Effects of Different Contents of Each Component on the Structural Stability and Mechanical Properties of Co-Cr-Fe-Ni High-Entropy Alloys
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Structural Stability
3.2. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Santodonato, L.J.; Zhang, Y.; Feygenson, M.; Parish, C.M.; Gao, M.C.; Weber, R.J.K.; Neuefeind, J.C.; Tang, Z.; Liaw, P.K. Deviation from high-entropy configurations in the atomic distributions of a multi-principal-element alloy. Nat. Commun. 2015, 6, 5964. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S. Alloyed pleasures: Multimetallic cocktails. Corros. Sci. 2003, 85, 1404–1406. [Google Scholar]
- Tsai, M.H.; Yeh, J.W.; Gan, J.Y. Diffusion barrier properties of AlMoNbSiTaTiVZr high-entropy alloy layer between copper and silicon. Thin Solid Films 2008, 516, 5527–5530. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef]
- Smeltzer, J.A.; Marvel, C.J.; Hornbuckle, B.C.; Roberts, A.J.; Marsico, J.M.; Giri, A.K.; Darling, K.A.; Rickman, J.M.; Chan, H.M.; Harmer, M.P. Achieving ultrahard refractory multi-principal element alloys via mechanical alloying. Mater. Sci. Eng. A 2019, 763, 138140. [Google Scholar] [CrossRef]
- Zhang, J.B.; Li, X.; Zhang, Y.C.; Zhang, F.; Wu, H.X.; Wang, X.Z.; Zhou, Q.; Wang, H.F. Sluggish dendrite growth in an undercooled high entropy alloy. Intermetallics 2020, 119, 106714. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.J.; Wu, Q.F.; Li, J.J.; Wang, J.C.; Liu, C.T. Phase separation of metastable CoCrFeNi high entropy alloy at intermediate temperatures. Scr. Mater. 2017, 126, 15–19. [Google Scholar] [CrossRef]
- Cornide, J.; Calvo-Dahlborg, M.; Chambreland, S.; Asensio Dominguez, L.; Leong, Z.; Dahlborg, U.; Cunliffe, A.; Goodall, R.; Todd, I. Combined atom probe tomography and TEM investigations of CoCrFeNi, CoCrFeNi-Pdx (x 0.5, 1.0, 1.5) and CoCrFeNi-Sn. Acta Phys. Pol. A 2015, 128, 557–560. [Google Scholar] [CrossRef]
