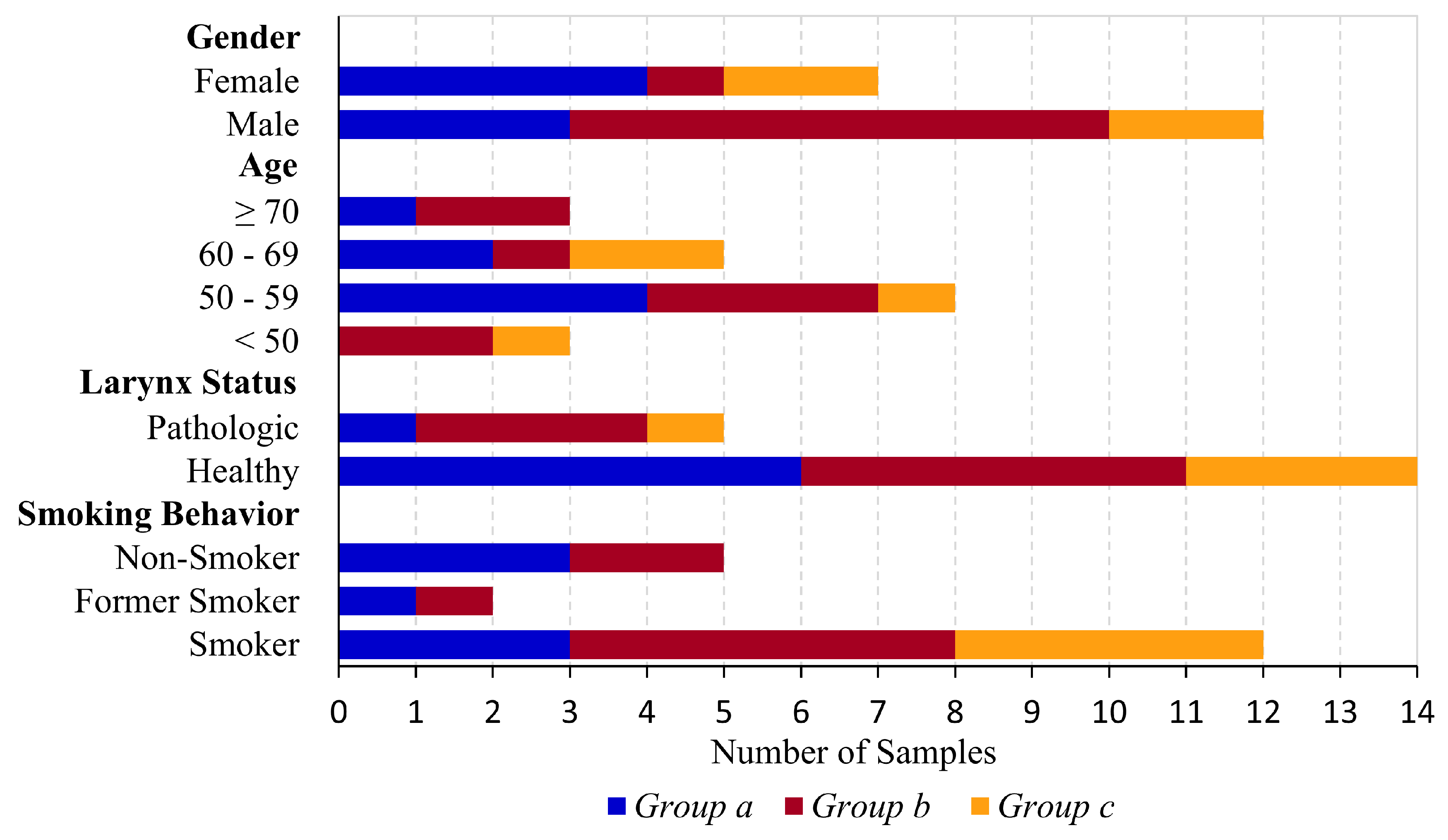
| Gender | Male | Female | ||
| 12 | 7 | |||
| Age | <50 | 50–59 | 60–69 | ≥70 |
| 3 | 8 | 5 | 3 | |
| Larynx Status | Healthy | Pathologic | ||
| 14 | 5 | |||
| Smoking Behavior | Non-Smokers | Former Smokers | Smokers | |
| 5 | 2 | 12 | ||
| Group a | Group b | Group c | ||
|---|---|---|---|---|
| MSD Parameters | ||||
| = 5.14 ms: | MSD [nm] | 73 ± 41 | 451 ± 128 | 1420 ± 341 |
| 0.33 ± 0.04 | 0.57 ± 0.07 | 0.76 ± 0.04 | ||
| = 1662 ms: | MSD [nm] | 300 ± 191 | 3489 ± 1709 | 34036 ± 8632 |
| 0.21 ± 0.03 | 0.22 ± 0.04 | 0.32 ± 0.07 | ||
| Viscoelasticity Parameters | ||||
| G′ [Pa] | 12.28 ± 12.89 | 0.80 ± 0.43 | 0.05 ± 0.02 | |
| = 0.6 s (at rest): | G″ [Pa] | 4.19 ± 4.60 | 0.28 ± 0.15 | 0.03 ± 0.00 |
| tan | 0.34 ± 0.05 | 0.36 ± 0.06 | 0.55 ± 0.15 | |
| = 195 s: | tan | 0.58 ± 0.09 | 1.21 ± 0.30 | 2.57 ± 0.46 |
| Post Hoc Tests (Mann–Whitney-U-Test; p < 0.017) | Kruskal Wallis (p < 0.05) | ||||
|---|---|---|---|---|---|
| Group a vs. b | Group a vs. c | Group b vs. c | |||
| MSD Parameters | |||||
| = 5.14 ms: | MSD | 0.000 | 0.006 | 0.004 | 0.000 |
| 0.000 | 0.006 | 0.004 | 0.000 | ||
| = 1662 ms: | MSD | 0.000 | 0.006 | 0.004 | 0.000 |
| - | - | - | 0.149 | ||
| Viscoelasticity Parameters | |||||
| G″ | 0.000 | 0.006 | 0.004 | 0.000 | |
| = 0.6 s (at rest): | G′ | 0.000 | 0.006 | 0.004 | 0.000 |
| tan | - | - | - | 0.149 | |
| = 195 s: | tan | 0.000 | 0.006 | 0.004 | 0.000 |
| = 0.06 rad/s | = 10 rad/s | |||||
|---|---|---|---|---|---|---|
| Sample | G′ [Pa] | G″ [Pa] | tan | G′ [Pa] | G″ [Pa] | tan |
| m1 | 0.63 | 0.33 | 0.52 | 1.08 | 0.50 | 0.46 |
| m2 | 1.32 | 0.80 | 0.61 | 3.80 | 2.14 | 0.56 |
