Abstract
Objective: Cluster of differentiation (CD14) is an important protein involved in activating toll-like receptors by bacterial components. It exists as either a transmembrane or soluble protein, called mCD14 or sCD14, respectively. Several studies show that CD14 regulates the inflammatory response to periodontal pathogens, and its expression is altered in periodontitis, an inflammatory disease of tooth-supporting tissues. It is the intent of this review to investigate the levels of expression of mCD14 and sCD14 in peripheral blood monocytes, saliva, gingival crevicular fluid, and gingival tissue biopsies in periodontitis patients. Methods: PubMed, Scopus, Ovid/Medline, Embase, and the Cochrane Library were consulted for the online literature search. To ensure methodical quality, titles and abstracts were reviewed in accordance to the PRISMA guidelines. Data extraction and evaluation of the full texts were executed in agreement with the GRADE approach. Results: This systematic review shows that mCD14 levels are decreased in peripheral blood monocytes of periodontitis patients in comparison to healthy patients, while sCD14 levels in sera, gingival crevicular fluid (GCF), and biopsies of periodontitis patients have a tendency to be increased in comparison to healthy controls. The evaluation of CD14 in gingival biopsies and periodontal tissues elucidated the fact that interpretation of the data obtained with qPCR, ELISA, and flow cytometry is questionable.
1. Introduction
Periodontitis is a multifactorial, inflammatory disease that causes the destruction of the periodontal tissues and, as the ultimate endpoint, can even lead to the loss of the tooth [1]. According to a recent study by Tonetti, et al. [2], the global burden of periodontal diseases remains high, with the most severe form affecting 11.2% of the world’s population [3]. Porphyromonas gingivalis, a gram-negative bacterium, is currently considered a keystone pathogen [4] and shows a strong association with periodontitis [5]. Recent studies have underscored that the pathogenicity of P. gingivalis lies in its constituents: Lipopolysaccharide (LPS), gingipains, and fimbriae/pili [6]. Each factor has been widely studied, and integral roles in the progression of periodontitis have been elucidated [6]. The human innate immune system comprises a family of pattern recognition receptors, termed toll-like receptors (TLRSs), which recognize various bacterial components and initiate the inflammatory reaction in response [7]. The recognition of bacterial components by TLRs is facilitated by numerous co-receptors, with the cluster of differentiation (CD) 14 being one of them [8].
Originally described as a myeloid differentiation antigen detected on mature monocytes/macrophages [9] and neutrophils, the CD14 molecule is a key factor for the innate recognition of bacteria [9,10]. CD14, a 55-kDa glycoprotein, exists in two forms, a soluble form (sCD14) [11] and a membrane bound form (mCD14) [11]. The membrane bound form is found mainly on mature monocytes, macrophages, and activated neutrophils, and is immobile due to the glycosylphosphatidylinositol tail [10,11]. The circulating form found in serum and other body fluids, is thought to be the result of protease-mediated shedding/cleavage of mCD14 [12]. sCD14 provides cells such as fibroblasts, and epithelial and endothelial cells that lack the membrane bound form of CD14, a sensitivity to bacterial products [12]. Thus, ultimately both mCD14 and sCD14 can function as a receptor for LPS of gram-negative bacteria and for various cell wall products of gram-positive bacteria [10]. In addition to its role in LPS/cell wall products signaling, sCD14 might play a part in inflammatory diseases by regulating the immune systems response [13]. In periodontal derived mesenchymal stromal cells, sCD14 drastically enhances inflammatory response to various bacterial components, such as lipopolysaccharide, lipoteichoic acid, and lipoproteins [14,15].
Since CD14 is an important regulator of the inflammatory response to bacteria, it is hypothesized to play an essential role in regulating periodontitis pathogenesis. However, the exact role of CD14 in periodontal health and periodontitis is not well defined. This systematic review aims to examine the currently available literature with reference to CD14 and periodontitis, focusing on how its role pertains to the pathogenesis of periodontitis. This review explicitly explores the levels of expression of mCD14 and sCD14 in peripheral blood monocytes, saliva, gingival crevicular fluid, and gingival tissue biopsies to allow a more in-depth understanding of the mechanism of periodontitis and provide information for future outcome measures for periodontal disease.
2. Materials and Methods
2.1. Eligibility Criteria
Experimental investigations, randomized controlled clinical trials, and available literature were screened with reference to CD14 and periodontal disease. In accordance with the Cochrane standards for systematic review, the Preferred Reporting Items for systematic reviews and Meta-Analysis (PRISMA) guidelines were adhered to [16].
2.2. Search Strategy for Identification of Studies
To identify preliminary relevance for studies to be included, a comprehensive research methodology was developed for each database. The search terms (MeSH terms) were “CD14”, “sCD14”,” soluble CD14”,” mCD14”, “cluster of differentiation 14” AND “periodontal disease”, “periodontitis”, “periodontal pathogenesis” to find as many relevant articles as possible.
2.3. Electronic Literature Search
Five databases: PubMed, Ovid/Medline, Embase, The Cochrane Library, and Scopus; were searched. The search was conducted from October 2019 to December 2020. All studies published until December 2020 were considered. No language restriction was set, however, included studies were solely written in English. The titles and records were screened for duplicates and consequently assessed in regard to the specified inclusion and exclusion criteria, as well as relevancy to topic. After fulfilling these benchmarks, full texts were examined and re-assessed. Furthermore, by manually reviewing lists of references of other scientific articles, supplementary records were sought for. Grey literature was not included.
