Abstract
The bone tissue destruction during drilling is still one of the crucial problems in implantology. In this study, the influence of drilling speed, coolant presence, and its temperature on bone tissue was tested using swine rib as a biological model of human jaws. The same method of drilling (with or without coolant) was used in all tested samples. The microscopic investigation estimated the size of the destruction zone and morphology of bone tissue surrounding the drilling canal. The achieved results were statistically elaborated. The study proved that the optimal drilling speed was ca. 1200 rpm, but the temperature of the used coolant had no significant influence on provoked bone destruction. Simultaneously, the drilling system without coolant compared to this with coolant has statistical importance on drilling results. Further in vivo studies will verify the obtained results.
1. Introduction
The first reports of implantology were recorded in ancient Egypt, where two teeth were connected by a gold wire passed through holes. This discovery was made by the Egyptologist Hermann Junker in 1914, and it is still the best-known proof of advanced dentistry that thrived from those times [1]. Currently, implantology is an ever-growing branch of dentistry, and is a contemporary part of human and veterinary medicine. In order to implant a tooth prosthesis, an implant site in the bone must be prepared. This must be done using special drilling systems. The mentioned procedure destroys the surrounding tissues mechanically and by the heat generated by the rotating drill. During osteointegration, the bone temperature may not exceed the temperature of 47 °C to prevent thermal osteonecrosis [2]. Earlier study showed that the temperature development and its influence on the bone tissue is proportional to the time of exposure to the friction during the drilling procedure [3]. Excessive destruction of the surrounding tissues like crushed bone, necrosis, or bone architecture annihilation influences the possibility of achieving good osteointegration, which is mandatory for dental implant success [4,5]. This bone change can vary according to the drill’s design, parameters, and cooling system utilized. The ideal method of drilling is difficult to define due to the anisotropic structure of bone, which connects organic and inorganic components [6]. Swine rib has a similar micro- and macrostructure to human mandible and maxilla and it can be an adequate in vitro model for biological studies [3]. The process of bone remodeling seems to be similar in human and other animals (pigs) including trabecular and intra-cortical based remodeling as well as cortical mineralization rate [7].
The expression of “osteointegration” describes a rigid, clinically asymptomatic connection between a bone tissue and implant, which has an integrity, allowing it to fulfil its function. The process of osseointegration is divided into primary and secondary phases. The primary phase is the connection between the implant and surrounding tissue, whilst, the second phase is based on the biologic activity of bone cells causing local inflammation, which enables healing and intensifies the contact of bone and implant [8]. In vitro, the bone damage surrounding the implant can be estimated microscopically (size and morphology of destruction zone around drilled hole).
The aim of the study was to describe the damage zone caused by drilling using pig ribs as a model for human bones of the splanchnocranium. Accessible literature lack larger studies comparing the changes of bone tissues surrounding implantation site with regard to the drilling system used, diameter of the drill, drilling temperature, and cooling system.
Optimal elaboration method of the bone bed before implantation determines the achievement of proper implant osteointegration. A crucial element of the preparation procedure of the bed is to minimalize thermic and mechanical injuries of the bone. Increase in temperature during preparation over 47 °C (over 1 min) leads to complete denaturation of the organic component in bone cell matrix, which can negatively affect the osteointegration process [9,10,11]. The main cause of this phenomenon is the disturbance of the collagen fibers’ structure and their cracking, thus implant bone connection stiffness changes and also increases vulnerability for bone tissue compression. Kim et al. and Bachus et al. proved the local thermal necrosis occurrence within the bone tissue after crossing the limit of temperature (over 50 °C) and prolonging procedure time during bone bed preparation [12,13].
2. Material and Methods
In this study, a total of 81 dental implant sites were made. For this purpose, nine fresh swine ribs were obtained from the Polish Large White (PLW) swine breed. The method of sample collection did not require permission from the ethical committee. The size (length, width, and thickness) of all samples were comparable, with the mean values of 146.2 mm × 24.6 mm × 21.3 mm, respectively. Implant sites were prepared using the following dental systems (Table 1):

Table 1.
Variants of the used drilling systems.
