Abstract
Animal waste is generated at an increased rate, and its disposal is attracting wide public attention. Anaerobic digestion is considered the most promising option for reducing this waste, and simultaneously, it produces renewable energy. Lignin contained in lignocellulosic biomass is hardly biodegradable, thus pre-treatment has to be considered prior to digestion. The possibility of biological pre-treatment of chicken manure with sawdust using Pleurotus ostreatus fungi was investigated in our study. This animal waste was used as a substrate for further biogas production. To provide a better nutrient balance, we added two different co-substrates, wheat straw and Miscanthus. Mixtures with different mass ratios of chicken manure with sawdust/ordinary wheat straw, as well as chicken manure with sawdust/pre-treated wheat straw were incubated for 30 d. The same experiments were performed with Miscanthus. During incubation, samples were taken at predetermined time intervals, and the concentration of acid-insoluble lignin was determined. Additionally, concentrations of glucose and xylose in the filtrate taken at the end of the Klasson procedure were determined in the initial samples and in the samples after 30 d of incubation. Despite our expectations, almost no lignin degradation was observed. Insignificant decreases in glucose and xylose concentrations after 30 d is attributed to fungi ingestion. Obtained results show that Pleurotus ostreatus, as a white-rot fungi with a unique enzymatic system and as generally preferred organisms for lignin degradation, is, therefore, not suitable for delignification of this particular animal waste.
1. Introduction
Renewable and sustainable bioenergy production has received a great deal of attention over the last several decades because of increasing energy demand and popular awareness of global warming caused by greenhouse gases [1,2,3,4]. Depletion of fossil fuels can be reduced with the use of renewable energy sources, such as biofuels and biogas [5]. In addition, from a waste management point of view, the conversion of bio-waste (including garden and agricultural waste, foliage, kitchen waste, different manures and sewage) to bioenergy is more acceptable and preferable to landfill disposal or other kind of waste treatment [6,7].
Increasing demand for food is reflected in expanded poultry rearing operations, which produce large amounts of miscellaneous waste, including feathers, fats and chicken manure. Because of environmental concerns (odor, greenhouse gas emissions, pathogens spreading and pollution of water), chicken manure cannot be disposed of in landfill sites [8,9]. Therefore, various approaches have been employed to solve this problem, including composting [10,11,12,13], pyrolysis [14,15,16], combustion [17,18], gasification [19,20,21], hydrothermal carbonization [22,23] and anerobic fermentation [24,25,26].
Anaerobic fermentation is a renewable and sustainable process for biogas production. Chicken manure can be used as a substrate in this process. When chickens are reared, sawdust is applied for litter. Anaerobic microorganisms are not capable of degrading this lignocellulosic biomass; however, some kind of pre-treatment must be performed. Lignocellulosic residues are widely available and are mainly composed of cellulose, hemicelluloses and lignin. All of them are rich in carbohydrates, which can be converted into biomass during the anaerobic digestion of both cellulose and hemicelluloses. However, the presence of lignin is well known to limit the accessibility and, thus, the biodegradation of polysaccharides in lignocellulosic materials. Due to the complicated structure of the cell walls, lignin is difficult to hydrolyze.
Pre-treatment techniques include physical, thermal, chemical and biological [3,4,27,28,29]. The use of white-rot fungi for pre-treatment of lignocellulosic biomass is gaining attention among researchers, because it is an environmentally acceptable biological process [4]. For instance, Pleurotus ostreatus has been used for the pre-treatment of rice straw [30,31], corn stover [32], Miscanthus [33] and wheat straw [34]; Trametes versicolor for wheat straw, barley straw [35] and Miscanthus [36]; Polyporus brumalis for wheat straw [37]; Ganoderma lucidum for rice straw [38]. White-rot fungi are generally preferred, as these organisms are most efficient in delignification. Their unique enzymatic system gives them the capacity to attack phenolic structures and to transform lignin. Although other microorganisms than white-rot fungi can be of interest in a pretreatment step for anaerobic digestion, they do not appear to outcompete them. White-rot fungi also have a strong potential to increase methane or bioethanol production.