Screening: Inclusion and Exclusion Criteria
The full texts were included if they pertained to periodontal disease and additionally explored the relation with CD14. The eligibility criteria were liberally applied at the start to warrant as many studies as possible, and no study was excluded without assessment. In developing a pool of studies that is relevant to the purpose of this review, these inclusion and exclusion criteria were specifically set forth. The study population should be human samples specifically (gingival crevicular fluid (GCF), Tissue, Serum, Blood), no sole in vitro studies, cell culture, or animal studies. There were no limits set on gender, grade, language, region, or age of the study population. However, studies with diseased individuals (i.e., HIV, immune deficiencies, diabetic populations, etc.) that had no healthy controls were emitted. In regard to the nature of the intervention/reference test, no limits were set, though Western blots, flow cytometry analysis, enzyme-linked immunosorbent assay (ELISA), and polymerase chain reaction (PCR) were the reference tests of choice. Additionally, singular polymorphism PCR studies were excluded, as this was not the sole focus of the review and would overextend the scope of this paper.
2.4. Quality and Eligibility Assessment
To limit the risk of bias caused by insufficiencies in design, conduct, or analysis of the study, the studies were individually assessed in accordance to the Cochrane Reviewers Handbook. For this review, each record was assessed on four different points in accordance with the GRADE (Grades of Recommendation Assessment Development and Evaluation) approach [17]. This method describes a structured procedure that permits the appraisal of quality of evidence and provides a scale for the strength of recommendation, given a set of outlined factors [17]. In this review, we had a few moderate-quality studies, which are categorized as downgraded randomized trials, or upgraded observational studies, as well as studies that include minimal factors which could reduce the general findings. Multiple studies contained in this review were low quality studies, which through this approach are seen as double-downgraded randomized trails that contain numerous factors that weaken the overall judgement quality, as well as studies that are of similar quality, contradictory, or sole observational studies. Very Low GRADE quality of evidence is given to downgraded observational studies, case series, or case reports that lack of evidence. Additionally, it is strongly suggested that the quality level of evidence can be reduced by additional factors, which are lack of evidence, limitations in the design and precision, inconsistency, and risk of bias [17].
2.5. Data Synthesis and Sensitivity Analysis
In adherence to the above outlined procedure, 20 relevant studies that met the criteria were included: [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Detailed information of these studies is listed in Table 1, Table 2, Table 3 and Table 4. The following predefined information points were obtained and summarized for each of the included studies:

Table 1.
Summary of studies on CD14 expression in peripheral blood monocytes.

Table 2.
Summary of studies on sCD14 levels in serum.

Table 3.
Summary of studies on sCD14 levels in saliva and gingival crevicular fluid (GCF).

Table 4.
Summary of studies of CD14 expression in periodontal tissue biopsies.
- -
- Study Details (Title, Author, Year)
- -
- Study Design (Methods/Materials)
- -
- Sample (Size and Type)
- -
- Outcome and General Findings
Due to the heterogenicity of the findings a statistical analysis and/or additional pooling of the retrieved data was deemed infeasible. This difficulty in comparable parameters also made sensitivity analyses not possible. Therefore, only a narrative analysis of the compiled data seemed reasonable. The results were tabularized and classified into four sub categories (CD14 in peripheral monocytes, serum sCD14, saliva/GCF, and biopsy studies) and analyzed accordingly (Table 1, Table 2, Table 3 and Table 4).
3. Results
Figure 1 shows a flowchart of the searching process. A total of 20 studies were identified for the inclusion in the review. Originally, the initial search in the databases of Medline, Embase, PubMed, Cochrane, and Scopus resulted in 1878 citations. After duplicates were removed, a total of 570 records remained. Of these 570, 338 were discarded because after the assessment of the abstract, the studies did not fit into the stipulated criteria. For the remaining 232 citations, the full text versions were obtained and examined more precisely. Multiple studies focused solely on the polymorphism of CD14, and thus were excluded [38,39,40,41,42,43,44,45,46,47]. Additional studies were excluded in the analysis because the levels of CD14 were not specifically retrieved in human patients, but in human cell cultures obtained from a Research center [48,49] and in vitro dental mesenchymal stromal cells or cell line studies [39]. Furthermore, subsequent reports were not included due to the fact that the tested or the control group were known to be individuals with compromised health (i.e., renal transplant patients [50,51] or HIV [52]. Finally, overview articles [53] and literature reviews of markers in periodontitis [54] provided great general information, but lacked the specificity in providing case control study examples, and thus results as such were excluded as well.
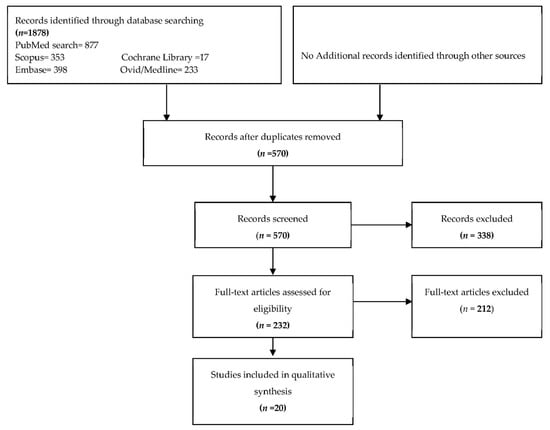
Figure 1.
Flowchart of the screening process.
3.1. Quality of Included Studies
In general, the studies included in the compilation were cross-sectional or case-control type studies. Individually, the studies explored different testing methodologies and biological substrates (GCF, blood, saliva, etc.) to compare the CD14 expression. Thus, as mentioned earlier, the results were tabularized and categorized into the following four substrate subgroups: CD14 in peripheral monocytes, serum sCD14, saliva/GCF, and biopsy studies. In order to allow a more specific assessment and evaluation of the variations in technical or interexperimental design, samples, and outcomes.