- BEGO Semados® RS (BEGO Implant Systems GmbH & Co., Bremen, Germany)—drills: pilot, 𝜙2.5, and final, 𝜙3.0;
- BIOMET 3i® T3 (Biomet 3i LLC, Palm Beach Gardens, FL, USA)—drills: pilot, 𝜙2.3, and final, 𝜙2.75); and
- NEO BIOTECH® IS-III Active (Neo Biotech Co., Ltd., Seoul, Korea)—drills: pilot, 𝜙2.2, and final, 𝜙2.9.
Each of the drills was tested with different cooling methods and drill rotation speed. A cooling system was supplied with:
- 0.9% NaCl solution (temperature approx. 20 °C);
- 0.9% NaCl solution (temperature approx. 3 °C); and
- without any cooling liquid supplementation.
Drilling was carried out with the following speeds (by the same person):
- 800 rpm;
- 1200 rpm; and
- 1500 rpm.
In order to prepare the implant sites, the NeoSurge implant micromotor was set at a contra angle with a 32:1 gear reduction. In all caused, the pilot drilling was used before the final drilling, not only because of dental systems requirements, but also to evaluate the drill diameter as an injury factor acting in bone model. Each of the drills were used only once at each drilling speed (800, 1200, and 1500 rpm) to avoid it becoming blunt. This may in turn generate additional heat during this procedure. All the implant sites were made on the anterior side of the swine ribs, reaching the depth of 10 mm. The same trained surgeon prepared each of the dental implants maintaining the optimal drilling force. Additionally, the ribs were fixed to the table mount to ensure the stability and repeatability. Drilling was done with an infrared thermographic camera ThermaCAM P640 [14] that registered the heat produced.
The samples were fixed in a 4% formaldehyde solution of pH 7.2 for two days and were rinsed under running water. Subsequently, the ribs were cut using a low-speed, water cooled diamond saw to obtain an opened implant site with the biggest possible diameter.
The obtained material was rinsed in a shaker at 25 °C for 10 min. A 0.9% sodium chloride solution was used to extract clear bone tissue. The material was examined under a fluorescent microscope Nikon Eclipse 80i using UV-2A (EX:330-380, DM:400, BA:420) and B-2A (EX: 450-490, DM:505, BA:520) filters with a magnification of 40× and 100×. Then, the rib was observed directly under fluorescence microscope and pictures were taken. Thanks to this method, we avoided a number of potential unwanted changes in the tissue adjacent to the drill hole. Moreover, it allowed almost direct observation of the borehole after the experiment.
The morphometric analysis was carried out using NIS Elements AR software. Additionally, the performed measurements were done in a closed room under the same conditions, maintaining the temperature and humidity, without additional air flow.
All data were subjected to statistical analysis using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA) software. The D’Agostino and Pearson normality test were used to determine the normality of the data. Based on the data distribution, either a one-way ANOVA with the Bonferroni post-hoc test or the Kruskal–Wallis with the post-hoc Dunns test were used to compare more than two groups of data. Correlations between the groups were determined using the Spearman correlation coefficient. p < 0.05 was considered statistically significant.
3. Results
The tissue destruction zone was significantly lower when saline was used to cool down the drill in all three drill rotation speeds and both drilling sites (apex: 800 rpm: p = 0.0001; 1200 rpm: p < 0.0001; 1500 rpm: p < 0.0001; side: 800 rpm: p = 0.0001; 1200 rpm: p = 0.0002; 1500 rpm: p < 0.0001). A post-hoc analysis showed no differences between 20 °C and 3 °C saline, regardless of drill rotation speed and drilling site (Figure 1). Spearman correlation coefficients between the actual temperature and tissue destruction were calculated for each drilling site, drilling speed, and cooling method (Table 2).
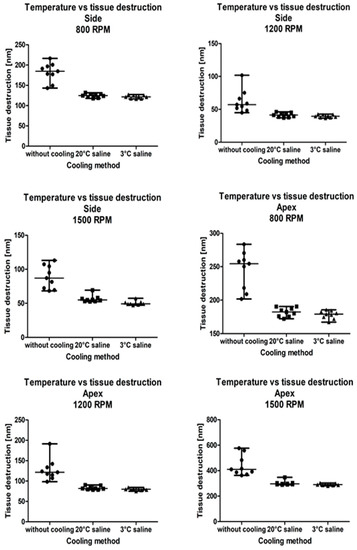
Figure 1.