- Niu, C.; Zaddach, A.J.; Oni, A.A.; Sang, X.; Hurt, J.W., III; LeBeau, J.M.; Koch, C.C.; Irving, D.L. Spin-driven ordering of Cr in the equiatomic high entropy alloy NiFeCrCo. Appl. Phys. Lett. 2016, 106, 161906. [Google Scholar] [CrossRef]
- Zhuang, C.; Qi, H.; Cheng, X.; Chen, G.; Gao, C.; Wang, L.; Sun, S.; Zou, J.; Han, X. In-situ observation of dynamic galvanic replacement reactions in twinned metallic nanowires by liquid cell transmission electron microscopy. Angew. Chem. Int. Ed. 2019, 58, 18627. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhang, X.C.; Zhou, J.; Cao, S.; Yu, H.; Hu, Q.M.; Sun, Z.M. Influence of lattice distortion on stacking fault energies of CoCrFeNi and Al-CoCrFeNi high entropy alloys. J. Alloy. Compd. 2020, 846, 156321. [Google Scholar] [CrossRef]
- Liu, J.P.; Guo, X.X.; Lin, Q.Y.; He, Z.B.; An, X.H.; Li, L.F.; Liaw, P.K.; Liao, X.Z.; Yu, L.P.; Lin, J.P.; et al. Excellent ductility and serration feature of metastable CoCrFeNi high-entropy alloy at extremely low temperatures. Sci. China Mater. 2019, 62, 853–863. [Google Scholar] [CrossRef]
- Salishchev, G.A.; Tikhonovsky, M.A.; Shaysultanov, D.G.; Stepanov, N.D.; Kuznetsov, A.V.; Kolodiy, I.V.; Tortika, A.S.; Senkov, O.N. Effect of Mn and V on structure and mechanical properties of high-entropy alloys based on CoCrFeNi system. J. Alloys Compd. 2014, 591, 11–21. [Google Scholar] [CrossRef]
- Xu, J.; Wang, S.R.; Shang, C.Y.; Huang, S.F.; Wang, Y. Microstructure and properties of CoCrFeNi(WC) high-entropy alloy coatings prepared using mechanical alloying and hot pressing sintering. Coatings 2019, 9, 16. [Google Scholar] [CrossRef]
- Huo, W.Y.; Zhou, H.; Fang, F.; Hu, X.J.; Xie, Z.H.; Jiang, J.Q. Strain-rate effect upon the tensile behavior of CoCrFeNi high-entropy alloys. Mater. Sci. Eng. A 2017, 689, 366–369. [Google Scholar] [CrossRef]
- Geng, Y.S.; Chen, J.; Tan, H.; Cheng, J.; Yang, J.; Liu, W.M. Vacuum tribological behaviors of CoCrFeNi high entropy alloy at elevated temperatures. Wear 2020, 456–457, 203368. [Google Scholar] [CrossRef]
- Zhang, A.J.; Han, J.S.; Su, B.; Meng, J.H. A novel CoCrFeNi high entropy alloy matrix self-lubricating composite. J. Alloy. Compd. 2017, 725, 700–710. [Google Scholar] [CrossRef]