Of the 20 included studies ([18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]), 14 ([18,19,20,21,22,24,27,28,30,32,34,35,36,37]) were deemed of moderate quality, and 6 were categorized as low quality [23,24,25,29,31,33]. Reasons for classifying as low-quality involved indirectness of evidence (missing controls), extreme small sample sizes, and/or limitations or errors in design.
3.2. mCD14 in Peripheral Monocytes
Buduneli et al. [19], Nagasawa et al. [22], Pietruska et al. [21], Nicu et al. [20], Cheng et al. [18], and Shapira, Soskolne and Van Dyke [24] analyzed the expression of mCD14 in peripheral monocytes (Table 1). The expression of mCD14 in peripheral monocytes was measured and analyzed through in-cell ELISA and/or three-color flow cytometry [18,19,20,21,22]. Shapira, Soskolne and Van Dyke [24] found a decrease in mCD14 expression on monocytes in aggressive periodontitis (AP) patients compared to healthy control subjects as determined by in-cell ELISA. Nicu et al. [20] measured mCD14 expression in polymorphonuclear cells (PMNs) and monocytes in response to bacterial stimulation with Aggregatibacter actinomycetemcomitans and P. gingivalis by flow cytometry and found that cells isolated from periodontal patients show concomitant low mCD14 [20]. Buduneli et al. [19], using flow cytometry to analyze peripheral blood mononuclear cells (PBMCs) from patients and control subjects, found that the level of mCD14 in early onset periodontitis patients (7.18%, age 15–35 years) was lower than that of adult periodontitis patients (9.3%, age 38–57 years) and the control subjects (9.2%, age 22–54 years). Nagasawa et al. [22] using a similar method found that the percentage of CD14bright monocytes in chronic periodontitis (CP) patients was significantly lower than healthy subjects. Pietruska, Zak, Pietruski and Wysocka [21] explored the expression of mCD14 and reported that the expression of mCD14 on monocytes in generalized AP patients and control subjects were comparable [21]. Finally, Cheng, Saleh, Abuaisha Karim, Hughes and Taams [18] found no significant difference in mCD14 expression in PBMCs from patients with CP or AP patients and periodontally healthy control patients.
3.3. CD14 and Serum
Hayashi, Masaka and Ishikawa [36], Nicu, Laine, Morré, Van der Velden and Loos [35], Raunio, Knuuttila, Karttunen, Vainio and Tervonen [37], and Pietruska et al. [21] analyzed the expression of sCD14 in serum using ELISA (Table 2). Hayashi, Masaka and Ishikawa [36] showed that the sCD14 levels in the sera of patients with periodontitis were significantly higher than those of healthy subjects [36]. Furthermore, periodontal therapy resulted in a decrease of sera sCD14 levels in periodontitis patients [36]. Nicu, Laine, Morré, Van der Velden and Loos [35] showed that periodontitis patients have a tendency towards increased sCD14 levels in comparison to healthy controls (p = 0.061). However, the application of frequency distribution analysis illustrated that sCD14 levels of moderate and severe periodontitis patients had higher sCD14 values above threshold of 2.03 mg/L compared to healthy controls [35]. Moreover, patients with moderate and severe periodontitis exhibited a positive correlation between sera sCD14 levels and the severity of the periodontal destruction [35]. Finally, Raunio, Knuuttila, Karttunen, Vainio and Tervonen [37] reported that the mean concentration of sCD14 in serum was also significantly higher in subjects with periodontitis than in control subjects, and was significantly associated with the extent of advanced periodontal disease [37]. In contrast, Pietruska et al. reported similar levels of sCD14 in serum of periodontitis patients and healthy subjects.
3.4. CD14 in Saliva and Gingival Crevicular Fluid
Isaza-Guzman, Aristizabal-Cardona, Martinez-Pabon, Velasquez-Echeverri and Tobon-Arroyave [26], Prakasam and Srinivasan [27], Jin and Darveau [25], and Duncan, Yoshioka, Chandad and Grenier [23] analyzed CD14 in saliva and GCF (Table 3). The analysis of sCD14 was done in whole saliva samples [26,27] and gingival crevicular fluid (GCF) samples [23,25].
Isaza-Guzman, Aristizabal-Cardona, Martinez-Pabon, Velasquez-Echeverri and Tobon-Arroyave [26] obtained unstimulated whole saliva samples from patients with CP and AP, and healthy controls and measured salivary levels of sCD14 by ELISA. The levels of sCD14 were found significantly different among the three clinical groups: Significantly greater values were found in the CP and AP groups compared with healthy controls, whereas no significant difference between CP and AP patients was observed. Furthermore, a highly significant correlation between salivary sCD14 levels and the clinical measurements, such as clinical attachment loss, mean pocket depth and the percentage of periodontal pocket ≥4 mm deep and attachment loss ≥2 mm was observed. Prakasam and Srinivasan [27] analyzed sCD14 in unstimulated whole saliva by ELISA and found significantly higher sCD14 levels in CP patients than healthy controls. Scaling and root planning led to the gradual decrease in salivary sCD14 levels within 6 weeks post-therapy [27].
Jin and Darveau [25] measured the levels of sCD14 in GCF by an ELISA based capture assay in 15 Chinese adults with untreated moderate to advanced periodontitis. The results indicated that sCD14 was detected in all 15 subjects and sCD14 concentration had a significant negative correlation with pocket depth. Duncan, Yoshioka, Chandad and Grenier [23] analyzed the presence of soluble CD14 in GCF by Western immunoblotting. In this case, results showed that 3 out of 8 GCF samples from healthy periodontal sites had traces of soluble CD14, whereas 9 out of 16 samples from patients affected by moderate or advanced periodontitis contained a higher amount of soluble CD14.
