Abstract
The efficient performance of metal-ion batteries strongly depends on electrode materials characteristics. Two-dimensional (2D) materials are among promising electrode materials for metal-ion battery cells, owing to their excellent structural and electronic properties. Two-dimensional graphdiyne has been recently fabricated and revealed unique storage capacities and fast charging rates. The current study explores the performance of the novel phosphorated-triphenylene graphdiyne (P-TpG) monolayer as an anode material for Li-, Na-, K-, Mg-, and Ca-ions storage via extensive density functional theory (DFT) simulations. Our results reveal that the stable structure of P-TpG monolayers delivers ultra-high storage capacities of ~2148, ~1696, ~1017, and ~2035 mA·h·g−1 for Li-, Na-, K-, and Ca- ions, respectively. Notably, the metallic electronic behavior is illustrated by adsorbing metal-ions on the P-TpG nanosheets, suggesting a good electronic conductivity. The NEB results demonstrate that P-TpG can serve as an outstanding candidate for the optimal charging/discharging process. This theoretical study suggests P-TpG nanosheets as a highly promising candidate for the design of advanced metal-ion batteries with remarkable charge capacities and optimal charging/discharging rates.
1. Introduction
Performance improvement of energy storage devices is a demanding task of advanced electrified instruments and vehicles. Batteries are among the primary solutions for energy storage devices that must satisfy the high energy density, safety, and low-cost criteria [1,2,3]. High-performance batteries strongly depend on the invention of novel electrode materials. Two-dimensional materials such as graphene [4,5] have gained remarkable attention as novel electrode materials owing to their excellent mechanical and electronic properties. Specifically, 2D Intercalation-based electrode materials have shown great performance in batteries [6,7,8]. The energy density and ion diffusion rate in electrodes determine the performance of metal-ion batteries. Graphite is a standard commercial electrode material, which is electrochemically stable. However, it has a low ion transmission rate and charge capacity [9,10]. Lithium-ion batteries (LIB) are classified among the best available cells due to their high power density and long-lasting battery life [11,12]. However, the scarcity of lithium reserves restricts their usage [13,14] and increases their price. A limited amount of Li resources in the Earth’s crust (20 ppm) [15,16] necessitates exploring non-Li storage systems. The importance of the non-Li rechargeable batteries should not be ignored [17]. Two-dimensional transition metal disulfides have gained great attention as anode materials for Na-ion batteries due to their high capacities and long operation life [18]. Xie et al. [19,20] investigated the Na, K, Mg, Ca and Al storage on MXene sheets using DFT method. They reported high volumetric capacities for these ions. VS2 and VSe2 exhibited promising performances as anode material for Ca- or Na- ions batteries as well. First-principles analysis revealed that VS2 and VSe2 have considerable charge capacities of 257 mA·h·g−1 for Ca- or Na-ion storages, which are very promising as non-Li batteries [21]. Wu et al. [22] investigated hierarchical MoS2/Ti3C2Tx composites structures. Their results revealed that MoS2/Ti3C2Tx electrodes have a good cycling stability with 250.9 mA·h·g−1 at 100 mA·g−1 after 100 cycles with 88% retention of the initial reversible capacity. Mg-ion batteries have confirmed a high cell performance using a VS2 nanosheet and with 0.4 M (PhMgCl)2-AlCl3/tetrahydrofuran (APC/THF) and 50 wt.% 1-butyl-1-methylpiperidinium chloride (PP14Cl) as the electrolyte. These batteries exhibited 348 mA·h·g−1 storage capacity at 20 mA·g−1 current density and excellent rate capability with a 214 mA·h·g−1 capacity at 2.0 A·g−1 current density [23]. Electrodes have an essential role in the performance improvement of metal-ion batteries. Hence, searching for optimal electrode materials is one of the significant research challenges in battery development.