- Zhang, A.J.; Han, J.S.; Su, B.; Li, P.D.; Meng, J.H. Microstructure, mechanical properties and tribological performance of CoCrFeNi high entropy alloy matrix self-lubricating composite. Mater. Des. 2017, 114, 253–263. [Google Scholar] [CrossRef]
- Wang, W.R.; Qi, W.; Xie, L.; Yang, X.; Li, J.T.; Zhang, Y. Microstructure and corrosion behavior of (CoCrFeNi)95Nb5 high-entropy alloy coating fabricated by plasma spraying. Materials 2019, 12, 694. [Google Scholar] [CrossRef]
- Muangtong, P.; Rodchanarowan, A.; Chaysuwan, D.; Chanlek, N.; Goodall, R. The corrosion behaviour of CoCrFeNi-x (x = Cu, Al, Sn) high entropy alloy systems in chloride solution. Corros. Sci. 2020, 172, 108740. [Google Scholar] [CrossRef]
- Zhang, B.L.; Zhang, Y.; Guo, S.M. A thermodynamic study of corrosion behaviors for CoCrFeNi-based high-entropy alloys. J. Mater. Sci. 2018, 53, 14729–14738. [Google Scholar] [CrossRef]
- Yang, H.O.; Shang, X.L.; Wang, L.L.; Wang, Z.J.; Wang, J.C.; Lin, X. Effect of constituent elements on the corrosion resistance of single-phase CoCrFeNi high-entropy alloys in NaCl solution. Acta Metall. Sin. 2018, 54, 905–910. [Google Scholar]
- Wang, W.R.; Wang, J.Q.; Yi, H.G.; Qi, W.; Peng, Q. Effect of molybdenum additives on corrosion behavior of (CoCrFeNi)100−xMox high-entropy alloys. Entropy 2018, 20, 908. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Chiang, W.C.; Wu, J.K. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005, 92, 112–117. [Google Scholar] [CrossRef]
- Lucas, M.S.; Belyea, D.; Bauer, C.; Bryant, N.; Michel, E.; Turgut, Z.; Leontsev, S.O.; Horwath, J.; Semiatin, S.L.; McHenry, M.E.; et al. Thermomagnetic analysis of FeCoCrxNi alloys: Magnetic entropy of high-entropy alloys. J. Appl. Phys. 2013, 113, 17A923. [Google Scholar] [CrossRef]
- Lucas, M.S.; Mauger, L.; Muñoz, J.A.; Xiao, Y.M.; Sheets, A.O.; Semiatin, S.L.; Horwath, J.; Turgut, Z. Magnetic and vibrational properties of high-entropy alloys. J. Appl. Phys. 2011, 109, 07E307. [Google Scholar] [CrossRef]