3.5. CD14 in Biopsy/Tissues Studies
Sumedha, Kotrashetti, Nayak, Nayak and Raikar [28], Nibali, Novoa, Donos, Henderson, Blanco and Tomas [29], Li, Chen, Ng, Fung, Xu, Cheng, Tsao and Leung [30], Roberts, McCaffery and Michalek [34], Scheres, Laine, Sipos, Bosch-Tijhof, Crielaard, de Vries and Everts [31], Ren, Leung, Darveau and Jin [32], and Jin, Ren, Leung and Darveau [33] analyzed the expression of CD14 in gingival biopsies and periodontal tissues. More precisely, the expression of CD14 was explored in gingival tissue samples [28,29,30,34], periodontal ligament fibroblasts [31], and gingival pocket biopsies [32,33].
Sumedha et al. reported that CD14 expression in gingival tissues as assessed by immunohistochemistry was greatest in the periodontitis group that was classified as a severe grade, followed by moderate and mild grades expression and minimal in healthy gingiva [28]. Nibali et al. showed that gingival biopsies of diseased sites of periodontitis patients contained an enhanced number of CD14 compared to the control sites [29]. Furthermore, it was also found that sampled sites with less bleeding on probing exhibited higher expression of CD14 than those from 2–3 bleeding sites [29]. Li, Chen, Ng, Fung, Xu, Cheng, Tsao and Leung [30] performed immunohistochemistry, RT-PCR, and immunoblotting on gingival biopsies of patients with advanced CP and control sample biopsies. They showed that CD14 was detected in both inflamed and healthy gingival tissue. Even though the authors did not attempt to quantify CD14 expression, the research group mentions it did not observe any varied detectability of CD14 in healthy and periodontitis tissue biopsies [30]. Finally, a reverse transcription PCR assessment of gingival mononuclear cells and whole gingival biopsies from 32 adult periodontitis patients and five healthy individuals for inflammatory cytokines showed that CD14 was present in both samples [34]. Tissue section histochemistry illustrated that CD14 was detected in very low numbers in healthy biopsies and more prevalently in diseased biopsies [34]. Explaining further that the CD14-positive cells were primarily in the connective tissue and in close association with the lymphoid aggregate [34].
Scheres et al. analyzed the gene expression of CD14 in the primary periodontal ligament and gingival fibroblast cells from periodontitis patients and healthy controls [31]. Periodontal ligament fibroblasts from periodontitis patients had a higher basal and P. gingivalis induced mRNA expression of CD14 than healthy controls, whereas no difference in gingival fibroblasts was detected [31]. Ren et al. used immunohistochemical staining of gingival biopsies and periodontal pocket tissue from subjects with CP to explore the location of mCD14 and related proteins [32]. Outcomes illustrated that mCD14 was mainly confined to the cells around epithelium-connective tissue interface in all samples [32]. Moreover, CD14 was co-detected in 89% of the samples with lipopolysaccharide binding protein, and mCD14 peptide expression levels were significantly higher in healthy controls than those in diseased tissues [32]. These results were similar to Jin, Ren, Leung and Darveau [33], where gingival biopsies were obtained and mCD14 was also detected by immunohistochemistry. This study showed that the mCD14-positive cells were mainly confined to the gingival epithelium-connective tissue interface. However, the expression levels in periodontally healthy tissues showed greater levels of mCD14 than periodontal pocket tissues and granulation tissues [33]. mCD14 was frequently expressed in both healthy and diseased gingival tissues and was primarily confined to the epithelium-connective tissue boundary [33].
4. Discussion
Disease manifestations through bacterial infections can take various forms including, but not limited to, direct effects of bacterial products, alterations of the host in response to the organism, and/or the persistent response of the hosts immune system after the clearance of the organism [55]. Though the mechanisms of the inflammatory host response have been extensively studied, CD14 remains a fascinating molecule whose functions and associations in role to periodontitis remain somewhat elusive. CD14 serves as an assessor molecule for different TLRs and facilitates the recognition of different bacterial components [56,57]. Additionally, CD14+ monocytes are important precursor cells from osteoclast development, which subsequently, in combination with osteoblasts, play an integral role in bone homeostasis. Hence further solidifying its role periodontal disease. Recently the role of sCD14 transducing and activating passage ways that could influence osteoclast genesis have been proposed [58].
In this systematic review, peripheral monocytes, serum, saliva, GCF, and tissue biopsies were chosen as subsets, as they are all thought to reflect different aspects of inflammatory response. Prior studies suggest that there is an increase in the percentage of monocytes in the peripheral blood of patients with chronic periodontitis [59]. Saliva has been actively scrutinized for its potential in primary diagnostics, exposing possible markers for early diagnosis of periodontal disease [60]. GCF is a filtrate of blood and an exudate of the inflamed periodontal tissue [61]. It has been used as a promising site-specific intermediate that exposes how the local host responses react to microbial challenges such as it can be found in periodontitis [62]. Thus, it was the goal of this systematic review to assess and examine local sCD14 and mCD14 levels in different tissues, physiological fluids, and periodontal compartments.
Membrane-bound CD14 has been thought to only be expressed on circulating monocytes and tissue macrophages [9]. It is one of the factors, which facilitates the recognition of LPS by TLR-4 of host cells [10]. The expression of mCD14 in peripheral monocytes was analyzed, and results indicate that in four reports the expression of mCD14 on monocytes is decreased in periodontitis [19,20,22,24], while two additional reports note no difference [18,21].
