Gross Ammonification and Nitrification Rates in Soil Amended with Natural and NH4-Enriched Chabazite Zeolite and Nitrification Inhibitor DMPP
Abstract
1. Introduction
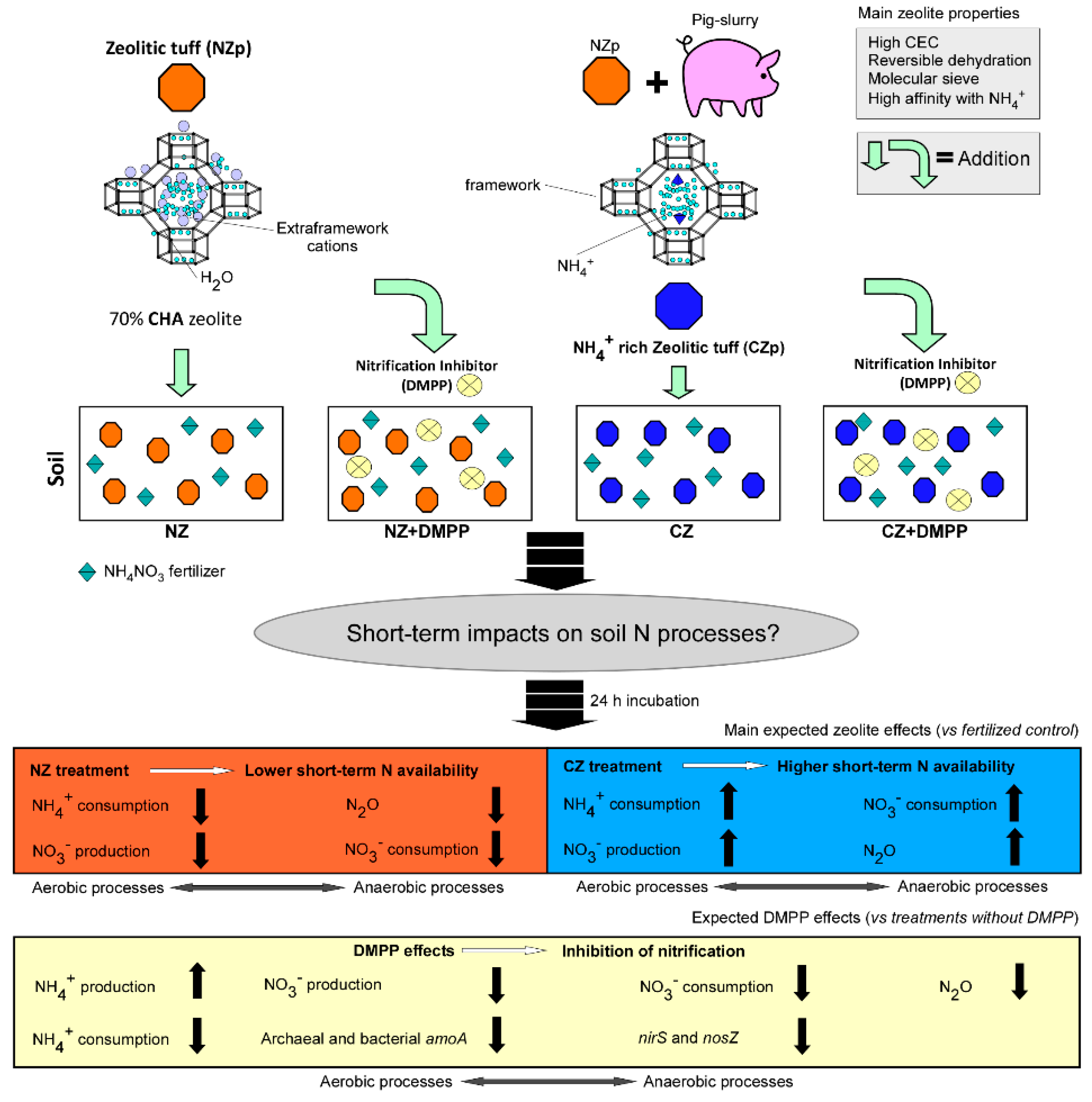
2. Materials and Methods
2.1. Zeolites
2.2. Soil
2.3. Experimental Setup
- unamended soil (CNTR);
- unamended soil (CNTR) + DMPP;
- soil with 10 wt% of NZp (NZ);
- soil with 10 wt% of NZp (NZ) + DMPP;
- soil with 10% of CZp (CZ);
- soil with 10% of CZp (CZ) + DMPP.