| m3 | 2.75 | 1.38 | 0.50 | 4.15 | 2.10 | 0.51 |
| m4 | 0.54 | 0.46 | 0.85 | 1.23 | 0.97 | 0.79 |
| m5 | 0.48 | 0.32 | 0.66 | 0.92 | 0.73 | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, G.; Wendler, O.; Böhringer, D.; Gostian, A.-O.; Müller, S.K.; Canziani, H.; Hesse, N.; Semmler, M.; Berry, D.A.; Kniesburges, S.; et al. Human Laryngeal Mucus from the Vocal Folds: Rheological Characterization by Particle Tracking Microrheology and Oscillatory Shear Rheology. Appl. Sci. 2021, 11, 3011. https://doi.org/10.3390/app11073011
Peters G, Wendler O, Böhringer D, Gostian A-O, Müller SK, Canziani H, Hesse N, Semmler M, Berry DA, Kniesburges S, et al. Human Laryngeal Mucus from the Vocal Folds: Rheological Characterization by Particle Tracking Microrheology and Oscillatory Shear Rheology. Applied Sciences. 2021; 11(7):3011. https://doi.org/10.3390/app11073011
Chicago/Turabian StylePeters, Gregor, Olaf Wendler, David Böhringer, Antoniu-Oreste Gostian, Sarina K. Müller, Herbert Canziani, Nicolas Hesse, Marion Semmler, David A. Berry, Stefan Kniesburges, and et al. 2021. "Human Laryngeal Mucus from the Vocal Folds: Rheological Characterization by Particle Tracking Microrheology and Oscillatory Shear Rheology" Applied Sciences 11, no. 7: 3011. https://doi.org/10.3390/app11073011
APA StylePeters, G., Wendler, O., Böhringer, D., Gostian, A.-O., Müller, S. K., Canziani, H., Hesse, N., Semmler, M., Berry, D. A., Kniesburges, S., Peukert, W., & Döllinger, M. (2021). Human Laryngeal Mucus from the Vocal Folds: Rheological Characterization by Particle Tracking Microrheology and Oscillatory Shear Rheology. Applied Sciences, 11(7), 3011. https://doi.org/10.3390/app11073011