In contrast to mCD14 expression in peripheral blood monocytes, the majority of studies on sCD14 levels in various biological fluids show a positive association between sCD14 levels and periodontal disease. This might be interpreted as an increase in production of sCD14 in cases with moderate-to-severe and generalized periodontal breakdown. The local levels of sCD14 measured in saliva and GCF are also affected by periodontitis. In two studies enhanced levels of sCD14 in periodontitis patients compared to healthy controls were found [26,27]. Furthermore, scaling and root-planning led to the decrease of salivary sCD14 levels, which underlies a positive association with periodontal disease [27]. The data on sCD14 levels in GCF are less conclusive [23,25].These inconsistencies could be explained by differences in small sample size, variations in individual profiles, cite specific difference in GCF composition and generally complicated procedure of GCF collection [63].
Interestingly, one study also hypothesized and explored the concept of in vivo cleavage of CD14 during periodontitis [23]. Particularly, immunoreactive CD14 fragments were present in certain GCF samples of advanced (pocket depth ≥7 mm) periodontitis patients. Further, susceptibility to degradation by P.gingivalis outer membrane vesicles was demonstrated, indicating that the CD14 receptor was highly sensitive to the proteinases associated with P. gingivalis. Thus, one could hypothesize that cleaving of mCD14 from monocytes might result in decreased phagocytosis and pathogen elimination, subsequently allowing easier reproduction of bacteria through a “weakening” of CD14 efficacy. Recent studies could support this ideas, as one study indicated that P. gingivalis gingipains can selectively reduce the responsiveness of macrophages to P. gingivalis infection [64]. Another recent study reported that the amount of mCD14 on peripheral blood monocytes treated with P. gingivalis proteinases was reduced [65]. In turn, increase of sCD14 or its fragments due to degradation of mCD14 by P. gingivalis might sensitize connective tissue cells to LPS and lipoproteins [14,15]. This will result in promoting inflammatory response and tissue destruction, and subsequently an enrichment with host derived protein as nutrition for bacteria.
Studies on the tissue biopsies report different results regarding the expression of CD14, yet suggest in most cases an enhanced CD14 expression in periodontitis. It is important here to note the differences in methodology applied, the type of biopsy and tissue tested as well as the type of CD14 molecule explored. Moreover, the expression of CD14 might be tissue specific.
Noteworthy, none of these five biopsy studies discriminated between sCD14 and mCD14. Although some studies mentioned that they use anti-mCD14 antibodies (e.g., [32,33]), it is difficult to imagine that these antibodies might differentiate between mCD14 and sCD14. Similarly, a distinguishing between these two CD14 forms is hardly imaginable in studies using PCR technique [31,34].
Taken together, these are all cross-sectional studies and limitations are inherent with their nature. The frequent small sample sizes, differences or flaws in sample collection, or lack of inclusion of healthy control subjects can limit studies. Additionally, contributing factors, processing errors, or limitations due to unavailable data and the mere adaptions in definitions each research team choose to qualify as healthy or diseased could influence subsequent outcomes. For example, in the saliva/GCF studies the method and timing of sample selection comes with great outcome variability, it has been shown that accurate analysis is difficult [66]. GCF can also contain molecular products from various sources including, but not limited to, epithelium cells, inflammatory cells, and bacterial products from the crevice and surrounding tissue [25] that could cause discrepancies. Confirmatory studies with increased sample sizes and longitudinal evaluations with lengthier follow ups would need to be evaluated to prove reliability. Furthermore, correlation doesn’t always apply cause and effect, thus randomized clinical studies are needed to clarify these points.
5. Conclusions
This review warrants that additional studies with greater sample populations and methodically standardized procedures are needed to further elucidate the molecular mechanisms.
Nonetheless, this review does highlight a few important concepts. One, that CD14 is frequently detected in healthy and diseased tissues, but CD14+ cells are predominantly confined in the gingival epithelial connective tissue interface and in lower quantities in the diseased state. Possible downregulation of mCD14 expression could be due to shedding regulated by P. gingivalis gingipains and cleaving of mCD14 from peripheral monocytes, consequently resulting in a slower responsiveness of macrophages and monocytes as well as reduced phagocytosis and pathogen elimination of P. gingivalis. This decline in CD14 efficacy could be advantageous for the proliferation of bacteria, consequently leading to an increase in inflammation. Moreover, the findings that sCD14 levels are increased in serum, GCF, and tissue biopsies could show that it serves as a sensitizing molecule for connective tissue, which further promotes the inflammatory response, osteoclast formation, and destruction of bone and soft tissue. Increased levels of sCD14 in serum could also be due to the fact that it has been postulated that the mechanism of bacterial lytic activity is withstood in blood serum. Lastly, measuring pre- and post-treatment sCD14 concentration could perhaps make sCD14 a useful marker for monitoring development of periodontal treatment.
Considering different experimental methods, cell types, media, and reagents, this review details the alterations in CD14 in the periodontal pathogenesis, however, also reveals the contradictions and controversies that still remain. These findings further reflect the dynamic nature of the local host inflammatory response to microbial challenge and the awareness that there still are gaps in our comprehension.