2.4. Basic Soil and Amendment Properties
2.5. 15N Pool Dilution
2.6. DNA Extraction and Quantitative Polymerase Chain Reaction (qPCR) of Functional Marker Genes
2.7. N2O and CO2 Measurements
2.8. Calculations and Statistical Analysis
3. Results
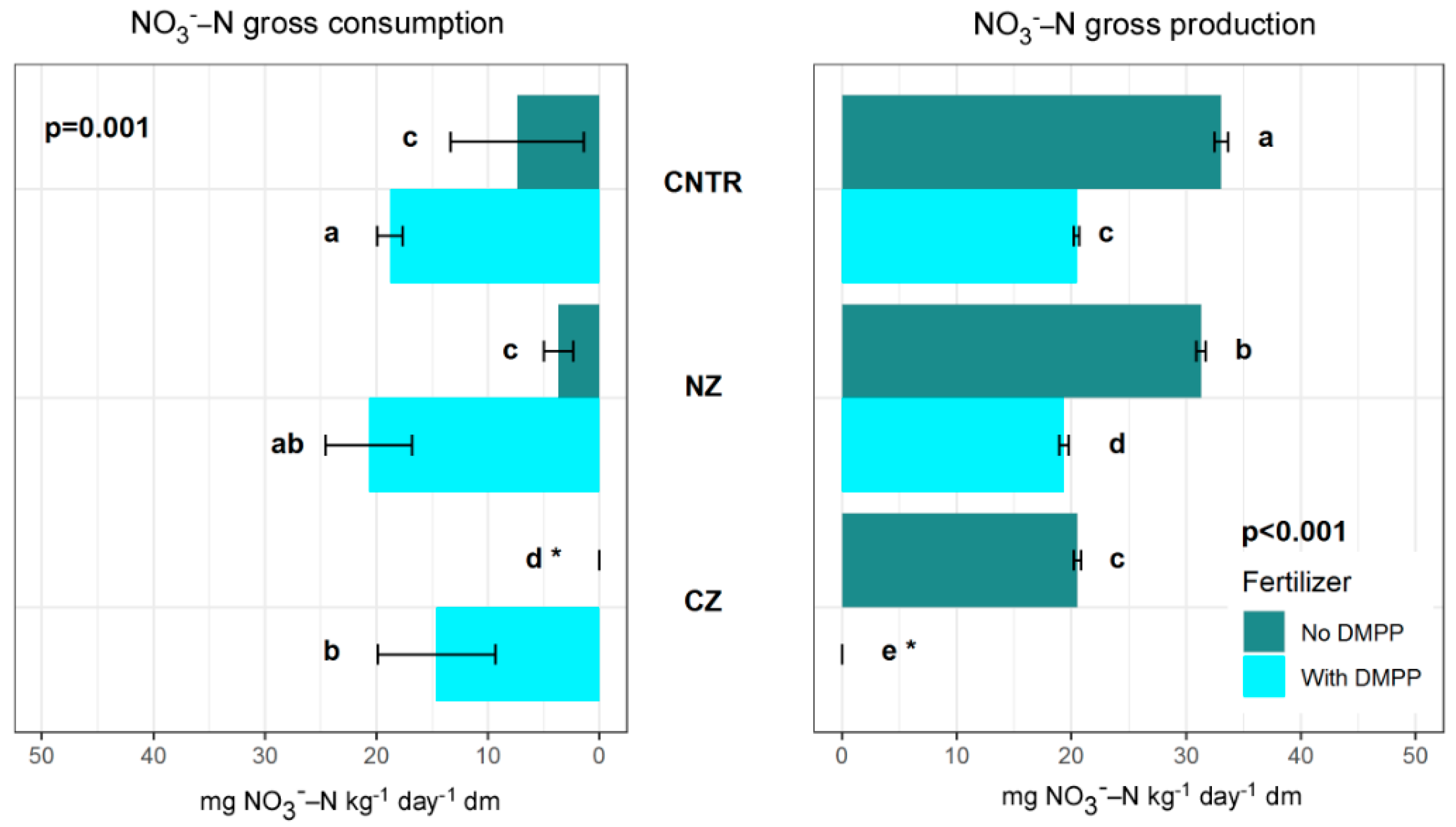
3.1. NH4+-N and NO3−-N Concentrations and Transformation Rates without DMPP
3.2. NH4+-N and NO3—N Concentrations and Transformation Rates with DMPP
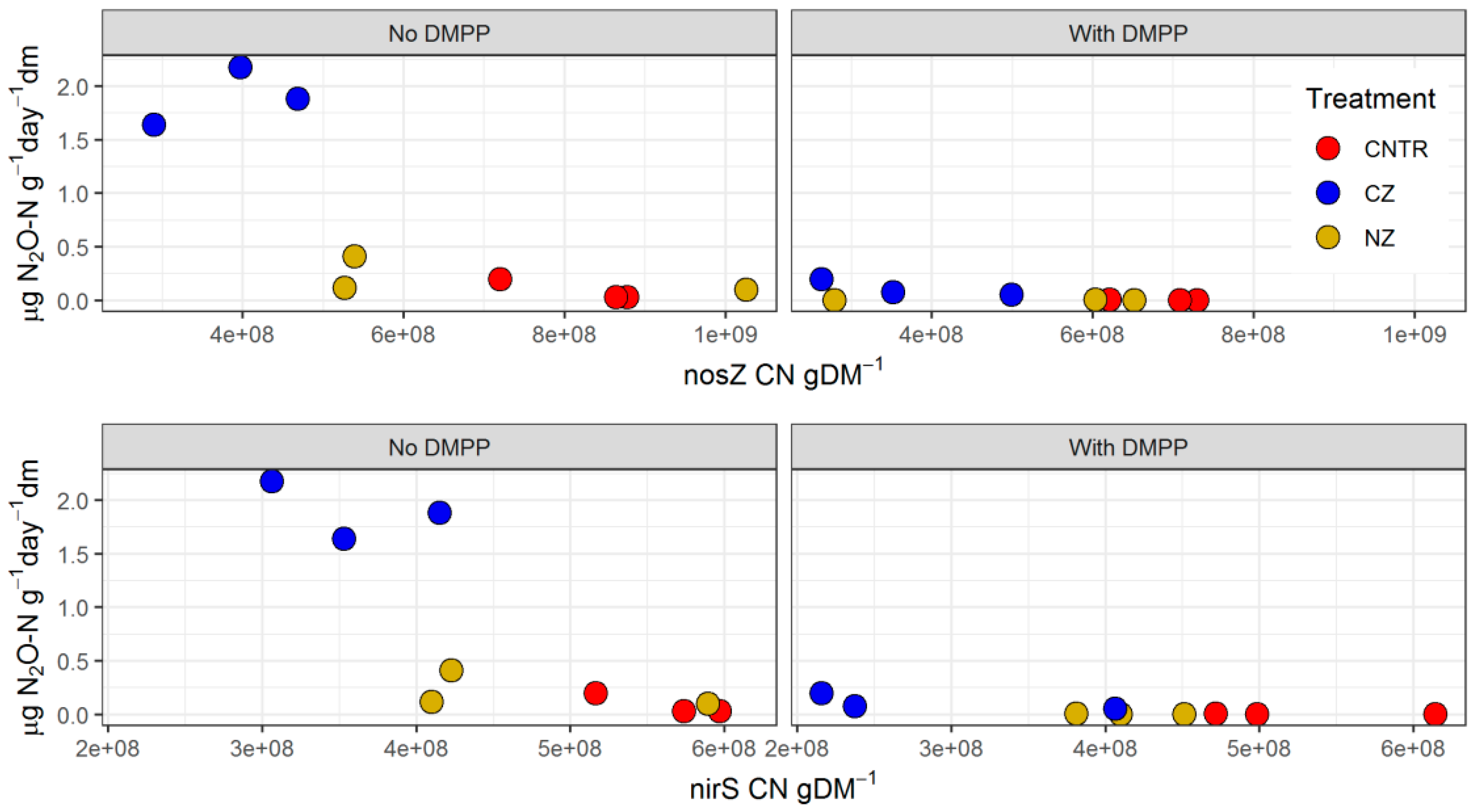
3.3. Abundance of Functional Genes (amoA AOA, amoA AOB, nirS, and nosZ)
3.4. N2O and CO2 Emissions
4. Discussion
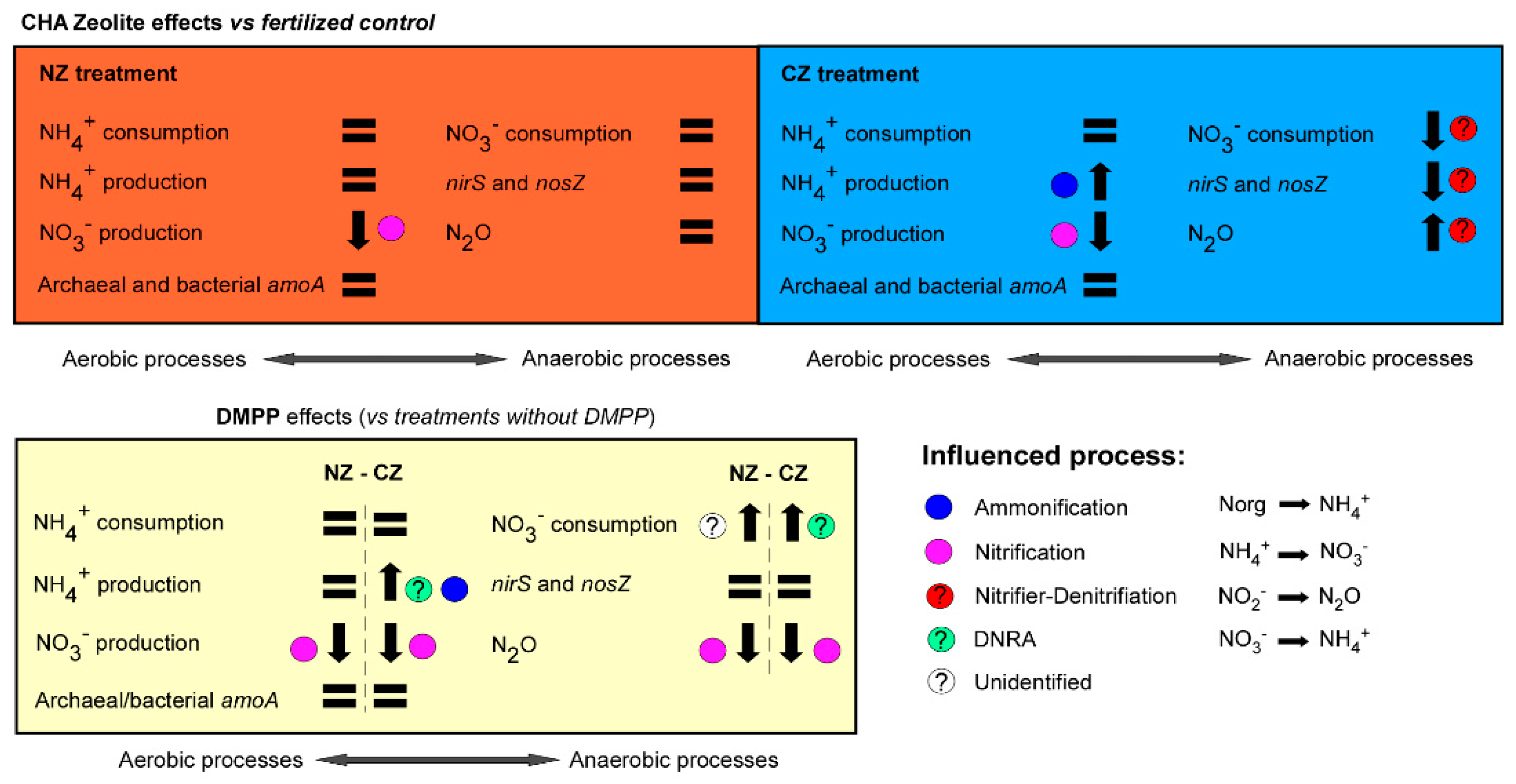
4.1. Effects of CHA in Natural State (without DMPP Addition)
4.2. Effects of CHA at NH4+-Enriched State (without DMPP Addition)
4.3. DMPP Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keiblinger, K.M.; Kral, R.M. Sustainable intensification of agricultural production: A review of four soil amendments. Bodenkultur 2018, 69, 141–153. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Ghalavand, A.; Khodaei-Joghan, A.; Dolatabadian, A.; Zakikhani, H.; Farmanbar, E. Zeolite-amended cattle manure effects on sunflower yield, seed quality, water use efficiency and nutrient leaching. Soil Tillage Res. 2013, 126, 193–202. [Google Scholar] [CrossRef]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Di Giuseppe, D.; Ferretti, G.; Faccini, B.; Blasi, E.; Passeri, N.; Bianchini, G.; Coltorti, M. Is it possible to cultivate corn in a sustainable way using a quarry waste? Period. Mineral. 2016, 85, 179–183. [Google Scholar]
- Sepaskhah, A.R.; Barzegar, M. Yield, water and nitrogen-use response of rice to zeolite and nitrogen fertilization in a semi-arid environment. Agric. Water Manag. 2010, 98, 38–44. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Sahrawat, K.L.; Nakahara, K.; Rao, I.M.; Ishitani, M.; Hash, C.T.; Kishii, M.; Bonnett, D.G.; Berry, W.L.; Lata, J.C. A paradigm shift towards low-nitrifying production systems: The role of biological nitrification inhibition (BNI). Ann. Bot. 2013, 112, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Di Giuseppe, D.; Natali, C.; Faccini, B.; Bianchini, G.; Coltorti, M. C-N elemental and isotopic investigation in agricultural soils: Insights on the effects of zeolitite amendments. Geochemistry 2017, 77, 45–52. [Google Scholar] [CrossRef]
- Faccini, B.; Di Giuseppe, D.; Ferretti, G.; Coltorti, M.; Colombani, N.; Mastrocicco, M. Natural and NH4+-enriched zeolitite amendment effects on nitrate leaching from a reclaimed agricultural soil (Ferrara Province, Italy). Nutr. Cycl. Agroecosyst. 2018, 110, 327–341. [Google Scholar] [CrossRef]
- Lima, D.L.D.; Santos, S.M.; Scherer, H.W.; Schneider, R.J.; Duarte, A.C.; Santos, E.B.H.; Esteves, V.I. Effects of organic and inorganic amendments on soil organic matter properties. Geoderma 2009, 150, 38–45. [Google Scholar] [CrossRef]
- Ferretti, G.; Keiblinger, K.M.; Di Giuseppe, D.; Faccini, B.; Colombani, N.; Zechmeister-Boltenstern, S.; Coltorti, M.; Mastrocicco, M. Short-Term Response of Soil Microbial Biomass to Different Chabazite Zeolite Amendments. Pedosphere 2018, 28, 277–287. [Google Scholar] [CrossRef]
- Biederman, L.A.; Stanley Harpole, W. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Ming, D.W.; Allen, E.R. Use of natural zeolites in agronomy, horticulture, and environmental soil remediation. Rev. Mineral. Geochem. 2001, 45, 618–654. [Google Scholar] [CrossRef]
- Ahmed, O.H.; Braine Yap, C.H.; Nik Muhamad, A.M. Minimizing ammonia loss from urea through mixing with zeolite and acid sulphate soil. Int. J. Phys. Sci. 2010, 5, 2198–2202. [Google Scholar]