The comparison of bone tissue destruction with regard to the drilling speed and the cooling system. Apex—at apical part of drill; Side—at lateral part of drill.

Table 2.
Spearman correlation coefficients between the actual temperature and tissue destruction. Apex—at apical part of drill; Side—at lateral part of drill.
The analysis of microanatomical structure showed the different morphological changes in samples referring to the drilling speed and cooling method used. During drilling, with a speed of 800 rpm and cooling liquid at temperature 3 °C, the zone of compressed bone tissue was visible (Figure 2). Simultaneously, the same speed of drilling without any cooling caused the moderate destruction of bone tissue and bone marrow (Figure 3). The microanatomical picture of changes generated by drilling with a speed of 1200 rpm (cooling liquid at 20 °C) showed the apical zone of rib with visible connective tissue without any areas of strong tissue compression (Figure 4). The same drilling speed, but with a cooling method at 3 °C, caused total destruction of the bone tissue and bone marrow. The empty bone marrow cavity was clearly distinguished from other parts of the rib’s wall (Figure 5). Finally, the drilling speed of 1500 rpm resulted in the lack of strong compression of the bone tissue and the apical area of rib filled with the connective tissue (Figure 6), when any cooling system was not used. In contrast, the use of cooling liquid at 20 °C changed the result of drilling, causing total bone tissue and bone marrow destruction and the presence of damaged trabecular architecture of bone with empty bone marrow cavity (Figure 7). The histometry of the damage zone at the wall of the drilling canal (Figure 8) and the thickness of compressed bone tissue at the top of drill (Figure 9) proved that the use of an intermediate speed of drilling (1200 rpm) was the least traumatic for the model.
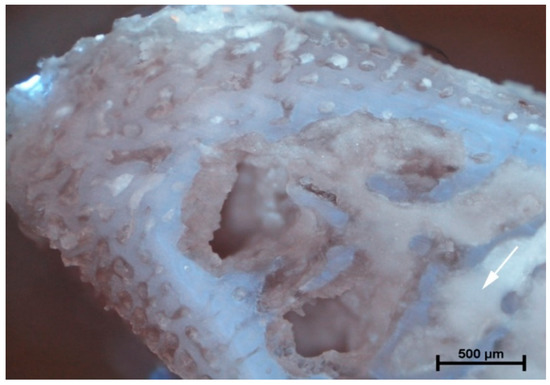
Figure 2.
Rotation of 800 rpm (3 °C). The apical area of rib filled with connective tissue. Visible area of tissue compression caused by the drill (arrow).
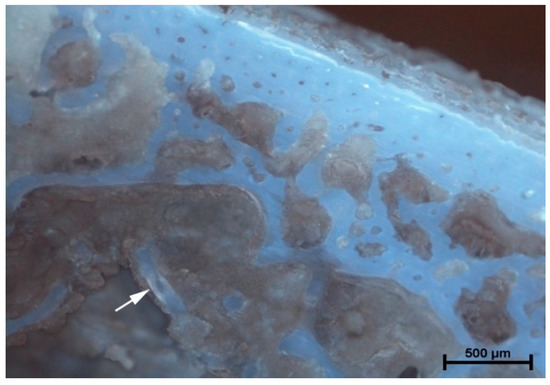
Figure 3.
Rotation of 800 rpm (without cooling). Moderate destruction of the bone tissue and bone marrow. Visible debris of bone trabecular architecture and bone marrow (arrow).

Figure 4.
Rotation of 1200 rpm (20 °C). The apical area of rib filled with connective tissue. The lack of strong tissue compression areas caused by the drill.