- Xia, S.Q.; Yang, X.; Yang, T.F.; Liu, S.; Zhang, Y. Irradiation resistance in AlxCoCrFeNi high entropy alloys. JOM 2015, 67, 2340–2344. [Google Scholar] [CrossRef]
- Li, Y.; Gao, C.; Jiang, W.; Zhuang, C.; Tan, W.; Li, W.; Li, Y.; Wang, L.; Liao, X.; Sun, Z.; et al. A game-changing design of low-cost, large-size porous cocatalysts decorated by ultra-small photocatalysts for highly efficient hydrogen evolution. Appl. Catal. B Environ. 2021, 286, 119923. [Google Scholar] [CrossRef]
- Zhang, F.X.; Zhao, S.J.; Jin, K.; Bei, H.; Popov, D.; Park, C.Y.; Neuefeind, J.C.; Weber, W.J.; Zhang, Y.W. Pressure-induced fcc to hcp phase transition in Ni-based high entropy solid solution alloys. Appl. Phys. Lett. 2017, 110, 011902. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wu, Q.F.; Zhou, W.Q.; He, F.; Yu, C.Y.; Lin, D.Y.; Wang, J.C.; Liu, C.T. Quantitative determination of the lattice constant in high entropy alloys. Scr. Mater. 2019, 162, 468–471. [Google Scholar] [CrossRef]
- Anand, G.; Eisenbach, M.; Goodall, R.; Freeman, C.L. Electron spin mediated distortion in metallic systems. Scr. Mater. 2020, 185, 159–164. [Google Scholar] [CrossRef]
- Song, W.W.; Radulescu, A.; Liu, L.L.; Bleck, W. Study on a high entropy alloy by high energy synchrotron X-ray diffraction and small angle neutron scattering. Steel Res. Int. 2017, 88, 1700079. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, T.J.; Chen, S.K.; Yeh, J.W. Microstructure and mechanical property of as-cast, -homogenized, and-deformed AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. J. Alloy. Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Tian, F.Y.; Delczeg, L.; Chen, N.X.; Varga, L.K.; Shen, J.; Vitos, L. Structural stability of NiCoFeCrAlx high-entropy alloy from ab initio theory. Phys. Rev. B 2013, 88, 085128. [Google Scholar] [CrossRef]
- Niu, C.; Zaddach, A.J.; Koch, C.C.; Irvin, D.L. First principles exploration of near-equiatomic NiFeCrCo high entropy alloys. J. Alloy. Compd. 2016, 672, 510–520. [Google Scholar] [CrossRef]