2D monolayers and heterostructures are used in various applications [24,25] due to their high mechanical strength, electronic, and thermal conductivity [26]. Graphene itself has a high electronic conductivity with moderate charge capacities of 372 mA·h·g−1 [10]. Extensive studies have been conducted for novel 2D materials with high electronic conductivity and charge capacity [27,28,29,30,31,32] as the most critical parameters in various applications [28,33,34]. Matsuoka et al. [33] experimentally designed and fabricated a novel graphdiyne family structure called triphenylene graphdiyne (TpG). TpG nanosheets were synthesized using hexaethynyltriphenylene monomers within the modified liquid-liquid interfacial method [34]. This experimental realization was motivated to investigate the intrinsic material properties and conducting novel theoretical predictions [35,36,37]. Recent studies of graphdiyne family monolayers demonstrated the semiconducting electronic behavior and high charge capacities for metal ion storage [38,39,40,41].
Graphdiyne belongs to the class of 2D carbon allotropes that have been experimentally synthesized through a cross-coupling reaction using hexaethynylbenzene [42,43]. Graphdiyne nanosheets have shown outstanding properties include electronic conductivity, charge capacity, low thermal resistance, and high stretchability [44,45,46,47,48,49]. Recent studies suggested graphdiyne nanosheets for applications include energy storage -devices [27,31,32], electrochemical actuators [50], or catalysts [30,51]. Recent experimental studies [33,42] synthesized graphdiyne and a novel triphenylene graphdiyne (TpG) nanosheet fabricated by the liquid-liquid and gas-liquid interface methods. The TpG nanosheet exhibits a polymeric structure with excellent thermal durability (up to 250 °C). Theoretical studies [37,52] confirmed the lattice stability of novel nitrogenated-, phosphorated-, and arsenicated-TpG (N-TpG, P-TpG, As-TpG) nanosheets. In our recent study [53], we employed DFT calculations to investigate the application of N-TpG as an anode material for Na-, K-, Mg-, Ca-ions storages. In this study, we comprehensively investigate the potential use of P-TpG as anode material. P-TpG monolayer has shown a lower band-gap than N-TpG, which suggest better semiconducting electronic behavior [37,52]. We explored electronic properties, stable adsorption sites, open circuit voltage profiles, charge exchange gradient, energy band structures, electronic density of states (DOS), storage capacity, and diffusion-energy barriers for Li-, Na-, K-, Mg-, Ca-ions. First-principles results confirm structural stability and electronic conductivity [37] of P-TpG nanosheet essential for application as an anode/cathode material in batteries. The acquired results suggest the great performance of this novel class of 2D materials.
2. Computational Method
We conducted first-principles DFT simulations by employing the Vienna ab-initio simulation package (VASP) [54,55,56]. Generalized gradient approximation (GGA) is used with the exchange-correlation functional of Perdew-Burke-Ernzerhof (PBE) [57]. The unbounded free electrons in the solid occupy the higher energy levels while the lowest ground-state energy represents the stable structure and the desired solution is the lowest energy structure. Therefore, the energy should cut at some upper bound. While the free electron’s kinetic energy is the highest in a solid, the cutoff energy refers in fact to this kinetic energy. The cutoff energy is depending on a system and should be defined high enough to derive accurate results. The plane waves with smaller kinetic energy are included in calculations. We use the plane-wave basis set with an energy cut-off of 500 eV. The VESTA [58] package is used for visualizing the atomic lattice. A dispersion scheme of DFT-D2 [59] is employed to improve the binding energy calculations by accounting for the dispersion corrections. Periodic boundary conditions are imposed in planar directions with a vacuum layer of 16 Å along with the sheet thickness. Energy minimized structures are obtained by the conjugate gradient method with a convergence criterion of 10−4 eV and 0.01 eV/Å for the energies and forces. A 3 × 3 × 1 Monkhorst-Pack [60] k-point mesh size is employed for the finite number of points in the Brillouin zone (BZ). Compared to finer k-point grids, the employed 3 × 3 × 1 grid yields computationally efficient and accurate results for the adsorption energies. Gradual intercalation simulations were realized by adding metal atoms firstly at the predicted most stable binding sites and then randomly positioning on the surface until the full capacity is reached [28,61]. To obtain more accurate energy values, we conduct single-point calculations using the tetrahedron method with Blöchl corrections and 5 × 5 × 1 Monkhorst k-point mesh size. Bader charge analysis [62] is then achieved to predict the final charge capacities. Finally, nudged elastic band (NEB) method is employed to simulate the diffusion of a single adatom over P-TpG.