The main purpose of this study was to check the ability of Pleurotus ostreatus fungi to degrade the lignin present in the mixture of chicken manure with sawdust and wheat straw in one case and in the mixture of chicken manure with sawdust and Miscanthus in another case. For the comparison, ordinary (without fungi pre-treatment) wheat straw and ordinary Miscanthus were used as co-substrates. The addition of plant raw materials to animal waste is desired to balance nutrients, increase buffer capacity and improve possible biogas production. Pleurotus ostreatus was selected for the pre-treatment of wheat straw and Miscanthus, because it has a high potential for delignification. Mixtures with different mass ratios of chicken manure with sawdust and ordinary or pre-treated substrates (wheat straw or Miscanthus) were incubated for 30 d. During that time, samples were taken at different intervals for the determination of acid-insoluble lignin, glucose and xylose. The research was considered as a preliminary investigation prior to anaerobic digestion of existing animal waste.
2. Materials and Methods
2.1. Materials
All the reagents and solvents used were of analytical grade. Dry pyridine (PYR), methanol (MeOH) and toluene were obtained from Merck (Darmstadt, Germany); N-O-bis-trimethylsilyl trifluoroacetamide with 1% of trimethylchlorosilane (BSTFA + 1% TMCS) and CaCO3 were supplied by Fluka Chemie (Buchs, Switzerland); D-glucose (99.5%), D-xylose (98%) and phenyl-β-D-glucopyranoside (99%, ISTD) were purchased from Sigma-Aldrich (Darmstadt, Germany). Wheat straw and Miscanthus were harvested in local fields in Slovenia. The chicken manure mixed with sawdust was freshly collected from a nearby biogas plant (Perutnina Ptuj, Draženci, Slovenia).
2.2. Fungal Pre-Treatment
The detailed pre-treatment procedure used in our experiments has been described in a previous study [24]. The wheat straw was sterilized, cooled and inoculated under sterile conditions with white-rot wood decay fungi (Pleurotus ostreatus, isolate P.o./strain H35). The same procedure was repeated with Miscanthus.
After 3 weeks, chicken manure with sawdust was mixed in one treatment with ordinary and in another one with pre-treated wheat straw at different mass ratios calculated to dry mass (80:20 and 50:50). The desires of the existing biogas plant were to implement as much as possible of chicken manure with sawdust as a substrate for anaerobic digestion. That was the reason why the mass ratios were selected first with high content and secondly with a lower content of chicken manure with sawdust. The same procedure was performed with Miscanthus. The mixtures were further incubated for different periods of time. Samples were taken for analysis at 0, 5, 9, 14, 19, 23 and 30 d. For total solids (TS) determination, the material was dried for 24 h at 105 °C.
2.3. Analysis of Acid-Insoluble Lignin
The Klasson procedure was used for the measurement of acid-insoluble lignin. A known amount of a dry sample was placed in a filter tube and extracted using a Soxhlet apparatus. Acetone was used as a solvent. The tube containing the extracted sample was placed in a beaker, to which water was added. The beaker was covered with foil, and the contents were boiled for one hour. The tube containing the sample was then withdrawn from the beaker and dried. A small amount of the extracted dry sample was placed in a small beaker and hydrolyzed with a 72% sulfuric acid solution for 1 day. The resulting solution was then placed in a larger beaker, diluted with water to 3% sulfuric acid solution, covered with foil and boiled for 4 h. The solution was left to cool to room temperature, and then, it was filtered with suction filtration. The filter paper with the sample was dried to a constant mass, which represents the amount of acid-insoluble lignin. The filtrate was stored for later measurement of monosaccharide concentrations.
2.4. GC–FID Instrumentation and Working Conditions
An Agilent 6890 Gas Chromatograph, equipped with an Agilent 6890 Autosampler with split/splitless injector and flame ionization detector (FID), was employed for the analysis of trimethylsilyl (TMS) derivatives of monosaccharides. Samples were injected in split mode (split ratio 7:1). The gas chromatograph conditions used were as follows: an Agilent HP-5MS UI column, 30 m × 0.32 mm i.d., 0.25 μm film thickness, column oven was programmed to provide temperature ranges from 70 °C (1 min) to 200 °C at 2 °C/min, then to 320 °C at 10 °C/min and maintained at 320 °C for 3 min. The FID temperature was set at 250 °C with a hydrogen flow rate of 30 mL/min, air flow rate of 300 mL/min and nitrogen flow rate of 10 mL/min.