- Dwairi, I.M. Evaluation of jordanian zeolite tuff as a controlled slow-release fertilizer for NH4+. Environ. Geol. 1998, 34, 1–4. [Google Scholar] [CrossRef]
- Passaglia, E. Zeoliti Naturali, Zeolititi e Loro Applicazioni; Arvan: Mira, Venice, Italy, 2008; ISBN 9788887801194. [Google Scholar]
- Söderström, B.; Hedlund, K.; Jackson, L.E.; Kätterer, T.; Lugato, E.; Thomsen, I.K.; Bracht Jørgensen, H. What are the effects of agricultural management on soil organic carbon (SOC) stocks? Environ. Evid. 2014, 3, 2. [Google Scholar] [CrossRef]
- Ferretti, G.; Di Giuseppe, D.; Faccini, B.; Coltorti, M. Mitigation of sodium risk in a sandy agricultural soil by the use of natural zeolites. Environ. Monit. Assess. 2018, 190, 646. [Google Scholar] [CrossRef] [PubMed]
- Coombs, D.S.; Alberti, A.; Armbruster, T.; Artioli, G.; Colella, C.; Galli, E.; Grice, J.D.; Liebau, F.; Mandarino, J.A.; Minato, H.; et al. Recommended nomenclature for zeolite minerals: Report of the subcommittee on zeolites of the International Mineralogical Association, Commission on new Minerals and Mineral names. Can. Mineral. 1997, 35, 1571–1606. [Google Scholar]
- Moshoeshoe, M.; Silas Nadiye-Tabbiruka, M.; Obuseng, V. A Review of the Chemistry, Structure, Properties and Applications of Zeolites. Am. J. Mater. Sci. 2017, 7, 196–221. [Google Scholar]
- Vilcek, J.; Torma, S.; Adamisin, P.; Hronec, O. Nitrogen sorption and its release in the soil after zeolite application. Bulg. J. Agric. Sci. 2013, 19, 228–234. [Google Scholar]
- Omar, L.; Ahmed, O.H.; Majid, N.M.A. Improving ammonium and nitrate release from urea using clinoptilolite zeolite and compost produced from agricultural wastes. Sci. World J. 2015, 2015. [Google Scholar] [CrossRef]
- Ferretti, G.; Keiblinger, K.M.; Zimmermann, M.; Di Giuseppe, D.; Faccini, B.; Colombani, N.; Mentler, A.; Zechmeister-Boltenstern, S.; Coltorti, M.; Mastrocicco, M. High resolution short-term investigation of soil CO2, N2O, NOx and NH3 emissions after different chabazite zeolite amendments. Appl. Soil Ecol. 2017, 119, 138–144. [Google Scholar] [CrossRef]
- Kesraoui-Ouki, S.; Cheeseman, C.R.; Perry, R. Natural zeolite utilisation in pollution control: A review of applications to metals’ effluents. J. Chem. Technol. Biotechnol. 1994, 59, 121–126. [Google Scholar] [CrossRef]
- Galli, E.; Passaglia, E. Natural zeolites in environmental engineering. In Zeolites in Chemical Engineering; Holzapfel, H., Ed.; Verlag ProcessEng Engineering GmbH: Vienna, Austria, 2011; pp. 392–416. ISBN 3902655089. [Google Scholar]
- Delkash, M.; Ebrazi Bakhshayesh, B.; Kazemian, H. Using zeolitic adsorbents to cleanup special wastewater streams: A review. Microporous Mesoporous Mater. 2015, 214, 224–241. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Passaglia, E. Rietveld structure refinement of NH4-exchanged natural chabazite. Eur. J. Mineral. 2006, 18, 351–359. [Google Scholar] [CrossRef]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Faccini, B.; Antisari, L.V.; Di Giuseppe, D.; Coltorti, M. 15N natural abundance, nitrogen and carbon pools in soil-sorghum system amended with natural and NH4+-enriched zeolitites. Appl. Sci. 2019, 9, 4524. [Google Scholar] [CrossRef]
- Reháková, M.; Čuvanová, S.; Dzivák, M.; Rimár, J.; Gaval’Ová, Z. Agricultural and agrochemical uses of natural zeolite of the clinoptilolite type. Curr. Opin. Solid State Mater. Sci. 2004, 8, 397–404. [Google Scholar] [CrossRef]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Microporous Mesoporous Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and actual uses of zeolites in crop protection. Pest Manag. Sci. 2015, 71, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, J.A.; Tarkalson, D.D.; Lehrsch, G.A. Zeolite soil application method affects inorganic nitrogen, moisture, and corn growth. Soil Sci. 2011, 176, 136–142. [Google Scholar] [CrossRef]