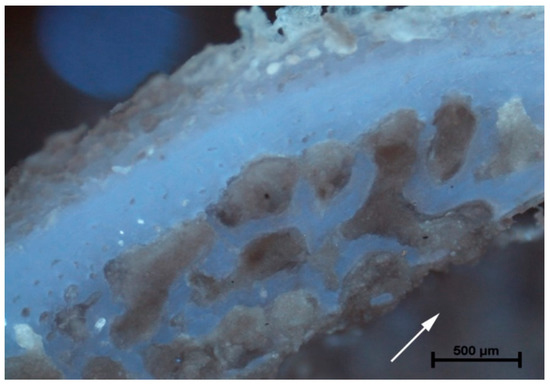
Figure 5.
Rotation of 1200 rpm (3 °C). Total destruction of bone tissue and bone marrow. Visible empty bone marrow cavity (arrow) clearly distinguished from other parts of unchanged rib tissue.
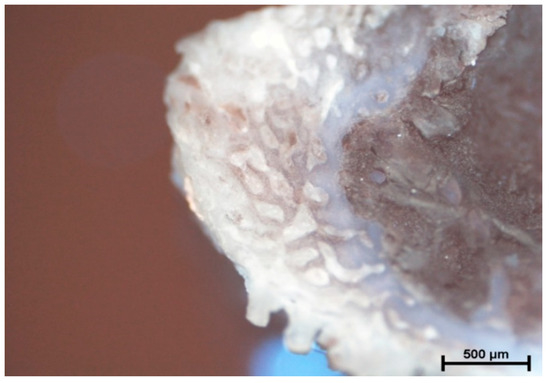
Figure 6.
Rotation of 1500 rpm (without cooling). The apical area of rib filled with connective tissue. The lack of strong tissue compression areas caused by the drill.
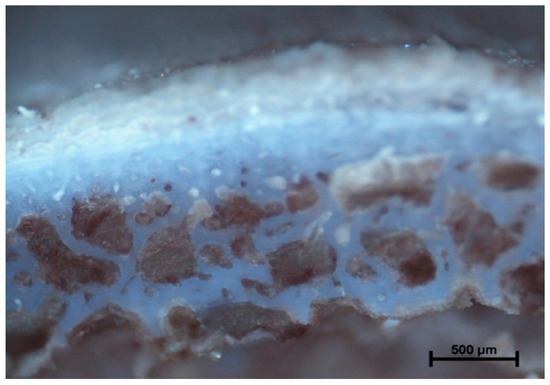
Figure 7.
Rotation 1200 rpm (20 °C). Total destruction of bone tissue and bone marrow. Visible empty bone marrow cavity (arrow) and debris of trabecular architecture of bone.
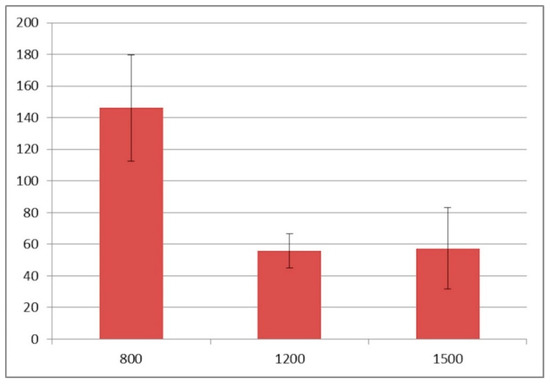
Figure 8.
The thickness of the damage zone in the tested drilling speeds [µm].
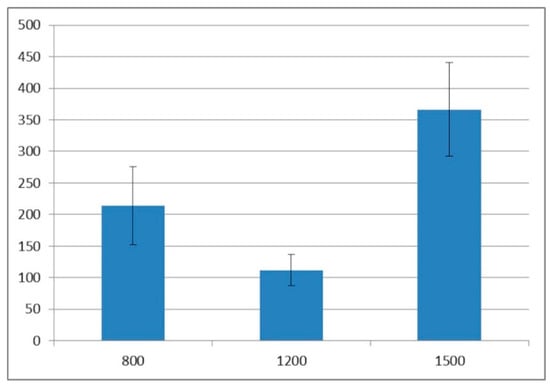
Figure 9.
The thickness of the compressed tissue area at the top drill [µm].