3. Results and Discussion
3.1. Lattice Structure and Electronic Properties
Figure 1 illustrates the energy-minimized P-TpG monolayer with a hexagonal lattice. The DFT algorithm employs electronic relaxation and optimized geometry structures. The hexagonal lattice parameter and the C-P bond’s average lengths are 14.41 Å and 1.74~1.78 Å, respectively. Unit-cell optimization results indicate the varying bond length of 1.23~1.44 Å for C-C bonds. The spatial-dependent electron localization function (ELF) [63] qualitatively describes the chemical bonding nature. We illustrated the ELF for the pristine nanosheet in Figure 1. It takes values between 0 and 1, indicating delocalized electrons (0), electron gas-like behavior (0.5), and localized electron regions (1). The electron localization intensity in the middle of the C-C bonds shows the covalent bonding features. However, the electron localization of the C-P bonds occurs mostly around the P atoms due to the higher electronegativity of the P atoms than C atoms.
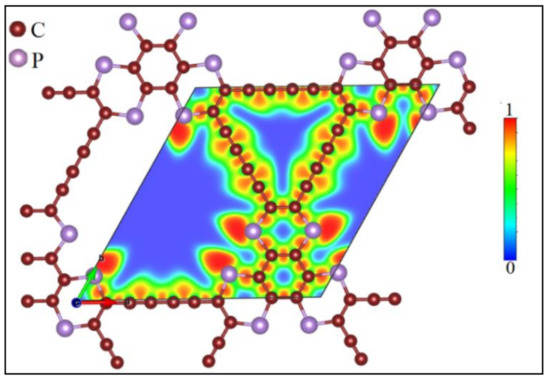
Figure 1.
The atomic lattice of P-TpG monolayer. Color contours refer to electron localization function in unit-cell.
Theoretical studies confirmed the lattice stability of novel nitrogenated-, phosphorated-, and arsenicated-TpG (N-TpG, P-TpG, As-TpG) nanosheets. According to reported PBE results, the band-gaps of TpG, As-TpG, N-TpG, and P-TpG monolayers have been predicted as ~1.26 eV, ~0.47 eV, ~1.24 eV, and ~1.00 eV, respectively, which makes them attractive semiconductor for the application as an electrode in rechargeable batteries [37,52]. The electronic properties of the single-layer pristine P-TpG can be found in Figure 2. It shows the band structure along with the high symmetry Γ-M-K-Γ directions in the irreducible Brillion zone. The atoms’ contribution to the band structure and electronic density of states (DOS) have been predicted by the PBE method. The results show that the top/bottom of the valence/conduction bands occurs at the Γ-point, leading to direct band-gaps of ~0.982 eV. P-TpG monolayer has shown a smaller band-gap value than N-TpG with ~0.982 eV and 1.24 [37,52] using the PBE method, respectively, which implies better semiconducting electronic characteristics of the P-TpG monolayer.
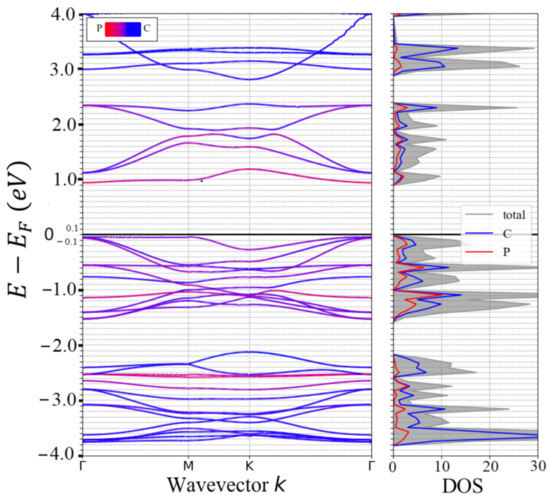
Figure 2.
P-TpG electronic band structure and density of states (DOS) are estimated employing PBE functional. The Fermi energy level is shifted to zero.