Author Contributions
Conceptualization, V.H., A.B. and O.A.; methodology, V.H. and O.A.; validation, V.H., A.B., C.B. and O.A.; formal analysis, V.H.; investigation, V.H.; resources, O.A.; data curation, V.H.; writing—original draft preparation, V.H.; writing—review and editing, A.B., C.B., and O.A.; visualization, V.H.; supervision, O.A.; project administration, O.A.; funding acquisition, O.A. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access Funding by the Austrian Science Fund (FWF), grant number P29440.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; Genco, R. Microbial pathogenicity black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: Virulence factors in colonization, survival, and tissue destruction. J. Dent. Res. 1984, 63, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Hasturk, H.; Kantarci, A. Activation and resolution of periodontal inflammation and its systemic impact. Periodontology 2000 2015, 69, 255–273. [Google Scholar] [CrossRef]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major periodontopathic pathogen overview. J. Immunol. Res. 2014, 2014. [Google Scholar] [CrossRef]
- Majewska, M.; Szczepanik, M. The role of Toll-like receptors (TLR) in innate and adaptive immune responses and their function in immune response regulation. Postepy Higieny i Medycyny Doswiadczalnej 2006, 60, 52–63. [Google Scholar]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273, Table of Contents. [Google Scholar] [CrossRef]
- Haziot, A.; Chen, S.; Ferrero, E.; Low, M.G.; Silber, R.; Goyert, S.M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J. Immunol. 1988, 141, 547–552. [Google Scholar]
- Wright, S.D.; Ramos, R.A.; Tobias, P.S.; Ulevitch, R.J.; Mathison, J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990, 249, 1431–1433. [Google Scholar] [CrossRef]
- Bažil, V.; Baudyš, M.; Hilgert, I.; Štefanová, I.; Low, M.G.; Zbrožek, J.; Hořejší, V. Structural relationship between the soluble and membrane-bound forms of human monocyte surface glycoprotein CD 14. Mol. Immunol. 1989, 26, 657–662. [Google Scholar] [CrossRef]
- Frey, E.A.; Miller, D.S.; Jahr, T.G.; Sundan, A.; Bazil, V.; Espevik, T.; Finlay, B.B.; Wright, S.D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J. Exp. Med. 1992, 176, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Bas, S.; Gauthier, B.R.; Spenato, U.; Stingelin, S.; Gabay, C. CD14 is an acute-phase protein. J. Immunol. 2004, 172, 4470–4479. [Google Scholar] [CrossRef] [PubMed]
- Behm, C.; Blufstein, A.; Gahn, J.; Noroozkhan, N.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Soluble CD14 Enhances the Response of Periodontal Ligament Stem Cells to Toll-Like Receptor 2 Agonists. Mediat. Inflamm. 2019, 2019, 8127301. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O.; Andrukhova, O.; Özdemir, B.; Haririan, H.; Müller-Kern, M.; Moritz, A.; Rausch-Fan, X. Soluble CD14 Enhances the Response of Periodontal Ligament Stem Cells to P. gingivalis Lipopolysaccharide. PLoS ONE 2016, 11, e0160848. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Schünemann, H.; Oxman, A.D.; Brozek, J.; Glasziou, P.; Jaeschke, R.; Vist, G.E.; Williams, J.W.; Kunz, R.; Craig, J.; Montori, V.M.; et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008, 336. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.C.; Saleh, F.; Abuaisha Karim, B.; Hughes, F.J.; Taams, L.S. Comparative analysis of immune cell subsets in peripheral blood from patients with periodontal disease and healthy controls. Clin. Exp. Immunol. 2018, 194, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Buduneli, N.; Bıçakçı, N.; Keskinogˇlu, A. Flow-cytometric analysis of lymphocyte subsets and mCD14 expression in patients with various periodontitis categories. J. Clin. Periodontol. 2001, 28, 419–424. [Google Scholar] [CrossRef]
- Nicu, E.A.; van der Velden, U.; Everts, V.; Loos, B.G. Expression of FcgammaRs and mCD14 on polymorphonuclear neutrophils and monocytes may determine periodontal infection. Clin. Exp. Immunol. 2008, 154, 177–186. [Google Scholar] [CrossRef]
- Pietruska, M.; Zak, J.; Pietruski, J.; Wysocka, J. Evaluation of mCD14 expression on monocytes and the blood level of sCD14 in patients with generalized aggressive periodontitis. Adv. Med. Sci. 2006, 51, 166–169. [Google Scholar]
- Nagasawa, T.; Kobayashi, H.; Aramaki, M.; Kiji, M.; Oda, S.; Izumi, Y. Expression of CD14, CD16 and CD45RA on monocytes from periodontitis patients. J. Periodontal. Res. 2004, 39, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Duncan, L.; Yoshioka, M.; Chandad, F.; Grenier, D. Loss of lipopolysaccharide receptor CD14 from the surface of human macrophage-like cells mediated by Porphyromonas gingivalis outer membrane vesicles. Microb. Pathog. 2004, 36, 319–325. [Google Scholar] [CrossRef]
- Shapira, L.; Soskolne, W.A.; Van Dyke, T.E. Prostaglandin E2 secretion, cell maturation, and CD14 expression by monocyte-derived macrophages from localized juvenile periodontitis patients. J. Periodontol. 1996, 67, 224–228. [Google Scholar] [CrossRef]