2.5. Preparation of Standard Solutions and Calibration Curves for Glucose and Xylose
Standard stock solutions of glucose, xylose and internal standard (ISTD) phenyl-β-D-glucopyranoside were prepared by weighing 10 mg of each into separate 10 mL glass volumetric flasks and dissolving them in methanol (γ = 1000 mg L−1). Afterwards, the flasks were placed in an ultrasonic bath for at least 15 min. Calibration solutions were prepared by combining different aliquots (20 to100 μL) of glucose and xylose with 50 μL of ISTD stock solution into separate conical glass flasks. These solutions were evaporated to absolute dryness using a rotary evaporator. Then, 100 µL of pyridine and 200 µL of BSTFA with 1% TMCS were added, and derivatization (silylation) was carried out by heating the samples for 90 min at 80 °C on a sand bath. Before the analysis, the solutions were diluted with toluene to the same final volume of 1 mL, and then, 1 µL of each solution was injected into the GC-FID system in triplicate. Linear curves (concentration range from 20 to 100 mg L−1) were constructed using linear regression of the peak–area ratio of individual monosaccharide to ISTD versus the concentration.
2.6. Preparation of Sample Extracts
Sample extracts for GC analysis were prepared using the same procedure as the standard solutions. After acid hydrolysis at the end of Klasson procedure, all filtrates contained H2SO4, and the pH of the samples was between 1 and 2. Because this could cause damage to the stationary phase in the GC column, the samples were neutralized using CaCO3 prior to derivatization and GC analysis. Before neutralization, the samples were spiked with an appropriate amount of ISTD. Cartridges for neutralization were prepared as follows: a filter paper was placed at the bottom of a 10 mL plastic syringe; 1 cm of cotton wool was put onto the filter paper, and about 1.5 cm of CaCO3 was added to the wool. The cartridges were then connected to the vacuum system. Two milliliters of each sample was carefully transferred into each cartridge using Pasteur pipettes, and the samples were slowly passed through the columns (at a flow rate 5 mL min−1). Two hundred microliter aliquots of the neutralized eluates were transferred into separate conical flasks and concentrated using rotary evaporation to dryness. Then, 100 µL of pyridine and 200 µL of BSTFA with 1% TMCS were added. Derivatization was carried out by heating for 90 min at 80 °C on a sand bath. Prior to analysis, the samples were diluted with toluene to 1 mL. One microliter of the final solution was injected into the GC-FID system in triplicate. The quantities of glucose and xylose from the sample extracts were determined from corresponding calibration curves.
3. Results and Discussion
3.1. Concentration of Acid-Insoluble Lignin
For verifying the efficiency of biomass delignification with Pleurotus ostreatus, we determined the concentration of acid-insoluble lignin according to the well-known Klasson procedure. The results for mixtures with different mass ratios of chicken manure with sawdust and pre-treated wheat straw (80:20, 50:50) and the same with Miscanthus are presented in Table 1 (abbreviations: CMS:S—chicken manure with sawdust and ordinary wheat straw, CMS:SP.o.—chicken manure with sawdust and wheat straw pre-treated with Pleurotus ostreatus fungi and CMS:M—chicken manure with sawdust and ordinary Miscanthus, CMS:MP.o.—chicken manure with sawdust and pre-treated Miscanthus with Pleurotus ostreatus fungi).

Table 1.
Concentrations of acid-insoluble lignin.