4. Discussion
Thermal osteonecrosis is a complex and multistage process, in which enzymatic and membrane proteins are destroyed, the osteoclastic and osteoblastic activity is significantly lowered, and dehydration as well as desiccation of the cell occurs. All these processes may lead to cell death, impaired healing, prolonged remodeling, and early implant failure. The first symptom of osteonecrosis is usually blood flow reduction, followed by osteoclastic resorption, which can no longer play their role in keeping a healthy structure cortical and spongy bone. Accurate temperature, which causes thermal damage, remains unclear [6,15].
The aim of the study was to measure the damage done during drilling to the tissues surrounding the implantation site in comparison to the drilling system, its diameter, drilling temperature, and cooling methods. The material was examined under a fluorescent microscope in order to receive the results, which accurately illustrated the thermal damage of the bone. Many factors have been described as the reasons for temperature rise during the dental implant site preparation including bone quality and its cortical thickness, drill type (material, design, diameter and wearing), drilling speed, feed rate and depth, pressure put while drilling, cooling approach and pre-drilling [6]. Previous studies showed that when the temperature during dental implant site preparation rises to 47 °C and remains so for 1 min, the bone is resorbed and replaced with fat tissue. We may therefore hypothesize, that a temperature under 47 °C combined with drilling time not exceeding 1 min is safe and protects against osteonecrosis. On the other hand, they revealed that heating the bone during the implant site preparation to the temperature of 50 °C caused resorption of the bone in the amount of 30% [16]. Many studies have been conducted in order to determine which cooling system and cooling conditions are the most efficient to avoid thermal necrosis. In general, cooling systems are divided into internal and external coolant approaches. Internal cooling systems deliver coolant (fluid or air) through the channel in the drill, whereas an external cooling system delivers it on the outer side of the drill or directly to the drilling point using fully automatic systems or a simple manual syringe [17]. As the depths are unknown for their infiltration sites, which are done by the external cooling systems, there is a limited possibility of cooling in the area where the drill has contact with the bone. In 1975, Kirschner and Mayer introduced the first internally cooled drills, so the coolant could also achieve the top of the rotating drill [18]. What is interesting to note is that another study performed many years after introducing the invention above-mentioned showed that more heat was generated in the superficial part of the bone than at the bottom of the drilling cavity. This means that the external delivery of the coolant should remain a sufficient method of lowering the temperature while drilling [19]. Therefore in our study, an external cooling system was used and evaluated. It was clearly visible that the lack of any coolant significantly increased the bone tissue destruction significantly in all three drill rotation speeds and both drilling sites (Figure 1 and Table 2).
Another extremely significant cooling practice, done while drilling, is irrigation and the amount of coolant. Several studies have compared the efficiency of lowering the temperature depending on the presence of coolant and its amount. Kalidindi compared the drilling with and without coolant, which showed a significant increase in temperature in cases without [20]. Sindel et al. compared irrigation volumes of 12 mL/min and 30 mL/min and revealed no significant difference between them [21]. In contrast, Mercan et al., who compared 32 mL/min, 44 mL/min, 56 mL/min, and 68 mL/min amounts of coolant, showed a visible reduction in thermal damages with volumes higher than 32 mL/min. They suggested that the most effective volume, which should be used to provide continuous cooling and limited thermal damage, is 56 mL/min [22]. Additionally, the temperature of the coolant may significantly impact the thermal necrosis and reduce its risk. Sener et al. showed that a lower temperature of the coolant (10 °C compared to 25 °C) may sufficiently decrease the final temperature and lower the risk of thermal damage [19]. In our study, we compared thermal damage in three conditions: without cooling and with cooling by 3 °C, and 20 °C of the 0.9% NaCl, which revealed a notable higher thermal damage in cases without coolant compared to cases with coolant, but the differences in damage zone between cases with 3 °C and 20 °C coolant temperatures were irrelevant.