3.2. Application as Anode Materials for Li, Na, K, Mg and Ca-Ions Batteries
We focus now on the application of P-TpG as anode material for batteries. We first estimate the adsorption-energy profiles in the most stable sites, i.e., S1, S2, S3, and S4, depicted in Figure 3. These bonding sites satisfy the highest adsorption-energy with minimum total energy conditions. S1 and S4 are placed inside the nanosheet plane hollow sections, while S2 is on top of the hexagonal part, while S4 can be found out-of-plane. The corresponding adsorption energies of Li-, Na-, K-, Mg-, and Ca-ions over P-TpG are calculated by:
where (EAd) denotes the adsorption energy, EP is the total energy of the pristine P-TpG nanosheet, ES the total energy of the system after metal atom adsorption and EAt refers to the per atom lattice energy of the metal adatoms (At = Li, Na, K, Mg, Ca). The S1 site exhibits the maximum adsorption energies for Li, Na, K, and Ca adatoms with −2.026 eV, −2.476 eV, −3.013 eV, and −2.428 eV, respectively. Detailed results are presented in Table 1 for all predicted/most stable adsorption sites. Note that the optimal adsorption energy for anode materials should not be close to zero or take positive values. Except for the Mg adatom, all others yield negative adsorption energy values indicating adequate bonding between the considered adatoms and P-TpG substrates. The results indicate that the considered configurations for the Mg atom storage are not suitable since the adsorption energies are very close to zero or positive. For all adatoms, the highest adsorption energies were found in the S1 site. According to Table 1, Bader charge exchange analysis revealed the transferred charge values (∆Q) from adsorbed atoms to the substrate for all configurations. The results illustrate that Li and Na adatoms were perfectly ionized over the P-TpG substrates.
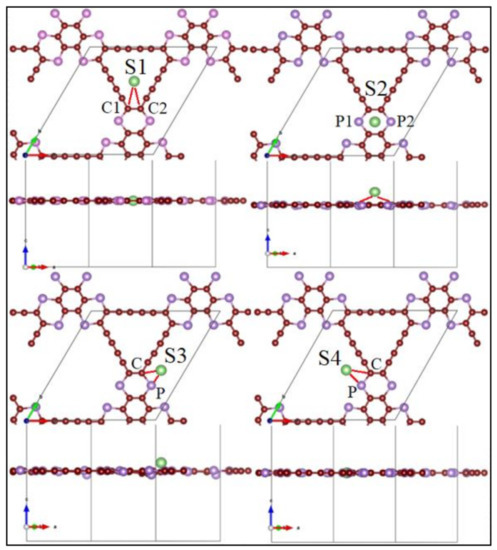
Figure 3.
Most stable adsorption sites over P-TpG.

Table 1.
Predicted most stable adsorption-sites (S1, S2, S3, S4) for Li, Na, K, and Ca adatoms over monolayer P-TpG substrate. EAds, Lx-y, and LZ referred to the corresponding adsorption-energy, the distance between the x and y atoms, the out-of-plane movement of an adatom on adsorption-sites (shown in Figure 3), respectively. ΔQ represents the charge transfer from a single adatom to the P-TpG monolayer according to the Bader charge analysis.
The charge transfers (∆ρq) between Li-, Na-, K-, and Ca-ions and the P-TpG nanosheet determine the configurations’ efficiency. Therefore, we calculated three charge densities of the pristine structure (ρS), single adatom (ρA), and system compositions (ρAS):
Figure 4 shows the Bader charge analysis [62] results, which gives insight about the charge transfer gradient variation in the presence of ions. We employ VESTA to visualize the variation of the charge transfer gradient. According to Table 1 and Figure 4, the charge transfer efficiencies from a single Li and Na adatom are always over 99%. These results suggest that a single Li and Na atom adsorbs effectively over the single-layer P-TpG. Moreover, according to Bader charge analysis results, upon the adsorption Li and Na adatoms are in the forms of Li+ and Na+, in agreement with the process in batteries, in which metal ions adsorb over the surface.

Figure 4.