- Zaman, M.; Nguyen, M.L.; Matheson, F.; Blennerhassett, J.D.; Quin, B.F. Can soil amendments (zeolite or lime) shift the balance between nitrous oxide and dinitrogen emissions from pasture and wetland soils receiving urine or urea-N? Aust. J. Soil Res. 2007, 45, 543–553. [Google Scholar] [CrossRef]
- Li, J.; Nedwell, D.B.; Beddow, J.; Dumbrell, A.J.; McKew, B.A.; Thorpe, E.L.; Whitby, C. amoA Gene Abundances and Nitrification Potential Rates Suggest that Benthic Ammonia-Oxidizing Bacteria and Not Archaea Dominate N Cycling in the Colne Estuary, United Kingdom. Appl. Environ. Microbiol. 2015, 81, 159–165. [Google Scholar] [CrossRef]
- Friedl, J.; Scheer, C.; Rowlings, D.W.; Mumford, M.T.; Grace, P.R. The nitrification inhibitor DMPP (3,4-dimethylpyrazole phosphate) reduces N2 emissions from intensively managed pastures in subtropical Australia. Soil Biol. Biochem. 2017, 8, 55–64. [Google Scholar] [CrossRef]
- Keuschnig, C.; Gorfer, M.; Li, G.; Mania, D.; Frostegård, Å.; Bakken, L.; Larose, C. NO and N2O transformations of diverse fungi in hypoxia: Evidence for anaerobic respiration only in Fusarium strains. Environ. Microbiol. 2020, 22, 2182–2195. [Google Scholar] [CrossRef]
- Olaya-Abril, A.; Hidalgo-Carrillo, J.; Luque-Almagro, V.M.; Fuentes-Almagro, C.; Urbano, F.J.; Moreno-Vivián, C.; Richardson, D.J.; Roldán, M.D. Exploring the Denitrification Proteome of Paracoccus denitrificans PD1222. Front. Microbiol. 2018, 9, 1137. [Google Scholar] [CrossRef] [PubMed]
- Friedl, J.; Scheer, C.; Rowlings, D.W.; Deltedesco, E.; Gorfer, M.; De Rosa, D.; Grace, P.R.; Müller, C.; Keiblinger, K.M. Effect of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on N-turnover, the N2O reductase-gene nosZ and N2O:N2 partitioning from agricultural soils. Sci. Rep. 2020, 10, 2399. [Google Scholar] [CrossRef] [PubMed]
- Hallin, S.; Philippot, L.; Löffler, F.E.; Sanford, R.A.; Jones, C.M. Genomics and Ecology of Novel N2O-Reducing Microorganisms. Trends Microbiol. 2018, 26, 43–55. [Google Scholar] [CrossRef]
- Smith, C.J.; Nedwell, D.B.; Dong, L.F.; Osborn, A.M. Diversity and abundance of nitrate reductase genes (narG and napA), nitrite reductase genes (nirS and nrfA), and their transcripts in estuarine sediments. Appl. Environ. Microbiol. 2007, 73, 3612–3622. [Google Scholar] [CrossRef]
- Bárta, J.; Melichová, T.; Vaněk, D.; Picek, T.; Šantrůčková, H. Effect of pH and dissolved organic matter on the abundance of nirK and nirS denitrifiers in spruce forest soil. Biogeochemistry 2010, 101, 123–132. [Google Scholar] [CrossRef]
- Galamini, G.; Ferretti, G.; Medoro, V.; Tescaro, N.; Faccini, B.; Coltorti, M. Isotherms, kinetics, and thermodynamics of nh4 + adsorption in raw liquid manure by using natural chabazite zeolite-rich tuff. Water 2020, 12, 2944. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Monsivais-Rocha, J.E.; Aragon-Piña, A.; Berber-Mendoza, M.S.; Guerrero-Coronado, R.M.; Alonso-Davila, P.; Mendoza-Barron, J. Removal of ammonium from aqueous solution by ion exchange on natural and modified chabazite. J. Environ. Manag. 2010, 91, 2662–2668. [Google Scholar] [CrossRef] [PubMed]
- Leggo, P.J. An investigation of plant growth in an organo-zeolitic substrate and its ecological significance. Plant Soil 2000, 219, 135–146. [Google Scholar] [CrossRef]