For the control, the concentrations of acid-insoluble lignin were determined in the untreated chicken manure with sawdust (16.42%), wheat straw (19.90%) and Miscanthus (18.43%). After an extensive literature review, it was clear that lignin concentration can vary significantly, depending on the maturity and origin of the crops. Data from the literature about lignin concentration are presented in continuation and compared to our values. Wheat straw contained 19.3, 23, 30 and 20% of lignin, according to Kaparaju et al. [39], Solé-Bundó et al. [40], Tsapekos et al. [41] and Rajput et al. [42], respectively. Li et al. determined the lignin content of different types of Miscanthus, where values varied from 8.58 to 14.44% [43]. Miscanthus contained 20, 25.2, 18.1 and 21.36% lignin, according to Zhou et al. [44], Guo et al. [45], Vasco-Correa et al. [46] and Li et al. [47], respectively. Vasco-Correa et al. performed an additional study of fungal pretreatment of Miscanthus for enhanced enzymatic hydrolysis and obtained lignin concentrations of raw Miscanthus and fungal colonized Miscanthus of 20.9 and 17.1%, respectively [48]. In our test, we compared the values of ordinary Miscanthus to the values of mixtures after incubation with fungi, and we conclude that the lignin concentrations of mixtures are only 3 to 9% lower. Lower values can also be attributed to the lower lignin content of chicken manure and sawdust. Vasco-Correa et al. found that if the inoculum ratio is more than 30% and the moisture content between 60 to 75%, then, lignin degradation could be 25 to 35% after 28 d. However, they observed almost no lignin degradation with a 10 to 20% inoculum ratio [48].
Table 1 shows that the values of lignin concentrations are dispersed and that there is no observable interdependence between the concentration of lignin and the mass ratio of the mixtures. Special care was taken in the preparation of mixtures; however, because of variations in the consistency of each component, it was difficult to prepare uniform mixtures. Chicken manure, among other things, contains sawdust and pebbles; pre-treated wheat straw and Miscanthus contain mycelium fungi; consequently, all ingredients are not evenly dispersed. The concentration of acid-insoluble lignin in all Miscanthus samples is 5–10% lower than that in the wheat straw ones. The average deviation in concentration of insoluble lignin in wheat straw and Miscanthus samples were 0.66 and 0.42%, respectively. We chose a time period of 30 d for performing our experiments, because we were focused on possible lignin degradation over a reasonable time span. However, lignin degradation was not evident during the 30 d of incubation.
The mycelium growth was satisfactory on both substrates, but it was more pronounced on wheat straw samples compared to Miscanthus samples. Van Kuijk et al. studied fungal treatment of lignocellulosic biomass and revealed that no mycelium growth of Pleurotus ostreatus was observed on Miscanthus after 4 weeks. The mycelium growth was observed later, and after 8 weeks, the growth was about double [49]. In another study, Trametes versicolor fungi caused a 46% mass loss of lignin in Miscanthus after 12 weeks of incubation at 20 °C [36].
3.2. Concentration of Glucose and Xylose
In addition to the insoluble lignin concentration, the concentrations of glucose and xylose remaining in the filtrate at the end of the Klasson procedure were measured. Lignin in plant cells is crosslinked to cellulose and hemicellulose. To make the cellulose and hemicellulose accessible, it is essential to break down the chemical bonds in lignin. One of the evidences of delignification is also the formation of sugars, which are the products of hydrolysis of cellulose and hemicellulose. The concentrations of both sugars in the initial samples and, after 30 d of incubation, were determined.
The results for wheat straw samples are presented in Figure 1 and for Miscanthus in Figure 2. For the control, ordinary wheat straw was mixed with chicken manure with sawdust in the same mass ratios as in the case of pre-treated samples. The same experiments were performed with both wheat straw and Miscanthus.
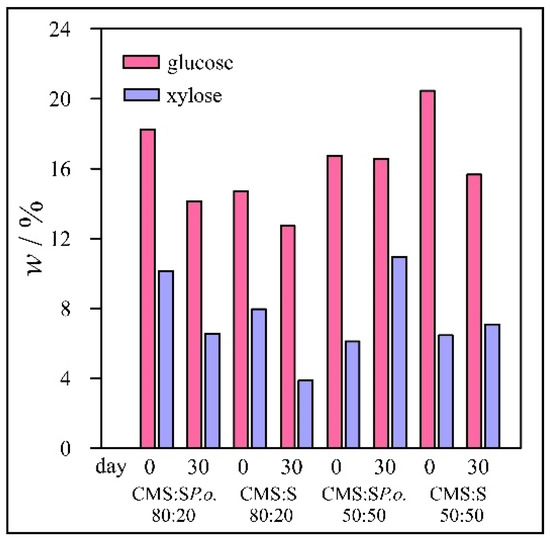
Figure 1.