Charge exchange between P-TpG and single Li, Na, K, and Ca-ions for S1 and S2 adsorption sites. Color coding represents the binding charge transfer, such that green and red show the charge losses and charge gains, respectively.
The adsorption energy evaluation—by increasing the adatom coverage—is essential for anode materials. The average adsorption energy () is calculated by:
where ESM, EP, EAt, and n are the system’s energy, pristine-substrate, single adatom, and the number of adsorbed-ions on the surface, respectively. Metal ions intercalation are simulated over P-TpG by gradually and consistently increase in ions coverage. Therefore, ions are positioned uniformly on both surfaces with a priority of predicted stable binding sites. Due to the surface energy-dependent and stochastic nature of the ions positioning, we constructed three different ion distributions over the considered substrate, where the lowest energy structure was selected to calculate the average adsorption energy. Electronic self-consistent iterations find out the ground state of the electrons at each fixed atom position. Then, geometry optimization is accomplished using the conjugate gradient approach, in order to reach the minimum energy configuration in which the forces on ever atoms fall below a predefined threshold of 10−4 eV eV/Å. Figure 5 demonstrates the average adsorption energy versus storage capacity over P-TpG. The adsorption of adatoms does not lead to new chemical bonds formation or substrate defects. According to Figure 5, at the initial storage levels, the adsorption energy decreases suddenly and then smoothly by increasing the adatoms’ coverage. Increasing the ion coverages leads to weaker interactions between adsorbed adatoms and the substrate. No ion agglomerations are observed due to the weak bonding or physisorption [64]. The Faraday equation calculates the storage capacity (Sc) for different ion coverage (n) and given by:
where n, F, Ve, and ma are the number of adatoms, Faraday constant, number of valance electrons of ion, and the unit-cell atomic mass, respectively. In this study, the ion coverage is calculated as 4, 7, 10,18, 22, 26, 30, and 38 adatoms for different ions. For all configurations, the absolute values of the adsorption energies decrease with increasing ion coverage. Subsequently, the repulsive forces increase hampering the adsorption for newly coming atoms. As shown in Figure 5, despite the low-coverage of Mg atoms, the adsorption energy quantity approaches zero or takes undesirable positive values, making Mg not suitable for adsorbing over P-TpG. For the remaining ions, the adsorption energies remain negative and far enough from zero. Interestingly, for ultra-high capacities of the Ca ion, the adsorption energy remains in the optimal range. However, the considered substrate’s structural stability should be demonstrated for a high amount of ion adsorption. The K adatom has shown maximum changes in the average adsorption energy. The N-TpG monolayers followed almost the same adsorption energy trends as the P-TpG nanosheets with different values [53].
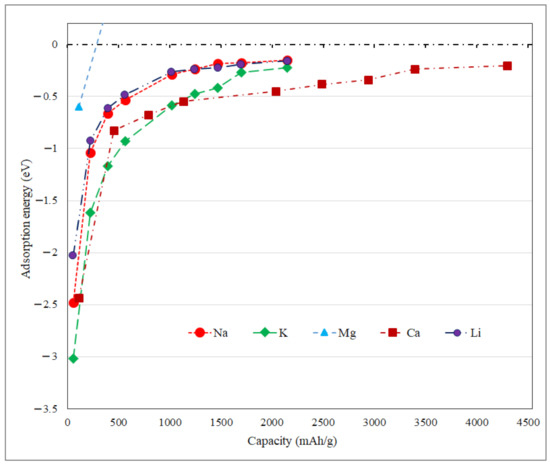
Figure 5.
The average adsorption energy versus storage capacity for Li, Na, Mg, K, and Ca-ions over P-TpG.