- Jin, L.; Darveau, R.P. Soluble CD14 levels in gingival crevicular fluid of subjects with untreated adult periodontitis. J. Periodontol. 2001, 72, 634–640. [Google Scholar] [CrossRef]
- Isaza-Guzman, D.M.; Aristizabal-Cardona, D.; Martinez-Pabon, M.C.; Velasquez-Echeverri, H.; Tobon-Arroyave, S.I. Estimation of sCD14 levels in saliva obtained from patients with various periodontal conditions. Oral Dis. 2008, 14, 450–456. [Google Scholar] [CrossRef]
- Prakasam, S.; Srinivasan, M. Evaluation of salivary biomarker profiles following non-surgical management of chronic periodontitis. Oral Dis. 2014, 20, 171–177. [Google Scholar] [CrossRef]
- Sumedha, S.; Kotrashetti, V.S.; Nayak, R.S.; Nayak, A.; Raikar, A. Immunohistochemical localization of TLR2 and CD14 in gingival tissue of healthy individuals and patients with chronic periodontitis. Biotech. Histochem. 2017, 92, 487–497. [Google Scholar] [CrossRef]
- Nibali, L.; Novoa, L.; Donos, N.; Henderson, B.; Blanco, J.; Tomas, I. Leukocyte receptor expression in chronic periodontitis. Clin. Oral Investig. 2016, 20, 2559–2564. [Google Scholar] [CrossRef]
- Li, J.P.; Chen, Y.; Ng, C.H.; Fung, M.L.; Xu, A.; Cheng, B.; Tsao, S.W.; Leung, W.K. Differential expression of Toll-like receptor 4 in healthy and diseased human gingiva. J. Periodontal Res. 2014, 49, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Scheres, N.; Laine, M.L.; Sipos, P.M.; Bosch-Tijhof, C.J.; Crielaard, W.; de Vries, T.J.; Everts, V. Periodontal ligament and gingival fibroblasts from periodontitis patients are more active in interaction with Porphyromonas gingivalis. J. Periodontal Res. 2011, 46, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Leung, W.K.; Darveau, R.P.; Jin, L. The Expression Profile of Lipopolysaccharide-Binding Protein, Membrane-Bound CD14, and Toll-Like Receptors 2 and 4 in Chronic Periodontitis. J. Periodontol. 2005, 76, 1950–1959. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Ren, L.; Leung, W.K.; Darveau, R.P. The in vivo expression of membrane-bound CD14 in periodontal health and disease. J. Periodontol. 2004, 75, 578–585. [Google Scholar] [CrossRef]
- Roberts, E.; McCaffery, K.A.; Michalek, S.M. Profile of Cytokine mRNA Expression in Chronic Adult Periodontitis. J. Dent. Res. 1997, 76, 1833–1839. [Google Scholar] [CrossRef]
- Nicu, E.A.; Laine, M.L.; Morré, S.A.; Van der Velden, U.; Loos, B.G. Soluble CD14 in periodontitis. Innate Immun. 2009, 15, 121–128. [Google Scholar] [CrossRef]
- Hayashi, J.; Masaka, T.; Ishikawa, I. Increased levels of soluble CD14 in sera of periodontitis patients. Infect. Immun. 1999, 67, 417–420. [Google Scholar] [CrossRef]
- Raunio, T.; Knuuttila, M.; Karttunen, R.; Vainio, O.; Tervonen, T. Serum sCD14, polymorphism of CD14(-260) and periodontal infection. Oral Dis. 2009, 15, 484–489. [Google Scholar] [CrossRef]
- Holla, L.I.; Buckova, D.; Fassmann, A.; Halabala, T.; Vasku, A.; Vacha, J. Promoter polymorphisms in the CD14 receptor gene and their potential association with the severity of chronic periodontitis. J. Med. Genet. 2002, 39, 844–848. [Google Scholar] [CrossRef]
- Donati, M.; Berglundh, T.; Hytönen, A.-M.; Hahn-Zoric, M.; Hanson, L.-Å.; Padyukov, L. Association of the −159 CD14 gene polymorphism and lack of association of the −308 TNFA and Q551R IL-4RA polymorphisms with severe chronic periodontitis in Swedish Caucasians. J. Clin. Periodontol. 2005, 32, 474–479. [Google Scholar] [CrossRef]
- Folwaczny, M.; Glas, J.; Török, H.-P.; Fricke, K.; Folwaczny, C. The CD14 −159C-to-T promoter polymorphism in periodontal disease. J. Clin. Periodontol. 2004, 31, 991–995. [Google Scholar] [CrossRef]
- Yamazaki, K.; Ueki-Maruyama, K.; Oda, T.; Tabeta, K.; Shimada, Y.; Tai, H.; Nakajima, T.; Yoshie, H.; Herawati, D.; Seymour, G.J. Single-nucleotide Polymorphism in the CD14 Promoter and Periodontal Disease Expression in a Japanese Population. J. Dent. Res. 2003, 82, 612–616. [Google Scholar] [CrossRef]
- Laine, M.L.; Morré, S.A.; Murillo, L.S.; van Winkelhoff, A.-J.; Peña, A.S. CD14 and TLR4 Gene Polymorphisms in Adult Periodontitis. J. Dent. Res. 2005, 84, 1042–1046. [Google Scholar] [CrossRef]
- Tervonen, T.; Raunio, T.; Knuuttila, M.; Karttunen, R. Polymorphisms in the CD14 and IL-6 genes associated with periodontal disease. J. Clin. Periodontol. 2007, 34, 377–383. [Google Scholar] [CrossRef]
- Schulz, S.; Zissler, N.; Altermann, W.; Klapproth, J.; Zimmermann, U.; Gläser, C.; Schaller, H.G.; Reichert, S. Impact of genetic variants of CD14 and TLR4 on subgingival periodontopathogens. Int. J. Immunogenet. 2008, 35, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Sahingur, S.E.; Xia, X.J.; Gunsolley, J.; Schenkein, H.A.; Genco, R.J.; De Nardin, E. Single nucleotide polymorphisms of pattern recognition receptors and chronic periodontitis. J Periodontal Res 2011, 46, 184–192. [Google Scholar] [CrossRef]
- Tarannum, F.; Faizuddin, M. Effect of gene polymorphisms on periodontal diseases. Indian J. Hum. Genet. 2012, 18, 9–19. [Google Scholar] [CrossRef]