- Zunger, A.; Wei, S.H.; Ferreira, L.G.; Bernard, J.E. Special quasirandom structures. Phys. Rev. Lett. 1990, 65, 353. [Google Scholar] [CrossRef]
- Tian, F.Y.; Varga, L.K.; Chen, N.X.; Shen, J.; Vitos, L. Empirical design of single phase high-entropy alloys with high hardness. Intermetallics 2015, 58, 1–6. [Google Scholar] [CrossRef]
- Tian, F.Y.; Varga, L.K.; Chen, N.X.; Shen, J.; Vitos, L. Ab initio design of elastically isotropic TiZrNbMoVx high-entropy alloys. J. Alloy. Compd. 2014, 599, 19–25. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B. Effect of the atomic size distribution on glass forming ability of amorphous metallic alloys. Mater. Res. Bull. 2001, 36, 2183–2198. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Quantitative evaluation of critical cooling rate for metallic glasses. Mater. Sci. Eng. A 2001, 304–306, 446–451. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Boer, F.R.; Perrifor, D.G. Cohesion in Metals; Elsevier Science Publishers, B.V.: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Miraclea, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condes. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Koval, N.E.; Juaristi, J.I.; Muiño, R.D.; Alducin, M. Structure and properties of CoCrFeNiX multi-principal element alloys from ab initio calculations. J. Appl. Phys. 2020, 127, 145102. [Google Scholar] [CrossRef]
- Anderson, O.L. A simplified method for calculating the debye temperature from elastic constants. J. Phys. Chem. Solids 1963, 24, 909–917. [Google Scholar] [CrossRef]
- Wu, Z.J.; Zhao, E.J.; Xiang, H.P.; Hao, X.F.; Liu, X.J.; Meng, J. Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys. Rev. B 2007, 76, 054115. [Google Scholar] [CrossRef]
- Ma, D.C.; Grabowski, B.; Körmann, F.; Neugebauer, J.; Raabe, D. Ab initio thermodynamics of the CoCrFeMnNi high entropy alloy: Importance of entropy contributions beyond the configurational one. Acta Mater. 2015, 100, 90–97. [Google Scholar] [CrossRef]