According to many different results published, the optimal speed for bone drilling remains unidentified. There is no compromise regarding the relationship between the drilling speed and temperature. The first research carried out in 1958 in order to examine the correlation between drilling rate and temperature revealed that the increase in drilling speed increased the temperature while drilling [23]. Many years later, these results were confirmed by Augustin et al., who evaluated five drill speeds between 188 and 1820 rpm, Nam et al., who used 600 and 1200 rpm drilling speeds, and Kalidindi, who investigated 1200, 1800, and 2200 rpm drilling rates. They all received the same result—the higher the speed, the higher the temperature [20,24,25]. As opposed to previously mentioned investigations, Sharawy et al. showed that the higher the speed, the lower the temperature. He obtained these research results using three different speeds: 1225, 1667, and 2500 rpm [26]. The latest results of the research carried out by Kim et al. showed that the optimal rate that stimulates osteointegration and minimalizes thermal damage ranged from 1000 rpm to 1500 rpm [12]. In our study, we used three rotation speeds: suboptimal (800 rpm), optimal (1200 rpm), and maximal (1500 rpm). The force applied during the creation of the implant site remains one of the factors that may influence the temperature while drilling. The average force put on the implant site during its preparation is 1.2 kg [27]. Brisman et al. compared drilling at 1200 and 2400 rpm under loads of 1.2 and 2.4 kg. It turned out that drilling at the speed of 1800 rpm at the load of 1.2 kg generated an identical amount of heat as that of the 2400 rpm speed at the load of 2.4 kg. To obtain the same conditions while making all the implant sites, all of them were made by the same researcher with a synonymous force. Furthermore, an increase of one of the parameters (speed or load) caused an increased heat generation. On the other hand, increasing both parameters (speed and load) caused an unnoticeable rise in temperature and allowed for more effective implant site preparation [28]. In our study, we also used pre-drilling as the method that allows for broadening of the drilling site and causing heat dissipation. During implant site preparation, the drill diameter was increased gradually from the smallest to the largest diameter. The method of pre-drilling was introduced in 1983 by Branemark and later confirmed by many researchers, but remains a rarely used procedure [20,29]. Our study showed that both speed and coolant used during drilling with constant force provoked significantly different changes in bone tissue. The bone tissue compression was visible after low speed (800 rpm), cooled drilling (Figure 2), and the lack of coolant caused only moderate destruction of bone tissue and bone marrow (Figure 3). Moderate speed drilling (1200 rpm) with coolant at room temperature microscopically showed no bone tissue compression (Figure 4), but if the temperature of coolant was lower (3 °C), the same drilling method caused total destruction of the bone tissue and bone marrow (Figure 5). Subsequently, high speed drilling (1500 rpm) did not give any intense compression of the bone tissue without coolant (Figure 6). The use of coolant increased the destructive activity of the drill (total bone tissue and bone marrow destruction together with the presence of damaged trabecular architecture of bone), being the most traumatic (Figure 7). These results proved that an increase in drilling speed and the level of bone tissue injury relation was interfered more by coolant existence than its temperature. Low speed drilling is the most predisposed to bone tissue compression and high speed drilling to bone tissue destruction. The microanatomical analysis of investigated material proved that the lowest bone tissue destruction zone was observed in moderate and high speed drilling (Figure 8), but simultaneously, the lowest bone tissue compression caused moderate speed drilling (Figure 9). Therefore we can conclude that the use of intermediate speed of drilling (1200 rpm) is the least traumatic, not only for the model, but also for further studies in vivo.
As well known, the existence of bone tissue within the drilling canal can be a facilitator in osteogenesis and consequently in implant integration with acceptor tissue. On the other hand, the same can increase removal of the cell debris process and decrease or prevent the healing process. In our model, both mentioned assumptions are impossible to verify per se. Further in vivo studies should be carried out to solve the hypothesis.
Moreover, we have to add that studies on the most important factors of successful implantation are not only narrowed to drilling and cooling methods. Modal analysis of implant stability brought new assumptions and point of view on osteointegration of various-shaped implants and thread pinches [6]. Similarly, the biomechanics of implant integrated with the bone tissue in vivo can be a sufficient evaluation method for experimental implantology [7].
5. Conclusions
In conclusion, the results of the study proved that the size of a drill does not affect the tissue destruction level, regardless of the use of saline as well as its temperature. Tissue destruction is, however, significantly lower when saline is used to cool down the drill, and it is notable that the temperature of saline does not influence the destruction zone. In our experiment, the drilling speed 1200 rpm was optimal due to the lowest influence on the surrounding bone tissue.