Concentrations of glucose and xylose in wheat straw samples.
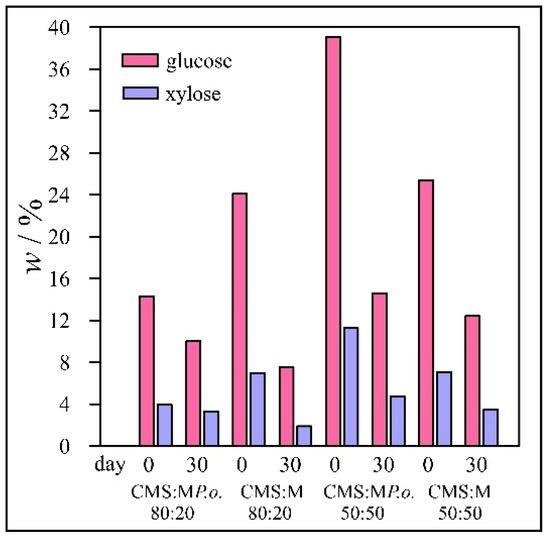
Figure 2.
Concentrations of glucose and xylose in Miscanthus samples.
The concentrations of xylose were about 6–10% lower than that of glucose, but in some samples, the difference was as much as 27%. The average deviations in concentration of glucose and xylose in wheat straw samples were 0.48 and 0.28% and in Miscanthus samples 0.63 and 0.39%, respectively. It is worth noting that after 30 d, the glucose and xylose concentrations were also lower for the control samples, which were not pre-treated with fungi. These results can be explained by the fact that microorganisms already present in chicken manure can consume glucose and xylose. Nevertheless, the decrease in sugar concentration during 30 d of incubation additionally indicated that selected fungi have almost no potential for degradation of lignin presented in studied mixture.
4. Conclusions
We explored the possibility of using Pleurotus ostreatus fungi for lignin degradation during incubation of mixtures of chicken manure and sawdust with pre-treated co-substrates: wheat straw, as agro-industrial waste, and Miscanthus, as biomass crop. There was no clear indication that lignin present in our mixtures would degrade during 30 d of incubation, thus making the cellulose and hemicellulose accessible for the hydrolysis to simpler monosaccharides. Instead of increasing, the concentrations of glucose and xylose even decreased compared to initial samples, which is most likely the consequence of fungi and microorganism consumption.
Our results show that the use of white-rot fungi obviously has its limitations. These organisms are generally known as most efficient in delignification process. However, preliminary studies about pre-treatment step for anaerobic digestion are always required. The results depend on the strain used and cultivation parameters. Some other microorganisms than white-rot fungi should be found for a pre-treatment procedure for anaerobic digestion of chicken manure with sawdust.