The open-circuit voltage is another significant parameter for anode substrates. Substrates with lower positive voltage (around 0.3 volts and far away from zero) could deliver higher output voltage in a battery cell. The voltage values should not be close to zero or take negative values; otherwise, resulting in ions clusterization and dendrites formation. The metallic-ion-clusters reduce the electrode adsorption capacity, and dendrites lead to thermal explosion and performance failure of batteries. The average voltage () can be estimated in a coverage domain of x1 ≤ x ≤ x2 as follows [65]:
where x1,2, , , and |e| indicate the number of adsorbed metal ions, total energies of the systems, single adatom energy, and the number of valence electrons, respectively. Equation (5) calculates the theoretical average voltage of a system. The maximum change of Gibbs free energy by an electrochemical cell is equal to the cell potential multiplied by the total charge that can be transferred during the charging/discharging. The energy obtained by discharging between anode with x1 and x2 number of adsorbed ions is equal to the integral of the product of the cell potential(voltage) and the displaced charge. Therefore, the theoretical average voltage could be calculated by dividing the maximum amount of work by the maximum charge (qmax = (x2 − x1)|e|) that can be transferred theoretically. Here |e| is the charge of an adatom and is equal to one for Li, Na, K ions, and for Ca is equal to two [34,66,67]. Figure 6 presents the open-circuit voltage profiles versus storage capacities. The voltage values drop immediately in the first coverage step (7 ions adsorption) and remain under the value of ~0.3. It is a desirable value for increasing the output voltage of a cell. However, there is no experimental support for P-TpG to compare open-circuit voltage results to our best knowledge, graphdiyne exhibited theoretical specific voltage value between 0.12–0.25 V [66] after the first coverage step, and the fabricated device with graphdiyne as the host active material for perovskite solar cell applications exhibits an open-circuit voltage of 1.073 V [67].
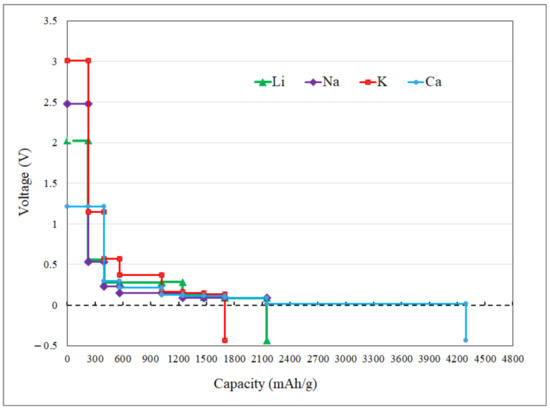
Figure 6.
Open-circuit voltage profiles versus storage capacity of Li, Na, K, and Ca ions over P-TpG.
The P-TpG nanosheet exhibits ultra-high capacities of ~2148, ~1696, ~1017, and ~2035 mA·h·g−1 for Li-, Na-, K-, and Ca- ions, respectively, while the same ion coverage for the N-TpG substrate revealed values of ~2160, ~1296, and ~2592 mA·h·g−1 for Na-, K-, and Ca-ions storage, respectively [53]. Besides, the P-TpG and N-TpG [53] nanosheets show a considerably higher storage capacity compared to the charge capacities of commercial graphite [68], and theoretical VS2/VSe2 [21] 2D structures for the Li-ion storage reported as ~372 mA·h·g−1, and ~466 mA·h·g−1, respectively.
Rechargeable batteries are continuously charged and discharged. The repeated increase/decrease in ion coverage may result in some structural changes in anode materials, which subsequently may reduce the battery performance. The structural stability of substrate materials during the ion adsorption plays a critical role in the application as a battery anode. Figure 7 depicts the stable structures of P-TpG for different ion coverages. Note that according to the ab-initio molecular dynamics calculations [37], the P-TpG nanosheets can stay intact at high temperatures of 2000 K, which is undoubtedly higher than the batteries’ working temperatures.
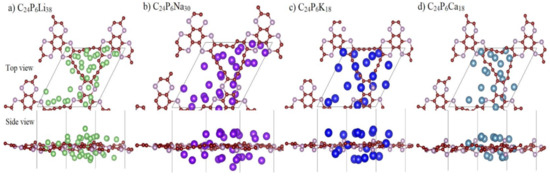
Figure 7.
Planar and lateral view of the most stable or lowest-energy structure of P-TpG nanosheet with (a) 38 Li; (b) 30 Na; (c) 18 K; (d) 18 Ca adatoms content.