- Zheng, J.; Hou, T.; Gao, L.; Wu, C.; Wang, P.; Wen, Y.; Guo, X. Association between CD14 gene polymorphism and periodontitis: A meta-analysis. Crit. Rev. Eukaryot. Gene Expr. 2013, 23, 115–123. [Google Scholar] [CrossRef]
- Hayashi, J.; Masaka, T.; Saito, I.; Ishikawa, I. Soluble CD14 mediates lipopolysaccharide-induced intercellular adhesion molecule 1 expression in cultured human gingival fibroblasts. Infect. Immun. 1996, 64, 4946–4951. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Sugiyama, A.; Nemoto, E.; Rikiishi, H.; Takada, H. Heterogeneous expression and release of CD14 by human gingival fibroblasts: Characterization and CD14-mediated interleukin-8 secretion in response to lipopolysaccharide. Infect. Immun. 1998, 66, 3043–3049. [Google Scholar] [CrossRef]
- Becerik, S.; Ozsan, N.; Gürkan, A.; Oztürk, V.Ö.; Atilla, G.; Emingil, G. Toll like receptor 4 and membrane-bound CD14 expressions in gingivitis, periodontitis and CsA-induced gingival overgrowth. Arch. Oral Biol. 2011, 56, 456–465. [Google Scholar] [CrossRef]
- Gong, Y.; Bi, W.; Cao, L.; Yang, Y.; Chen, J.; Yu, Y. Association of CD14-260 polymorphisms, red-complex periodontopathogens and gingival crevicular fluid cytokine levels with cyclosporine A-induced gingival overgrowth in renal transplant patients. J. Periodontal. Res. 2013, 48, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Moscicki, A.B.; Yao, T.J.; Russell, J.S.; Farhat, S.; Scott, M.; Magpantay, L.; Halec, G.; Shiboski, C.H.; Ryder, M.I. Biomarkers of oral inflammation in perinatally HIV-infected and perinatally HIV-exposed, uninfected youth. J. Clin. Periodontol. 2019, 46, 1072–1082. [Google Scholar] [CrossRef]
- Bascones-Martínez, A.; Muñoz-Corcuera, M.; Noronha, S.; Mota, P.; Bascones-Ilundain, C.; Campo-Trapero, J. Host defence mechanisms against bacterial aggression in periodontal disease: Basic mechanisms. Medicina Oral Patología Oral y Cirugía Bucal 2009, 14, e680–e685. [Google Scholar] [CrossRef][Green Version]
- Pussinen, P.J.; Paju, S.; Mäntylä, P.; Sorsa, T. Serum microbial- and host-derived markers of periodontal diseases: A review. Curr. Med. Chem. 2007, 14, 2402–2412. [Google Scholar] [CrossRef]
- Tobón-Arroyave, S.I.; Jaramillo-González, P.E.; Isaza-Guzmán, D.M. Correlation between salivary IL-1β levels and periodontal clinical status. Arch. Oral Biol. 2008, 53, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Avalos, A.M.; Ploegh, H.L. Accessory molecules for Toll-like receptors and their function. Nat. Rev. 2012, 12, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Kim, S.J.; Rah, S.H.; Kang, J.I.; Jung, H.E.; Lee, D.; Lee, H.K.; Lee, J.O.; Park, B.S.; Yoon, T.Y.; et al. Reconstruction of LPS Transfer Cascade Reveals Structural Determinants within LBP, CD14, and TLR4-MD2 for Efficient LPS Recognition and Transfer. Immunity 2017, 46, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Ichise, Y.; Saegusa, J.; Tanaka-Natsui, S.; Naka, I.; Hayashi, S.; Kuroda, R.; Morinobu, A. Soluble CD14 Induces Pro-inflammatory Cytokines in Rheumatoid Arthritis Fibroblast-Like Synovial Cells via Toll-Like Receptor 4. Cells 2020, 9, 1689. [Google Scholar] [CrossRef]
- Jagannathan, R.; Lavu, V.; Rao, S.R. Comparison of the Proportion of Non-Classic (CD14+CD16+) Monocytes/Macrophages in Peripheral Blood and Gingiva of Healthy Individuals and Patients With Chronic Periodontitis. J. Periodontol. 2014, 85, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.S.; Ramseier, C.A.; Giannobile, W.V. Oral fluid-based biomarkers of alveolar bone loss in periodontitis. Ann. N.Y. Acad. Sci. 2007, 1098, 230. [Google Scholar] [CrossRef]
- Griffiths, G.S. Formation, collection and significance of gingival crevice fluid. Periodontology 2000 2003, 31, 32–42. [Google Scholar] [CrossRef]
- Cimasoni, G.; Giannopoulou, C. Can crevicular fluid component analysis assist in diagnosis and monitoring periodontal breakdown? In Periodontology Today; Karger Publishers: Basel, Switzerland, 1988; pp. 260–270. [Google Scholar]
- Barros, S.P.; Williams, R.; Offenbacher, S.; Morelli, T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontol 2000 2016, 70, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Wilensky, A.; Tzach-Nahman, R.; Potempa, J.; Shapira, L.; Nussbaum, G. Porphyromonas gingivalis gingipains selectively reduce CD14 expression, leading to macrophage hyporesponsiveness to bacterial infection. J. Innate Immun. 2015, 7, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Nemoto, E.; Tada, H.; Miyake, K.; Imamura, T.; Takada, H. Proteolysis of human monocyte CD14 by cysteine proteinases (gingipains) from Porphyromonas gingivalis leading to lipopolysaccharide hyporesponsiveness. J. Immunol. 2000, 165, 411–418. [Google Scholar] [CrossRef]
- Nazar Majeed, Z.; Philip, K.; Alabsi, A.M.; Pushparajan, S.; Swaminathan, D. Identification of Gingival Crevicular Fluid Sampling, Analytical Methods, and Oral Biomarkers for the Diagnosis and Monitoring of Periodontal Diseases: A Systematic Review. Dis. Markers 2016, 2016, 1804727. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).