Author Contributions
Conceptualization, D.P.; methodology, D.P.; formal analysis, D.P. and M.I.R.; investigation, D.P. and M.I.R.; data curation, D.P. and M.I.R.; writing—original draft preparation, D.P. and M.I.R.; writing—review and editing, D.P., M.I.R. and A.G.; supervision, D.P. and A.G.; project administration, D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was supported by the Slovenian Research Agency within the project Design of Sustainable and Energy Self Sufficient Processes Based on Renewable Resources. ID L2-7633.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We are grateful to Bojan Pahor as well as biogas plant owners (Perutnina Ptuj, Draženci, Slovenia) for the supply of chicken manure with sawdust. The authors acknowledge Melisa Gjura for support in experimental work and Franc Pohleven for fungal pre-treatment.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Yi, S.; Yang, H.; Lee, S.H.; An, K.-J. Quantifying and managing regional greenhouse gas emissions: Waste sector of Daejeon, Korea. J. Environ. Sci. 2014, 26, 1249–1259. [Google Scholar] [CrossRef]
- Hosseini Koupaie, E.; Dahadha, S.; Bazyar Lakeh, A.A.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production—A review. J. Environ. Manag. 2019, 233, 774–784. [Google Scholar] [CrossRef]
- Rodriguez, C.; Alaswad, A.; Benyounis, K.Y.; Olabi, A.G. Pretreatment techniques used in biogas production from grass. Renew. Sustain. Energy Rev. 2017, 68, 1193–1204. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Awad, F.N.; Qi, X.; Sahu, J.N. Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew. Sustain. Energy Rev. 2019, 105, 105–128. [Google Scholar] [CrossRef]
- Rouches, E.; Zhou, S.; Steyer, J.P.; Carrere, H. White-Rot Fungi pretreatment of lignocellulosic biomass for anaerobic digestion: Impact of glucose supplementation. Process Biochem. 2016, 51, 1784–1792. [Google Scholar] [CrossRef]
- Mutschlechner, M.; Illmer, P.; Wagner, A.O. Biological pre-treatment: Enhancing biogas production using the highly cellulolytic fungus Trichoderma viride. Waste Manag. 2015, 43, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Lawlor, P.G.; Frost, P.; Dennehy, C.D.; Hu, Z.; Zhan, X. A pilot scale study on synergistic effects of co-digestion of pig manure and grass silage. Int. Biodeterior. Biodegrad. 2017, 123, 244–250. [Google Scholar] [CrossRef]
- Cárdenas-Aguiar, E.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. Thermogravimetric analysis and carbon stability of chars produced from slow pyrolysis and hydrothermal carbonization of manure waste. J. Anal. Appl. Pyrolysis 2019, 140, 434–443. [Google Scholar] [CrossRef]
- Dróżdż, D.; Wystalska, K.; Malińska, K.; Grosser, A.; Grobelak, A.; Kacprzak, M. Management of poultry manure in Poland—Current state and future perspectives. J. Environ. Manag. 2020, 264, 110327. [Google Scholar] [CrossRef]
- Chen, H.; Awasthi, S.K.; Liu, T.; Duan, Y.; Ren, X.; Zhang, Z.; Pandey, A.; Awasthi, M.K. Effects of microbial culture and chicken manure biochar on compost maturity and greenhouse gas emissions during chicken manure composting. J. Hazard. Mater. 2020, 389, 121908. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, A.; Chen, S.; He, X.; Jin, L.; Yu, X.; Yang, S.; Li, B.; Fan, L.; Ji, L.; et al. Heavy metals, antibiotics and nutrients affect the bacterial community and resistance genes in chicken manure composting and fertilized soil. J. Environ. Manag. 2020, 257, 109980. [Google Scholar] [CrossRef]
- Jung, J.-M.; Oh, J.-I.; Park, Y.-K.; Lee, J.; Kwon, E.E. CO2-mediated chicken manure biochar manipulation for biodiesel production. Environ. Res. 2019, 171, 348–355. [Google Scholar] [CrossRef]