Author Contributions
Conceptualization, K.K. and M.D.; Methodology, K.K. and A.C.; Software, J.J. and J.B.; Validation, A.C., M.D., and P.K.; Formal Analysis, K.K., A.C., D.P.; Investigation, K.K. and M.H.; Resources, A.C.; Data Curation, M.M. and W.B.; Writing—Original Draft Preparation, K.K., A.C., and D.P.; Writing—Review & Editing, A.C. and D.P.; Visualization, K.K. and P.K.; Supervision, A.C. and M.D.; Project Administration, K.K.; Funding Acquisition, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by Statutory Research and Development Activity funds assigned to the Faculty of Veterinary Medicine, Wroclaw University of Environmental and Life Sciences.
Institutional Review Board Statement
According to Polish law, this study did not need ethic committee approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Piotr Kosior, specialist in conservative dentistry and endodontics from Department of Conservative Dentistry with Endodontics, Faculty of Dentistry, Wrocław Medical University for the valuable cooperation and the realization of all experimental tasks.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Forshaw, R.J. The practice of dentistry in ancient Egypt. Br. Dent. J. 2009, 206, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, R.A.; Adell, R. Temperatures during drilling for the placement of implants using the osseointegration technique. J. Oral. Maxillofac. Surg. 1986, 44, 4–7. [Google Scholar] [CrossRef]
- Marheineke, N.; Scherer, U.; Rücker, M.; von See, C.; Rahlf, B.; Gellrich, N.-C.; Stoetzer, M. Evaluation of accuracy in implant site preparation performed in single or multi-step drilling procedures. Clin. Oral. Investig. 2018, 22, 2057–2067. [Google Scholar] [CrossRef]
- Pascoletti, G.; di Chio, G.; Marmotti, A.; Boero Baroncelli, A.; Costa, P.; Tancredi Lugas, A.; Serino, G. A novel technique for testing osteointegration in load-bearing conditions. WIT Trans. Eng. Sci. 2019, 124, 187–194. [Google Scholar]
- Zanetti, E.M.; Ciaramella, S.; Calì, M.; Pascoletti, G.; Martorelli, M.; Asero, R.; Watts, D.C. Modal analysis for implant stability assessment:Sensitivity of this methodology for differentimplant designs. Dent. Mater. 2018, 34, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Augustin, G.; Zigman, T.; Davila, S.; Udilljak, T.; Staroveski, T.; Brezak, D.; Babic, S. Cortical bone drilling and thermal osteonecrosis. Clin. Biomech. 2012, 27, 313–325. [Google Scholar] [CrossRef]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cell. Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mints, D.; Elias, C.; Funkenbusch, P.; Meirelles, L. Integrity of Implant Surface Modifications After Insertion. Int. J. Oral. Maxillofac. Implant 2014, 29, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, A.; Sivolella, S.; Stocco, E.; Favero, V.; Stellini, E. Experimental Analysis of Temperature Differences during Implant Site Preparation: Continuous Drilling Technique Versus Intermittent Drilling Technique. J. Oral Implantol. 2018, 44, 46–50. [Google Scholar] [CrossRef]
- Kosior, P.; Kuropka, P.; Janeczek, M.; Mikulewicz, M.; Zakrzewski, W.; Dobrzyński, M. The Influence of Various Preparation Parameters on the Histological Image of Bone Tissue during Implant Bed Preparation—An In Vitro Study. Appl. Sci. 2021, 11, 1916. [Google Scholar] [CrossRef]
- Marković, A.; Lazić, Z.; Mišić, T.; Šćepanović, M.; Todorović, A.; Thakare, K.; Janjić, B.; Vlahović, Z.; Glišić, M. Effect of Surgical Drill Guide and Irrigans Temperature on Thermal Bone Changes during Drilling Implant Sites—Thermographic Analysis on Bovine Ribs. Vojnosanit. Pregl. 2016, 73, 744–750. [Google Scholar] [CrossRef]