- Song, H.Q.; Tian, F.Y.; Hu, Q.M.; Vitos, L.; Wang, Y.D.; Shen, J.; Chen, N.X. Local lattice distortion in high-entropy alloys. Phys. Rev. Mater. 2017, 1, 023404. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Söderlind, P.; Eriksson, O.; Wills, J.M.; Boring, A.M. Theory of elastic constants of cubic transition metals and alloys. Phys. Rev. B 1993, 48, 5844. [Google Scholar] [CrossRef]
- Nong, Z.S.; Zhu, J.C.; Zhao, R.D. Prediction of structure and elastic properties of AlCrFeNiTi system high entropy alloys. Intermetallics 2017, 86, 134–146. [Google Scholar] [CrossRef]
- Yu, J.Y.; Wang, K.M.; Wang, Z.M. First principles calculation of elastic properties of high-entropy alloy CoCrFeNi. J. Univ. Sci. Technol. Liaoning 2018, 41, 357–361. [Google Scholar]
- Oh, H.S.; Sang, J.K.; Odbadrakh, K.; Ryu, W.H.; Yoon, K.N.; Mu, S.; Körmann, F.; Ikeda, Y.; Tasan, C.C.; Raabe, D.; et al. Engineering atomic-level complexity in high-entropy and complex concentrated alloys. Nat. Commun. 2019, 10, 2090. [Google Scholar] [CrossRef]
- Ishibashi, S.; Ikeda, Y.; Körmann, F.; Grabowski, B.; Neugebauer, J. Correlation analysis of strongly fluctuating atomic volumes, charges, and stresses in body-centered cubic refractory high-entropy alloys. Phys. Rev. Mater. 2020, 4, 023608. [Google Scholar] [CrossRef]
- Pugh, S.F. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Gu, X.J.; McDermott, A.G.; Joseph Poon, S. Critical Poisson’s ratio for plasticity in Fe–Mo–C–B–Ln bulk amorphous steel. Appl. Phys. Lett. 2006, 88, 211905. [Google Scholar] [CrossRef]
- Nguyen-Manh, D.; Mrovec, M.; Fitzgerald, S.P. Dislocation driven problems in atomistic modelling of materials. Mater. Trans. 2018, 49, 2497–2506. [Google Scholar] [CrossRef]
- Zhao, Q.K.; Li, J.; Fang, Q.H.; Feng, H. Effect of Al solute concentration on mechanical properties of AlxFeCuCrNi high-entropy alloys: A first-principles study. Phys. B 2018, 566, 30–37. [Google Scholar] [CrossRef]
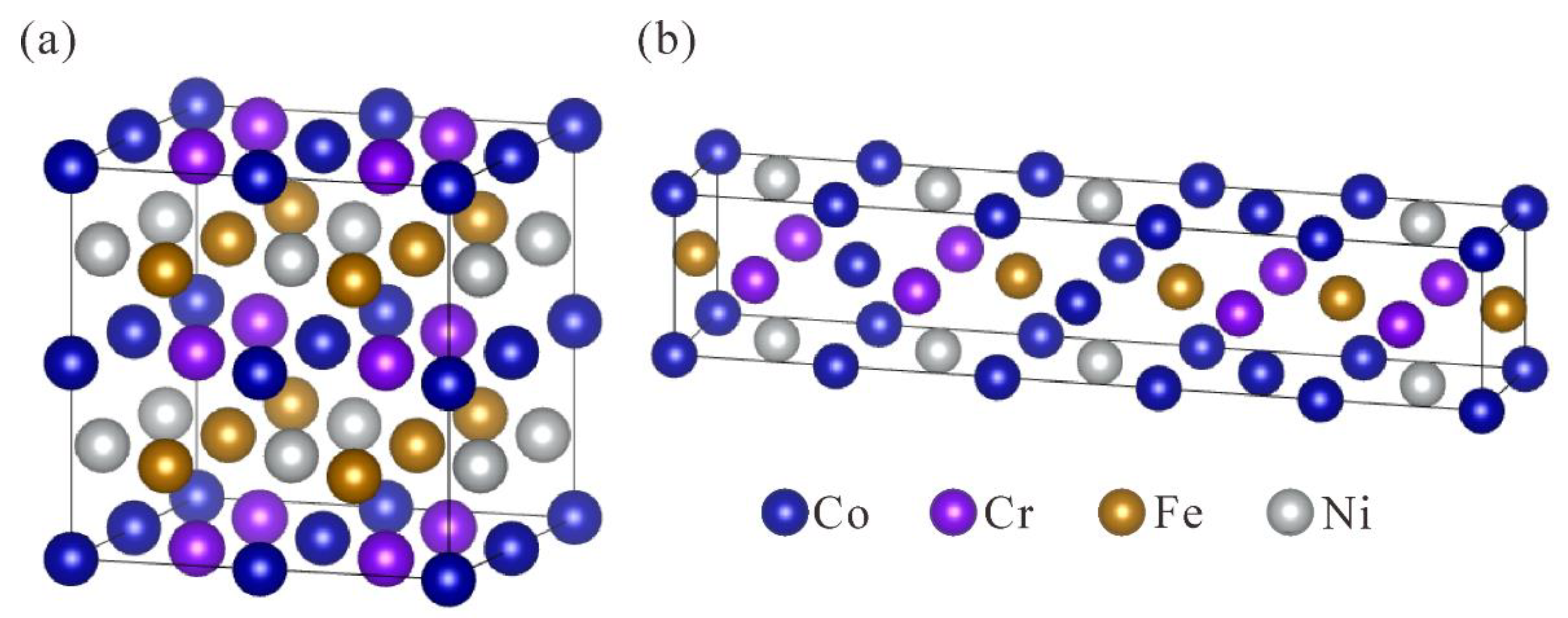
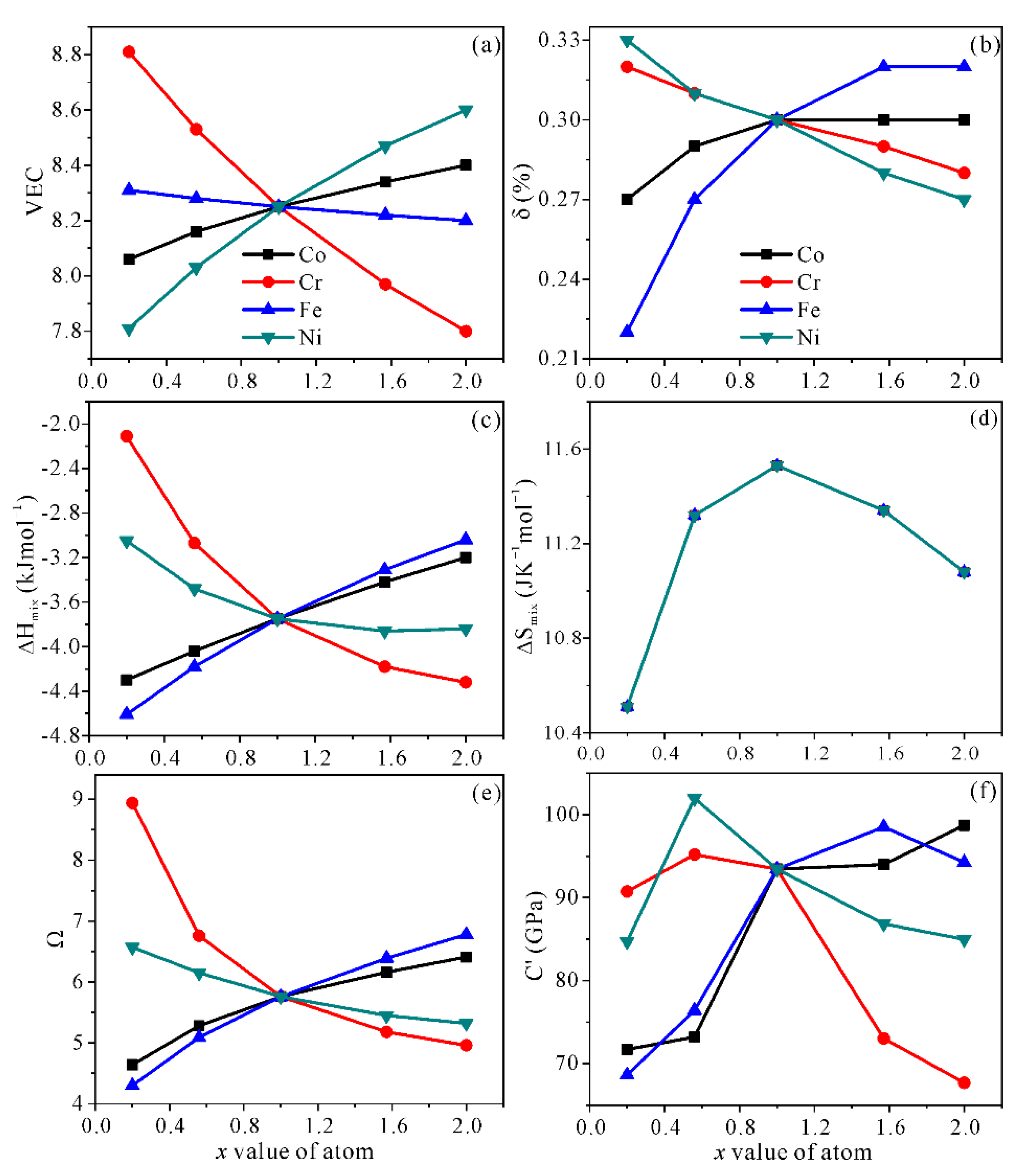
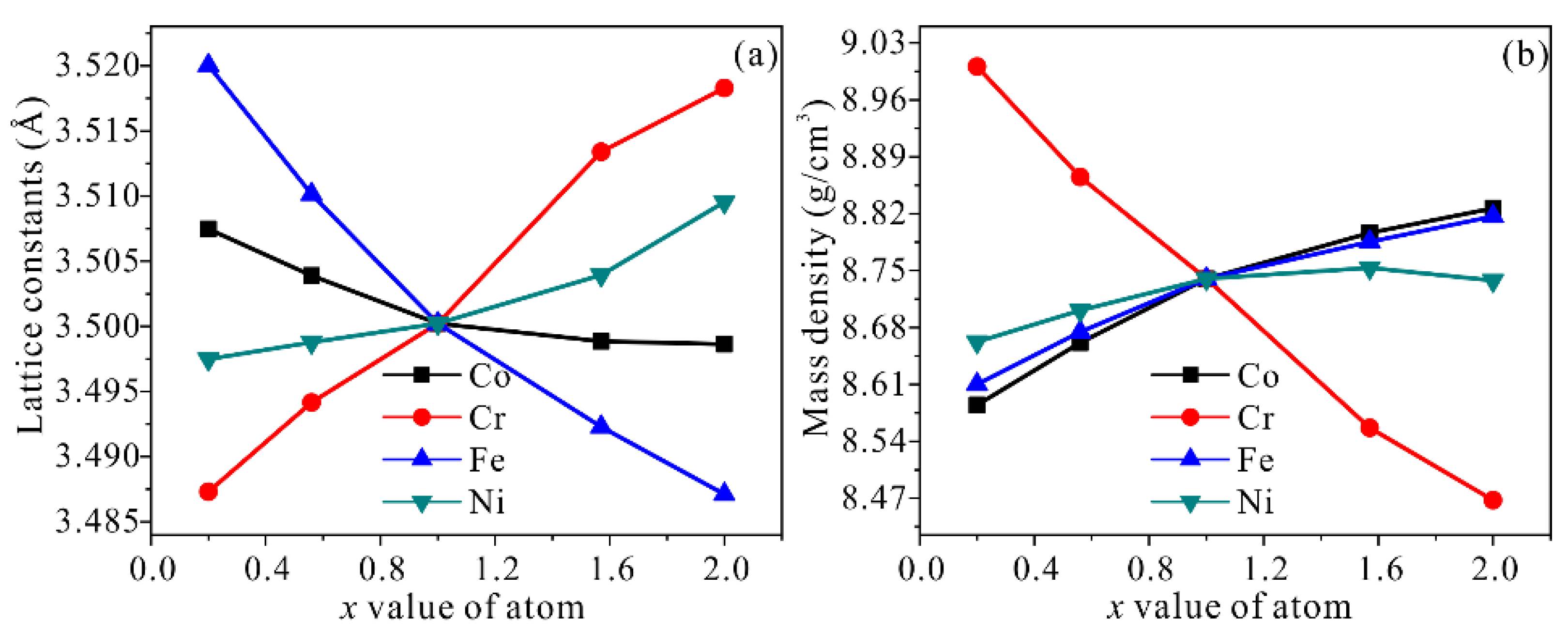
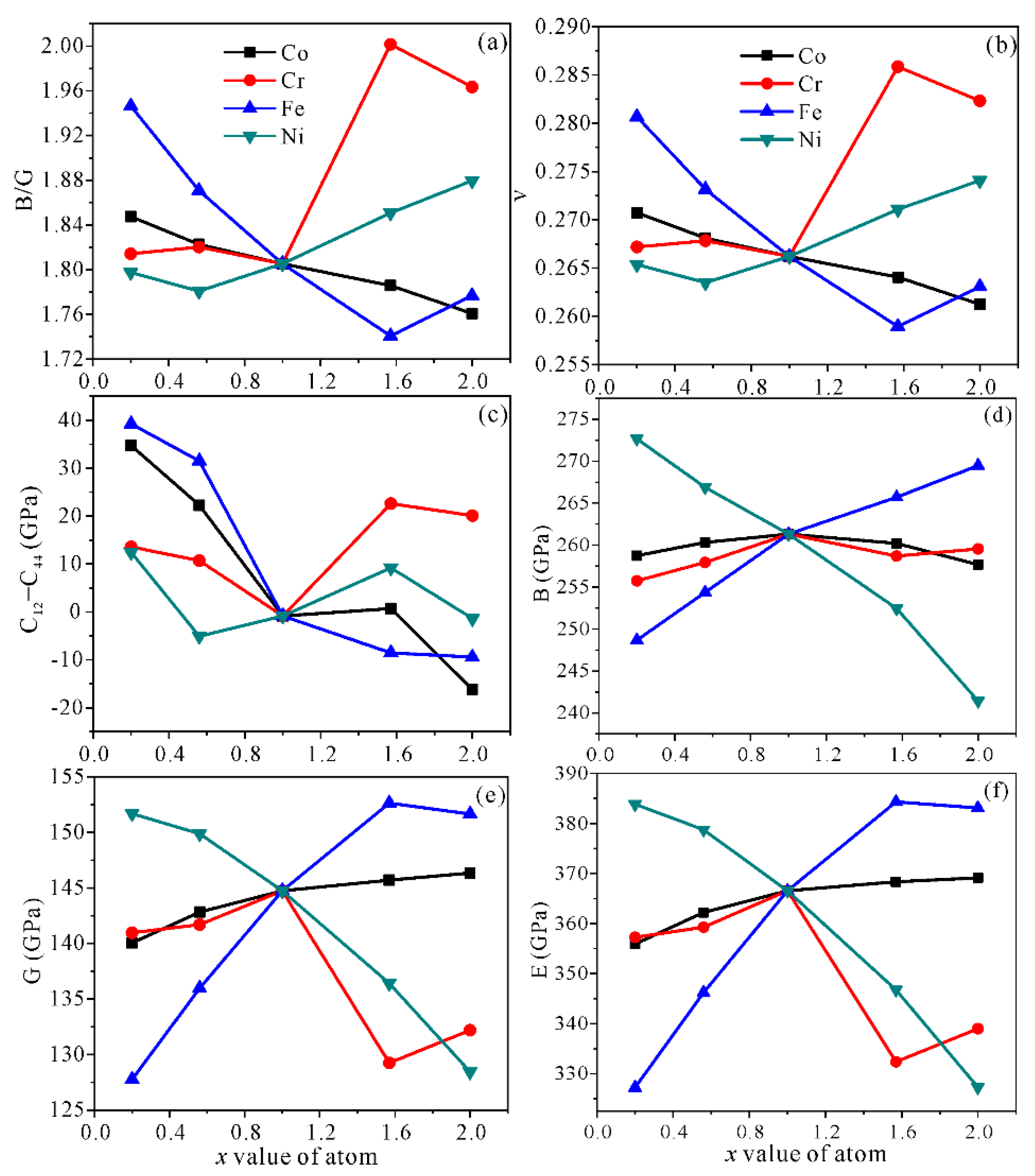
| Alloy | Alloy Model | x Value | x Elements/at.% | Other Elements/at.% |
|---|---|---|---|---|