- Hill, D.; Morra, M.J.; Stalder, T.; Jechalke, S.; Top, E.; Pollard, A.T.; Popova, I. Dairy manure as a potential source of crop nutrients and environmental contaminants. J. Environ. Sci. 2021, 100, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Das, D.D.; Schnitzer, M.I.; Monreal, C.M.; Mayer, P. Chemical composition of acid–base fractions separated from biooil derived by fast pyrolysis of chicken manure. Bioresour. Technol. 2009, 100, 6524–6532. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, Z.; Kaya, N.; Topcu, Y.; Uzun, H. Pyrolysis and optimization of chicken manure wastes in fluidized bed reactor: CO2 capture in activated bio-chars. Process Saf. Environ. Prot. 2019, 130, 297–305. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Raghavan, V. Pyrolysis in auger reactors for biochar and bio-oil production: A review. Biosyst. Eng. 2017, 161, 80–92. [Google Scholar] [CrossRef]
- Wagner, K.; Häggström, G.; Mauerhofer, A.M.; Kuba, M.; Skoglund, N.; Öhman, M.; Hofbauer, H. Layer formation on K-feldspar in fluidized bed combustion and gasification of bark and chicken manure. Biomass Bioenergy 2019, 127, 105251. [Google Scholar] [CrossRef]
- Thygesen, O.; Johnsen, T. Manure-based energy generation and fertiliser production: Determination of calorific value and ash characteristics. Biosyst. Eng. 2012, 113, 166–172. [Google Scholar] [CrossRef]
- Hussein, M.S.; Burra, K.G.; Amano, R.S.; Gupta, A.K. Temperature and gasifying media effects on chicken manure pyrolysis and gasification. Fuel 2017, 202, 36–45. [Google Scholar] [CrossRef]
- Hussein, M.S.; Burra, K.G.; Amano, R.S.; Gupta, A.K. Effect of oxygen addition in steam gasification of chicken manure. Fuel 2017, 189, 428–435. [Google Scholar] [CrossRef]
- Tańczuk, M.; Junga, R.; Werle, S.; Chabiński, M.; Ziółkowski, Ł. Experimental analysis of the fixed bed gasification process of the mixtures of the chicken manure with biomass. Renew. Energy 2019, 136, 1055–1063. [Google Scholar] [CrossRef]
- Kantarli, I.C.; Kabadayi, A.; Ucar, S.; Yanik, J. Conversion of poultry wastes into energy feedstocks. Waste Manag. 2016, 56, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Mau, V.; Quance, J.; Posmanik, R.; Gross, A. Phases’ characteristics of poultry litter hydrothermal carbonization under a range of process parameters. Bioresour. Technol. 2016, 219, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Pečar, D.; Pohleven, F.; Goršek, A. Kinetics of methane production during anaerobic fermentation of chicken manure with sawdust and fungi pre-treated wheat straw. Waste Manag. 2020, 102, 170–178. [Google Scholar] [CrossRef]
- Xie, T.; Xie, S.; Sivakumar, M.; Nghiem, L.D. Relationship between the synergistic/antagonistic effect of anaerobic co-digestion and organic loading. Int. Biodeterior. Biodegrad. 2017, 124, 155–161. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, K.; Sun, P.; Zhouyang, S.; Wang, Y.; Wang, H.; Zheng, Y.; Li, Q. Effects of substrate types on the transformation of heavy metal speciation and bioavailability in an anaerobic digestion system. J. Environ. Sci. 2021, 101, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, C.; Kim, T.-H.; Lee, M.; Kim, S.; Kim, S.-W.; Lee, J. Effects of various pretreatments for enhanced anaerobic digestion with waste activated sludge. J. Biosci. Bioeng. 2003, 95, 271–275. [Google Scholar] [CrossRef]
- Forgács, G.; Alinezhad, S.; Mirabdollah, A.; Feuk-Lagerstedt, E.; Horváth, I.S. Biological treatment of chicken feather waste for improved biogas production. J. Environ. Sci. 2011, 23, 1747–1753. [Google Scholar] [CrossRef]
- Xavier, C.A.N.; Moset, V.; Wahid, R.; Møller, H.B. The efficiency of shredded and briquetted wheat straw in anaerobic co-digestion with dairy cattle manure. Biosyst. Eng. 2015, 139, 16–24. [Google Scholar] [CrossRef]
- Huang, W.; Yuan, H.; Li, X. Multi-perspective analyses of rice straw modification by Pleurotus ostreatus and effects on biomethane production. Bioresour. Technol. 2020, 296, 122365. [Google Scholar] [CrossRef]
- Taniguchi, M.; Suzuki, H.; Watanabe, D.; Sakai, K.; Hoshino, K.; Tanaka, T. Evaluation of pretreatment with Pleurotus ostreatus for enzymatic hydrolysis of rice straw. J. Biosci. Bioeng. 2005, 100, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zeng, Y.; Ma, F.; Zhang, X.; Yu, H. Effect of biopretreatment on thermogravimetric and chemical characteristics of corn stover by different white-rot fungi. Bioresour. Technol. 2010, 101, 5475–5479. [Google Scholar] [CrossRef] [PubMed]