- Kim, S.-J.; Yoo, J.; Kim, Y.-S.; Shin, S.-W. Temperature change in pig rib bone during implant site preparation by low-speed drilling. J. Appl. Oral Sci. 2010, 5, 522–527. [Google Scholar] [CrossRef]
- Bachus, K.N.; Rondina, M.T.; Hutchinson, D.T. The effects of drilling force on cortical temperatures and their duration: An in vitro study. Med. Eng. Phys. 2000, 22, 685–691. [Google Scholar] [CrossRef]
- Kirstein, K.; Dobrzyński, M.; Kosior, P.; Chrószcz, A.; Dudek, K.; Fita, K.; Parulska, O.; Rybak, Z.; Skalec, A.; Szklarz, M.; et al. Infrared thermographic assessment of cooling effectiveness in selected dental implant sites. Biomed. Res. Int. 2016, 2016, 1879468. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Pandey, P.M.; Gupta, R.K.; Mridha, A.R. Rotary ultrasonic drilling on bone: A novel technique to put an end to thermal injury to bone. Proc. Inst. Mech. Eng. H 2017, 231, 189–196. [Google Scholar] [CrossRef]
- Eriksson, A.R.; Albrektsson, T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J. Prosthet. Dent. 1983, 50, 101–107. [Google Scholar] [CrossRef]
- Bagci, E.; Ozcelik, B. Effects of different cooling conditions on twist drill temperature. Int. J. Adv. Manuf. Technol. 2007, 34, 867–877. [Google Scholar] [CrossRef]
- Kirschner, H.; Meyer, W. Development of an internal cooling mechanism for surgical drills. Dtsch. Zahnarztl. Z. 1975, 30, 436–438. [Google Scholar] [PubMed]
- Sener, B.C.; Dergin, G.; Gursoy, B.; Kelesoglu, E.; Slih, I. Effects of irrigation temperature on heat control in vitro at different drilling depths. Clin. Oral Implants Res. 2009, 20, 294–298. [Google Scholar] [CrossRef]
- Kalidindi, V. Optimization of Drill Design and Coolant Systems during Dental Implant Surgery. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2004. [Google Scholar]
- Sindel, A.; Dereci, Ö.; Hatipoğlu, M.; Altay, M.-A.; Özalp, Ö.; Öztürk, A. The effects of irrigation volume to the heat generation during implant surgery. Med. Oral. Patol. Oral Cir. Bucal 2017, 22, e506–e511. [Google Scholar] [CrossRef]
- Mercan, U.; Sumer, M.; Kaya, O.A.; Keskiner, I.; Meral, D.G.; Erdogan, O. An In-vitro study on thermal changes during implant drilling with different irrigation volumes. Niger J. Clin. Pract. 2019, 22, 350–354. [Google Scholar]
- Thompson, H.C. Effect of drilling on bone. J. Oral Surg. 1958, 16, 22–30. [Google Scholar] [PubMed]
- Augustin, G.; Davila, S.; Mihoci, K.; Udiljak, T.; Vedrina, D.S.; Antabak, A. Thermal osteonecrosis and bone drilling parameters revisited. Arch. Orthop. Trauma Surg. 2008, 128, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Nam, O.; Yu, W.; Choi, M.Y.; Kyung, H.M. Monitoring of bone temperature during osseous preparation for orthodontic micro-screw implants: Effect of motor speed and pressure. Key Eng. Mater. 2006, 321–322, 1044–1047. [Google Scholar] [CrossRef]
- Sharawy, M.; Misch, C.E.; Weller, N.; Tehemar, S. Heat Generation during Implant Drilling: The Significance of Motor Speed. J. Oral Maxillofac. Surg. 2002, 60, 1160–1169. [Google Scholar] [CrossRef]
- Hobkirk, J.A.; Rusiniak, K. Investigation of variable factors in drilling bone. J. Oral Surg. 1977, 35, 968–973. [Google Scholar] [PubMed]
- Brisman, D.L. The effect of speed, pressure, and time on bone temperature during the drilling of implant sites. Int. J. Oral Maxillofac. Implants 1996, 11, 35–37. [Google Scholar]
- Brånemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 3. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).