Metallic electronic characteristics are highly desirable for electrode materials in batteries to reach internal electronic resistances. Metal atoms generally have low ionization energy and low electronegativity. Hence, they tend to lose electrons. Therefore, we conducted total electronic density of states (DOS) calculations for all the configurations with three coverage levels of 1, 18, and 38 atoms over the P-TpG for each Li, Na, K, and Ca adatom. Figure 8 confirms that at zero-state energy (Fermi level), the DOS is not zero, verifying the composite structures’ metallic electronic behaviour.
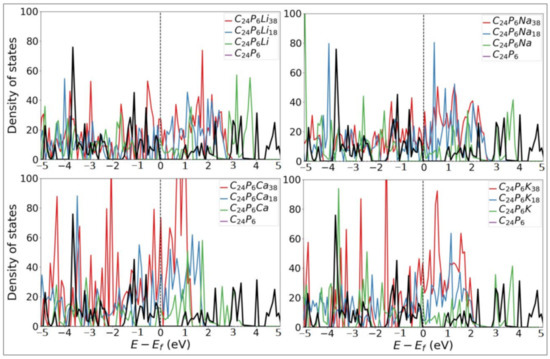
Figure 8.
Electronic density of states (DOS) of P-TpG nanosheets with and without diffusive ions. The vertical dashed line represents the Fermi level and is modified to zero.
The ion conductivity of electrode materials is among the most crucial parameters for the optimal charging and discharging rate. Fast charging processes are needed for electric vehicles and mobile devices. Therefore, we studied the ion conductivity over the P-TpG monolayer to explore Li, Na, K, and Ca ion diffusion rates. The climbing image NEB method is conducted for two hoping paths using most stable adsorption sites (Figure 3). Figure 9 shows the corresponding hoping diffusion paths and barrier energies for considered ions. Path 1 and path 2 start from the S1 adsorption site while it has the highest adsorption energy value for all considered ions. Path1 follows the hoping of the ions to the second most stable adsorption site (S2 adsorption site in Figure 3) over the center of the C4P2 hexagonal ring jumping over the full carbon hexagonal ring and finally to the next C4P2 hexagonal ring. Path2 tracks the hoping of the ions to the second most stable adsorption site then jumps to the S4 adsorption site in the hallowed part and follows the path to the top left site over the C4P2 hexagonal ring. Figure 9 depicts the predicted images between two hopping targets and the corresponding energy variations.
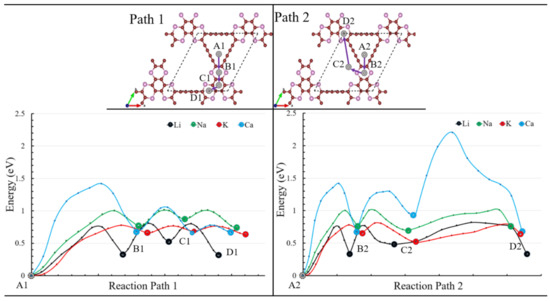
Figure 9.
Energy barriers along Path1 and Path2 are predicted by the nudged-elastic band method for a single Li, Na, K, Ca ions diffusion over the monolayer P-TpG.