- McGilloway, R.L.; Weaver, R.W.; Ming, D.W.; Gruener, J.E. Nitrification in a zeoponic substrate. Plant Soil 2003, 256, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Hu, H.W.; Müller, C.; He, J.Z.; Chen, D.; Suter, H.C. Effects of the nitrification inhibitor 3,4-dimethylpyrazole phosphate on nitrification and nitrifiers in two contrasting agricultural soils. Appl. Environ. Microbiol. 2016, 82, 5236–5248. [Google Scholar] [CrossRef] [PubMed]
- Marsden, K.A.; Marín-Martínez, A.J.; Vallejo, A.; Hill, P.W.; Jones, D.L.; Chadwick, D.R. The mobility of nitrification inhibitors under simulated ruminant urine deposition and rainfall: A comparison between DCD and DMPP. Biol. Fertil. Soils 2016, 52, 491–503. [Google Scholar] [CrossRef]
- Zerulla, W.; Barth, T.; Dressel, J.; Erhardt, K.; Horchler von Locquenghien, K.; Pasda, G.; Rädle, M.; Wissemeier, A. 3,4-Dimethylpyrazole phosphate (DMPP)—A new nitrification inhibitor for agriculture and horticulture. An introduction. Biol. Fertil. Soils 2001, 34, 79–84. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Zheng, X. Effects of nitrification inhibitors (DCD and DMPP) on nitrous oxide emission, crop yield and nitrogen uptake in a wheat-maize cropping system. Biogeosciences 2013, 10, 2427–2437. [Google Scholar] [CrossRef]
- Mahmood, T.; Ali, R.; Latif, Z.; Ishaque, W. Dicyandiamide increases the fertilizer N loss from an alkaline calcareous soil treated with 15N-labelled urea under warm climate and under different crops. Biol. Fertil. Soils 2011, 47, 619–631. [Google Scholar] [CrossRef]
- Benckiser, G.; Christ, E.; Herbert, T.; Weiske, A.; Blome, J.; Hardt, M. The nitrification inhibitor 3,4-dimethylpyrazole-phosphat (DMPP)—Quantification and effects on soil metabolism. Plant Soil 2013, 371, 257–266. [Google Scholar] [CrossRef]
- Fan, X.; Yin, C.; Chen, H.; Ye, M.; Zhao, Y.; Li, T.; Wakelin, S.A.; Liang, Y. The efficacy of 3,4-dimethylpyrazole phosphate on N 2 O emissions is linked to niche differentiation of ammonia oxidizing archaea and bacteria across four arable soils. Soil Biol. Biochem. 2019, 130, 82–93. [Google Scholar] [CrossRef]
- Hatch, D.; Trindade, H.; Cardenas, L.; Carneiro, J.; Hawkins, J.; Scholefield, D.; Chadwick, D. Laboratory study of the effects of two nitrification inhibitors on greenhouse gas emissions from a slurry-treated arable soil: Impact of diurnal temperature cycle. Biol. Fertil. Soils 2005, 41, 225–232. [Google Scholar] [CrossRef]
- Weiske, A.; Benckiser, G.; Ottow, J.C.G. The new nitrification inhibitor DMPP—Effects on gaseous emissions (N2O, CO2, CH4) from soil under field conditions. In Plant Nutrition; Horst, W.J., Schenk, M.K., Bürkert, A., Claassen, N., Flessa, H., Frommer, W.B., Goldbach, H., Olfs, H.-W., Römheld, V., Sattelmacher, B., et al., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 766–767. [Google Scholar]
- Zhu, G.; Ju, X.; Zhang, J.; Müller, C.; Rees, R.M.; Thorman, R.E.; Sylvester-Bradley, R. Effects of the nitrification inhibitor DMPP (3,4-dimethylpyrazole phosphate) on gross N transformation rates and N2O emissions. Biol. Fertil. Soils 2019, 55, 603–615. [Google Scholar] [CrossRef]
- Barth, G.; Von Tucher, S.; Schmidhalter, U. Influence of soil parameters on the effect of 3,4-dimethylpyrazole-phosphate as a nitrification inhibitor. Biol. Fertil. Soils 2001, 34, 98–102. [Google Scholar]
- Keiblinger, K.M.; Zehetner, F.; Mentler, A.; Zechmeister-Boltenstern, S. Biochar application increases sorption of nitrification inhibitor 3,4-dimethylpyrazole phosphate in soil. Environ. Sci. Pollut. Res. 2018, 25, 11173–11177. [Google Scholar] [CrossRef]
- Ferretti, G.; Keiblinger, K.M.; Faccini, B.; Di Giuseppe, D.; Mentler, A.; Zechmeister-Boltenstern, S.; Coltorti, M. Effects of Different Chabazite Zeolite Amendments to Sorption of Nitrification Inhibitor 3,4-Dimethylpyrazole Phosphate (DMPP) in Soil. J. Soil Sci. Plant Nutr. 2020, 20, 973–978. [Google Scholar] [CrossRef]