| CoxCrFeNi | Co2Cr10Fe10Ni10 | 0.2 | 6.25 | 31.25 |
| Co5Cr9Fe9Ni9 | 0.56 | 15.625 | 28.125 | |
| Co8Cr8Fe8Ni8 | 1 | 25 | 25 | |
| Co11Cr7Fe7Ni7 | 1.57 | 34.375 | 21.875 | |
| Co8Cr4Fe4Ni4 | 2 | 40 | 20 | |
| CoCrxFeNi | Co10Cr2Fe10Ni10 | 0.2 | 6.25 | 31.25 |
| Co9Cr5Fe9Ni9 | 0.56 | 15.625 | 28.125 | |
| Co8Cr8Fe8Ni8 | 1 | 25 | 25 | |
| Co7Cr11Fe7Ni7 | 1.57 | 34.375 | 21.875 | |
| Co4Cr8Fe4Ni4 | 2 | 40 | 20 | |
| CoCrFexNi | Co10Cr10Fe2Ni10 | 0.2 | 6.25 | 31.25 |
| Co9Cr9Fe5Ni9 | 0.56 | 15.625 | 28.125 | |
| Co8Cr8Fe8Ni8 | 1 | 25 | 25 | |
| Co7Cr7Fe11Ni7 | 1.57 | 34.375 | 21.875 | |
| Co4Cr4Fe8Ni4 | 2 | 40 | 20 | |
| CoCrFeNix | Co10Cr10Fe10Ni2 | 0.2 | 6.25 | 31.25 |
| Co9Cr9Fe9Ni5 | 0.56 | 15.625 | 28.125 | |
| Co8Cr8Fe8Ni8 | 1 | 25 | 25 | |
| Co7Cr7Fe7Ni11 | 1.57 | 34.375 | 21.875 | |
| Co4Cr4Fe4Ni8 | 2 | 40 | 20 |
| x | C11 | C22 | C33 | C44 | C55 | C66 | C12 | C13 | C23 |
|---|---|---|---|---|---|---|---|---|---|
| Co-0.2 | 364.3 | 397.3 | 386.4 | 186.2 | 193.4 | 188.7 | 220.9 | 207.4 | 163.0 |
| Co-0.56 | 358.0 | 399.9 | 412.2 | 189.4 | 186.9 | 188.0 | 211.6 | 200.1 | 175.0 |
| Co-1 | 379.5 | 400.6 | 400.3 | 193.5 | 188.6 | 184.4 | 192.6 | 216.2 | 179.3 |
| Co-1.57 | 382.0 | 401.0 | 395.1 | 193.3 | 186.4 | 188.0 | 194.0 | 206.8 | 181.0 |
| Co-2 | 380.6 | 402.8 | 384.9 | 199.4 | 188.1 | 187.8 | 183.2 | 209.4 | 182.9 |
| Cr-0.2 | 379.7 | 371.3 | 384.5 | 184.7 | 184.5 | 194.3 | 198.3 | 188.5 | 196.4 |
| Cr-0.56 | 386.8 | 372.1 | 386.4 | 185.7 | 186.3 | 194.0 | 196.4 | 186.6 | 205.0 |
| Cr-1 | 379.5 | 400.6 | 400.3 | 193.5 | 188.6 | 184.4 | 192.6 | 216.2 | 179.3 |
| Cr-1.57 | 347.4 | 387.9 | 365.0 | 178.8 | 189.6 | 175.7 | 201.4 | 228.1 | 184.6 |
| Cr-2 | 346.0 | 372.2 | 362.5 | 190.6 | 200.6 | 192.8 | 210.7 | 222.8 | 194.2 |
| Fe-0.2 | 347.8 | 358.8 | 374.7 | 171.3 | 161.7 | 182.1 | 210.5 | 190.5 | 177.4 |
| Fe-0.56 | 366.0 | 375.0 | 389.4 | 181.7 | 171.6 | 183.7 | 213.2 | 191.8 | 174.6 |
| Fe-1 | 379.5 | 400.6 | 400.3 | 193.5 | 188.6 | 184.4 | 192.6 | 216.2 | 179.3 |
| Fe-1.57 | 391.5 | 418.0 | 406.8 | 203.1 | 194.4 | 196.5 | 194.5 | 213.8 | 179.3 |