- Awedem Wobiwo, F.; Ercoli Balbuena, J.-L.; Nicolay, T.; Larondelle, Y.; Gerin, P.A. Valorization of spent coffee ground with wheat or miscanthus straw: Yield improvement by the combined conversion to mushrooms and biomethane. Energy Sustain. Dev. 2018, 45, 171–179. [Google Scholar] [CrossRef]
- Albornoz, S.; Wyman, V.; Palma, C.; Carvajal, A. Understanding of the contribution of the fungal treatment conditions in a wheat straw biorefinery that produces enzymes and biogas. Biochem. Eng. J. 2018, 140, 140–147. [Google Scholar] [CrossRef]
- Akyol, Ç.; Ince, O.; Bozan, M.; Ozbayram, E.G.; Ince, B. Biological pretreatment with Trametes versicolor to enhance methane production from lignocellulosic biomass: A metagenomic approach. Ind. Crop. Prod. 2019, 140, 111659. [Google Scholar] [CrossRef]
- Osono, T. Decomposition of grass leaves by ligninolytic litter-decomposing fungi. Grassl. Sci. 2010, 56, 31–36. [Google Scholar] [CrossRef]
- Rouches, E.; Zhou, S.; Sergent, M.; Raouche, S.; Carrere, H. Influence of white-rot fungus Polyporus brumalis BRFM 985 culture conditions on the pretreatment efficiency for anaerobic digestion of wheat straw. Biomass Bioenergy 2018, 110, 75–79. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V.; Goel, R. Fungal pretreatment and associated kinetics of rice straw hydrolysis to accelerate methane yield from anaerobic digestion. Bioresour. Technol. 2019, 286, 121368. [Google Scholar] [CrossRef]
- Kaparaju, P.; Serrano, M.; Thomsen, A.B.; Kongjan, P.; Angelidaki, I. Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour. Technol. 2009, 100, 2562–2568. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Eskicioglu, C.; Garfí, M.; Carrère, H.; Ferrer, I. Anaerobic co-digestion of microalgal biomass and wheat straw with and without thermo-alkaline pretreatment. Bioresour. Technol. 2017, 237, 89–98. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Angelidaki, I. Mechanical pretreatment for increased biogas production from lignocellulosic biomass; predicting the methane yield from structural plant components. Waste Manag. 2018, 78, 903–910. [Google Scholar] [CrossRef]
- Rajput, A.A.; Visvanathan, C. Effect of thermal pretreatment on chemical composition, physical structure and biogas production kinetics of wheat straw. J. Environ. Manag. 2018, 221, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Strömberg, S.; Liu, G.; Nges, I.A.; Liu, J. Assessment of regional biomass as co-substrate in the anaerobic digestion of chicken manure: Impact of co-digestion with chicken processing waste, seagrass and Miscanthus. Biochem. Eng. J. 2017, 118, 1–10. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Q.; Zhang, Y.; Gu, Y. Effect of hydrothermal pretreatment on Miscanthus anaerobic digestion. Bioresour. Technol. 2017, 224, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wu, Y.; Hong, C.; Chen, H.; Chen, X.; Zheng, B.; Jiang, D.; Qin, W. Enhancing digestibility of Miscanthus using lignocellulolytic enzyme produced by Bacillus. Bioresour. Technol. 2017, 245, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Vasco-Correa, J.; Luo, X.; Li, Y.; Shah, A. Comparative study of changes in composition and structure during sequential fungal pretreatment of non-sterile lignocellulosic feedstocks. Ind. Crop. Prod. 2019, 133, 383–394. [Google Scholar] [CrossRef]
- Li, H.-Q.; Li, C.-L.; Sang, T.; Xu, J. Pretreatment on Miscanthus lutarioriparious by liquid hot water for efficient ethanol production. Biotechnol. Biofuels 2013, 6, 76. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Ge, X.; Li, Y. Fungal pretreatment of non-sterile miscanthus for enhanced enzymatic hydrolysis. Bioresour. Technol. 2016, 203, 118–123. [Google Scholar] [CrossRef]
- Van Kuijk, S.J.A.; Sonnenberg, A.S.M.; Baars, J.J.P.; Hendriks, W.H.; Cone, J.W. Fungal treatment of lignocellulosic biomass: Importance of fungal species, colonization and time on chemical composition and in vitro rumen degradability. Anim. Feed Sci. Technol. 2015, 209, 40–50. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).