- Malferrari, D.; Laurora, A.; Brigatti, M.F.; Coltorti, M.; Di Giuseppe, D.; Faccini, B.; Passaglia, E.; Vezzalini, M.G. Open-field experimentation of an innovative and integrated zeolitite cycle: Project definition and material characterization. Rend. Lincei 2013, 24, 141–150. [Google Scholar] [CrossRef]
- Faccini, B.; Di Giuseppe, D.; Malferrari, D.; Coltorti, M.; Abbondanzi, F.; Campisi, T.; Laurora, A.; Passaglia, E. Ammonium-exchanged zeolitite preparation for agricultural uses: From laboratory tests to large-scale application in ZeoLIFE project prototype. Period. Mineral. 2015, 84, 303–321. [Google Scholar]
- Di Giuseppe, D.; Faccini, B.; Mastrocicco, M.; Colombani, N.; Coltorti, M. Reclamation influence and background geochemistry of neutral saline soils in the Po River Delta Plain (Northern Italy). Environ. Earth Sci. 2014, 72, 2457–2473. [Google Scholar] [CrossRef]
- Baxter, S. World Reference Base for Soil Resources; World Soil Resources Report 103; US$22.00 (paperback); Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; Volume 43, p. 132. ISBN 92-5-10511-4. [Google Scholar]
- Shand, C.A.; Williams, B.L.; Coutts, G. Determination of N-species in soil extracts using microplate techniques. Talanta 2008, 74, 648–654. [Google Scholar] [CrossRef]
- Hood-Nowotny, R.; Umana, N.H.-N.; Inselbacher, E.; Oswald-Lachouani, P.; Wanek, W. Alternative Methods for Measuring Inorganic, Organic, and Total Dissolved Nitrogen in Soil. Soil Sci. Soc. Am. J. 2010, 74, 1018–1027. [Google Scholar] [CrossRef]
- Masse, J.; Prescott, C.E.; Müller, C.; Grayston, S.J. Gross nitrogen transformation rates differ in reconstructed oil-sand soils from natural boreal-forest soils as revealed using a 15N tracing method. Geoderma 2016, 282, 37–48. [Google Scholar] [CrossRef]
- Harter, J.; Krause, H.M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J. 2014, 8, 660–674. [Google Scholar] [CrossRef]
- Wei, W.; Isobe, K.; Nishizawa, T.; Zhu, L.; Shiratori, Y.; Ohte, N.; Koba, K.; Otsuka, S.; Senoo, K. Higher diversity and abundance of denitrifying microorganisms in environments than considered previously. ISME J. 2015, 9, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Leitner, S.; Sae-Tun, O.; Kranzinger, L.; Zechmeister-Boltenstern, S.; Zimmermann, M. Contribution of litter layer to soil greenhouse gas emissions in a temperate beech forest. Plant Soil 2016, 403, 455–469. [Google Scholar] [CrossRef]
- Deltedesco, E.; Keiblinger, K.M.; Naynar, M.; Piepho, H.P.; Gorfer, M.; Herndl, M.; Bahn, M.; Pötsch, E.M.; Zechmeister-Boltenstern, S. Trace gas fluxes from managed grassland soil subject to multifactorial climate change manipulation. Appl. Soil Ecol. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Kaiser, C.; Fuchslueger, L.; Koranda, M.; Gorfer, M.; Stange, C.F.; Kitzler, B.; Rasche, F.; Strauss, J.; Sessitsch, A.; Zechmeister-Boltenstern, S.; et al. Plants control the seasonal dynamics of microbial N cycling in a beech forest soil by belowground C allocation. Ecology 2011, 92, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Comeau, L.-P.; Lai, D.Y.F.; Cui, J.J.; Hartill, J. Soil heterotrophic respiration assessment using minimally disturbed soil microcosm cores. MethodsX 2018, 5, 834–840. [Google Scholar] [CrossRef]
- Bodenhofer, U.; Kothmeier, A.; Hochreiter, S. Apcluster: An R package for affinity propagation clustering. Bioinformatics 2011, 27, 2463–2464. [Google Scholar] [CrossRef]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.2-0. 2014. Available online: http://CRAN.R-project.org/package=agricolae (accessed on 9 March 2021).