| Fe-2 | 388.9 | 412.7 | 392.9 | 209.8 | 205.8 | 205.5 | 200.4 | 222.9 | 192.2 |
| Ni-0.2 | 386.4 | 402.3 | 401.6 | 204.6 | 212.9 | 215.7 | 217.0 | 213.8 | 201.1 |
| Ni-0.56 | 391.9 | 412.7 | 393.6 | 193.1 | 201.0 | 203.2 | 188.0 | 220.7 | 193.3 |
| Ni-1 | 379.5 | 400.6 | 400.3 | 193.5 | 188.6 | 184.4 | 192.6 | 216.2 | 179.3 |
| Ni-1.57 | 365.5 | 382.2 | 374.7 | 182.6 | 177.7 | 177.9 | 191.8 | 202.8 | 180.4 |
| Ni-2 | 344.4 | 370.1 | 358.3 | 175.9 | 166.6 | 161.7 | 174.5 | 199.8 | 175.9 |
| x | C11 | C12 | C44 | B | G | E | ν | B/G | Ref. | Model | Package | Magnetism |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CoCrFeNi | 379.48 | 192.62 | 193.47 | 261.31 | 144.75 | 366.56 | 0.266 | 1.81 | SQS | CASTEP | FM | |
| 376.63 | 197.04 | 184.71 | 245.3 | 146.73 | 367.01 | 0.251 | 1.67 | [58] | Single | VASP | NM | |
| 271 | 175 | 189 | 207 | 110 | 280 | 0.275 | 1.88 | [37] | EMTO-CPA | PM | ||
| 204 | 118.6 | 298 | 0.256 | 1.71 | [50] | USPEX | VASP | NM | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Xin, C.; Liu, L.; Zhuang, C. Effects of Different Contents of Each Component on the Structural Stability and Mechanical Properties of Co-Cr-Fe-Ni High-Entropy Alloys. Appl. Sci. 2021, 11, 2832. https://doi.org/10.3390/app11062832
Liu H, Xin C, Liu L, Zhuang C. Effects of Different Contents of Each Component on the Structural Stability and Mechanical Properties of Co-Cr-Fe-Ni High-Entropy Alloys. Applied Sciences. 2021; 11(6):2832. https://doi.org/10.3390/app11062832
Chicago/Turabian StyleLiu, Haibo, Cunlin Xin, Lei Liu, and Chunqiang Zhuang. 2021. "Effects of Different Contents of Each Component on the Structural Stability and Mechanical Properties of Co-Cr-Fe-Ni High-Entropy Alloys" Applied Sciences 11, no. 6: 2832. https://doi.org/10.3390/app11062832
APA StyleLiu, H., Xin, C., Liu, L., & Zhuang, C. (2021). Effects of Different Contents of Each Component on the Structural Stability and Mechanical Properties of Co-Cr-Fe-Ni High-Entropy Alloys. Applied Sciences, 11(6), 2832. https://doi.org/10.3390/app11062832