- Zhang, J.; Sui, Q.; Li, K.; Chen, M.; Tong, J.; Qi, L.; Wei, Y. Influence of natural zeolite and nitrification inhibitor on organics degradation and nitrogen transformation during sludge composting. Environ. Sci. Pollut. Res. 2016, 23, 1324–1334. [Google Scholar] [CrossRef]
- Tiedje, J.M. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. Environ. Microbiol. Anaerobes 1988, 717, 179–244. [Google Scholar]
- Friedl, J.; De Rosa, D.; Rowlings, D.W.; Grace, P.R.; Müller, C.; Scheer, C. Dissimilatory nitrate reduction to ammonium (DNRA), not denitrification dominates nitrate reduction in subtropical pasture soils upon rewetting. Soil Biol. Biochem. 2018, 125, 340–349. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Jalota, S.K.; Vashisht, B.B.; Sharma, S.; Kaur, S. Chapter 1—Emission of Greenhouse Gases and Their Warming Effect. In Understanding Climate Change Impacts on Crop Productivity and Water Balance; Academic Press: Cambridge, MA, USA, 2018; pp. 1–53. ISBN 978-0-12-809520-1. [Google Scholar]
- Stojanovic, B.J.; Alexander, M. Effect of inorganic nitrogen on nitrification. Soil Sci. 1958, 86, 208–215. [Google Scholar] [CrossRef]
- Morrill, L.G.; Dawson, J.E. Patterns Observed for the Oxidation of Ammonium to Nitrate by Soil Organisms. Soil Sci. Soc. Am. J. 1967, 31, 757–760. [Google Scholar] [CrossRef]
- Breuillin-Sessoms, F.; Venterea, R.T.; Sadowsky, M.J.; Coulter, J.A.; Clough, T.J.; Wang, P. Nitrification gene ratio and free ammonia explain nitrite and nitrous oxide production in urea-amended soils. Soil Biol. Biochem. 2017, 111, 143–153. [Google Scholar] [CrossRef]
- Wrage-Mönnig, N.; Horn, M.A.; Well, R.; Müller, C.; Velthof, G.; Oenema, O. The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol. Biochem. 2018, 123, A3–A16. [Google Scholar] [CrossRef]
- McGeough, K.L.; Watson, C.J.; Müller, C.; Laughlin, R.J.; Chadwick, D.R. Evidence that the efficacy of the nitrification inhibitor dicyandiamide (DCD) is affected by soil properties in UK soils. Soil Biol. Biochem. 2016, 94, 222–232. [Google Scholar] [CrossRef]
- Tosi, M.; Brown, S.; Ferrari Machado, P.V.; Wagner-Riddle, C.; Dunfield, K. Short-term response of soil N-cycling genes and transcripts to fertilization with nitrification and urease inhibitors, and relationship with field-scale N2O emissions. Soil Biol. Biochem. 2020, 142, 107703. [Google Scholar] [CrossRef]
- Yang, M.; Fang, Y.; Sun, D.; Shi, Y. Efficiency of two nitrification inhibitors (dicyandiamide and 3,4-dimethypyrazole phosphate) on soil nitrogen transformations and plant productivity: A meta-analysis. Sci. Rep. 2016, 6, 22075. [Google Scholar] [CrossRef]
- Huang, T.; Gao, B.; Hu, X.-K.; Lu, X.; Well, R.; Christie, P.; Bakken, L.R.; Ju, X.-T. Ammonia-oxidation as an engine to generate nitrous oxide in an intensively managed calcareous Fluvo-aquic soil. Sci. Rep. 2015, 4, 3950. [Google Scholar] [CrossRef]

| Sample | pH | EC mS cm−1 | Total N g kg−1 | Total C g kg−1 | Total OC g kg−1 |
|---|---|---|---|---|---|
| CNTR | 7.59 ± 0.05 a | 1.44 ± 0.04 c | 2.09 ± 0.10 c | 33.47 ± 0.76 a | 21.04 ± 0.23 a |
| NZp | 7.58 ± 0.05 a | 0.54 ± 0.07 e | 0.10 ± 0.01 e | 0.80 ± 0.10 e | nd |
| CZp | 6.95 ± 0.09 b | 15.03 ± 0.63 a | 4.27 ± 0.12 a | 1.53 ± 0.08 d | 0.08 ± 0.01 d |
| NZ | 7.55 ± 0.03 a | 1.34 ± 0.02 d | 1.76 ± 0.01 d | 27.69 ± 0.75 c | 16.48 ± 0.03 c |
| CZ | 7.63 ± 0.04 a | 2.56 ± 0.11 b | 2.27 ± 0.07 b | 29.78 ± 0.78 b | 18.10 ± 0.01 b |
| Sample | NH4+-N t0 mg kg−1 | NH4+-N t24 mg kg−1 | NH4+-N Net mg kg−1day−1 | NO3−-N t0 mg kg−1 | NO3−-N t24 mg kg−1 | NO3−-N Net mg kg−1day−1 | |
|---|---|---|---|---|---|---|---|
| No DMPP | CNTR | 97.4 ± 2.8 b | 65.8 ± 1.3 e | −31.5 ± 3.8 e | 123 ± 4 b | 149 ± 4 c | 25.7 ± 5.8 a |
| NZ | 96.4 ± 1.0 b | 68.6 ± 1.9 d | −27.8 ± 1.9 e | 118 ± 2 bc | 146 ± 2 c | 27.6 ± 1.4 a | |
| CZ | 293 ± 22 a | 355 ± 15 a | 61.8 ± 26.9 b | 303 ± 9 a | 337 ± 2 a | 33.6 ± 8.9 a | |
| With DMPP | CNTR | 96.8 ± 2.3 b | 94.3 ± 1.1 b | −2.5 ± 1.9 c | 114 ± 1 d | 116 ± 2 d | 1.7 ± 1.2 b |
| NZ | 95.0 ± 2.8 b | 79.0 ± 4.5 c | −16.1 ± 3.4 d | 116 ± 0.4 c | 115 ± 5 d | −1.4 ± 4.2 b | |
| CZ | 269 ± 19 a | 368 ± 6 a | 98.3 ± 17 a | 296 ± 3 a | 281 ± 4 b | −14.6 ± 5.2 c |
| Sample | amoA (AOA) CN g−1 × 1012 | amoA (AOB) CN g−1 × 109 | nirS CN g−1 × 108 | nosZ CN g−1 × 108 | |
|---|---|---|---|---|---|
| No DMPP | CNTR | 10.3 ± 3.19 a | 2.43 ± 0.47 a | 5.62 ± 0.41 a | 8.20 ± 0.87 a |
| NZ | 8.00 ± 1.61 a | 2.56 ± 0.43 a | 4.74 ± 1.00 abc | 6.97 ± 2.85 a | |
| CZ | 9.30 ± 2.06 a | 2.61 ± 0.29 a | 3.58 ± 0.55 bc | 3.85 ± 0.90 b | |
| With DMPP | CNTR | 10.1 ± 1.32 a | 1.93 ± 0.55 a | 5.28 ± 0.76 ab | 6.86 ± 0.58 a |
| NZ | 10.4 ± 1.84 a | 1.60 ± 0.92 a | 4.14 ± 0.35 abc | 5.11 ± 2.03 ab | |
| CZ | 7.13 ± 3.18 a | 2.71 ± 0.19 a | 2.87 ± 1.04 c | 3.71 ± 1.19 b |
| Sample | N2O-N Emissions µg N2O-N g−1 day−1 | CO2-C Emissions µg CO2-C g−1 day−1 | |
|---|---|---|---|
| No DMPP | CNTR | 0.127 ± 0.108 b | 21.67 ± 19.55 bc |
| NZ | 0.196 ± 0.148 b | 25.15 ± 6.55 bc | |
| CZ | 1.876 ± 0.226 a | 46.43 ± 4.89 a | |
| With DMPP | CNTR | 0.0061 ± 0.0031 c | 32.62 ± 11.81 ab |
| NZ | 0.0049 ± 0.0034 c | 16.98 ± 1.55 c | |
| CZ | 0.122 ± 0.068 b | 28.88 ± 1.94 bc |
| Treatment | N Input NH4NO3 kg N ha−1 | N Input CZp kg N ha−1 | N2O-N/N Added µg g−1 day−1 (×100) | ¥ DMPP Reduction vs. CNTR Fertilized | ¥ µg N2O-N Inhibited by DMPP | |
|---|---|---|---|---|---|---|
| No DMPP | CNTR | 170 | 0 | 0.075 ± 0.064 bc | - | - |
| NZ | 170 | 0 | 0.115 ± 0.087 b | - | - | |
| CZ | 170 | 427 | 0.314 ± 0.043 a | - | - | |
| With DMPP | CNTR | 170 | 0 | 0.004 ± 0.002 d | 95% | 0.121 |
| NZ | 170 | 0 | 0.003 ± 0.002 d | 96% | 0.191 | |
| CZ | 170 | 427 | 0.020 ± 0.011 c | 72% | 1.754 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferretti, G.; Galamini, G.; Deltedesco, E.; Gorfer, M.; Fritz, J.; Faccini, B.; Mentler, A.; Zechmeister-Boltenstern, S.; Coltorti, M.; Keiblinger, K.M. Gross Ammonification and Nitrification Rates in Soil Amended with Natural and NH4-Enriched Chabazite Zeolite and Nitrification Inhibitor DMPP. Appl. Sci. 2021, 11, 2605. https://doi.org/10.3390/app11062605
Ferretti G, Galamini G, Deltedesco E, Gorfer M, Fritz J, Faccini B, Mentler A, Zechmeister-Boltenstern S, Coltorti M, Keiblinger KM. Gross Ammonification and Nitrification Rates in Soil Amended with Natural and NH4-Enriched Chabazite Zeolite and Nitrification Inhibitor DMPP. Applied Sciences. 2021; 11(6):2605. https://doi.org/10.3390/app11062605
Chicago/Turabian StyleFerretti, Giacomo, Giulio Galamini, Evi Deltedesco, Markus Gorfer, Jennifer Fritz, Barbara Faccini, Axel Mentler, Sophie Zechmeister-Boltenstern, Massimo Coltorti, and Katharina Maria Keiblinger. 2021. "Gross Ammonification and Nitrification Rates in Soil Amended with Natural and NH4-Enriched Chabazite Zeolite and Nitrification Inhibitor DMPP" Applied Sciences 11, no. 6: 2605. https://doi.org/10.3390/app11062605
APA StyleFerretti, G., Galamini, G., Deltedesco, E., Gorfer, M., Fritz, J., Faccini, B., Mentler, A., Zechmeister-Boltenstern, S., Coltorti, M., & Keiblinger, K. M. (2021). Gross Ammonification and Nitrification Rates in Soil Amended with Natural and NH4-Enriched Chabazite Zeolite and Nitrification Inhibitor DMPP. Applied Sciences, 11(6), 2605. https://doi.org/10.3390/app11062605