According to Table 2, the paths start from the first most stable site. The maximum barrier energies for Li, Na, K, and Ca ions take values of 0.75, 1, 0.78, 1.42 eV, respectively. The K ion has the lowest barrier energy for the rest of the hopping paths, which is also lower than the Li-ion barrier energy of graphene (0.37 eV) [69]. The Li-ion diffusion over the hexagonal ring in path 1 N-TpG (0.37 eV) [37] has a lower energy barrier than P-TpG (0.44 eV). Diffusion energy barriers play an important role in the diffusion rate of intercalation electrodes. The optimized charging/discharging rate strongly depends on the diffusive ions kinetic on an electrode. Intuitively lower energy barriers deliver a fast diffusion rate. However, diffusion rates can be affected by multiple diffusive ion interactions and stacking electrode materials [70,71]. As shown in Table 2, Li and K ions are energetically preferential ions to deliver a high diffusion rate in the first and second pathways. The stacking structure is an important influence on ions diffusion within changing/discharging and is one of the possible ways to enhance anode materials performance [70,71]. Bilayer structures of the graphene [72] considered as anode material for Li-ion batteries. Their results confirmed the higher diffusion rate in AB-stacking structure than AA-stacking structures. The dominant potential analysis indicated stacking structure highly affected the height of potential well. In other study, stacking structure effects of bilayer black phosphorene were investigated on Li-ions adsorption and diffusion [73]. The AC-stacking exhibited most favorable results for Li adsorption and diffusion. Their results revealed stacking structure has very important effects on ion adsorption, open-circuit voltage, and diffusion and indicated appropriate stacking structure improves the anodic behaviour of the material. Figure 9 shows various position-dependent energy barriers for different ions. The ions choose the paths with the lowest barrier energies for passing during charging/discharging. The porous structure of the TpG materials helps the ions’ in-plane paths, resulting in a faster charging process. Except for the Ca barrier energy over the last part of path 1, Li, Na, and K ions have acceptable barrier energies which make P-TpG as an excellent candidate for optimal charging/discharging processes.

Table 2.
Maximum barrier energies along Path1 and Path2 for a single Li, Na, K, Ca ions diffusion over the monolayer P-TpG.
4. Summary
This study investigated the possible application of P-TpG monolayer as anode materials for Li, Na, K, Mg, and Ca ion batteries. Our results confirmed the ultra-high charge capacity of P-TpG for Li-, Na-, K-, and Ca- ions. Predicted open circuit voltage profiles revealed the promising performance of P-TpG monolayer generally positive values below ~0.3 V after the adsorption of single adatoms. The NEB results illustrated P-TpG as electrode material can offer optimal conditions to achieve fast charging/discharging rates, which is highly desirable for some critical applications like electric vehicles. According to our current and previous studies [53], the adsorption-energy of the studied ions over the N-TpG monolayers followed almost the similar variation trends as the P-TpG nanosheets but with different adsorption energy values. The P-TpG nanosheet exhibits ultra-high capacities of ~2148, ~1696, ~1017, and ~2035 mA·h·g−1 for Li-, Na-, K-, and Ca- ions, respectively, while the same ion coverage for the N-TpG substrate revealed values of ~2160, ~1296, and ~2592 mA·h·g−1 for Na-, K-, and Ca-ions storage, respectively [53]. Besides, the P-TpG and N-TpG [53] nanosheets show a considerably higher storage capacity compared to the charge capacities of commercial graphite [68], and theoretical VS2/VSe2 [21] structures for the Li-ion storage reported as ~372 mA·h·g−1, and ~466 mA·h·g−1, respectively. The insight provided by the first-principles results highlights the outstanding prospect of P-TpG nanosheets as an anode material for future energy storage systems.
Author Contributions
M.S. worked out the technical details, and performed the computational calculations and wrote the manuscript. N.A. read the manuscript and contribute in final version of the manuscript. T.R. devised the project, and verified computational process and also contributed to the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Distinguished Scientist Fellowship Program (DSFP) at King Saud University for funding this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cao, W.; Zhang, J.; Li, H. Batteries with high theoretical energy densities. Energy Storage Mater. 2020, 26, 46–55. [Google Scholar] [CrossRef]
- Zhu, K.; Li, Q.; Xue, Z.; Yu, Q.; Liu, X.; Shan, Z.; Liu, K. Mesoporous TiO2 Spheres as Advanced Anodes for Low-Cost, Safe, and High-Areal-Capacity Lithium-Ion Full Batteries. ACS Appl. Nano Mater. 2020. [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Huskinson, B.; Marshak, M.P.; Suh, C.; Er, S.; Gerhardt, M.R.; Galvin, C.J.; Chen, X.; Aspuru-Guzik, A.; Gordon, R.G.; Aziz, M.J. A metal-free organic-inorganic aqueous flow battery. Nature 2014, 505, 195–198. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Magasinski, A.; Dixon, P.; Hertzberg, B.; Kvit, A.; Ayala, J.; Yushin, G. High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nat. Mater. 2010, 9. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.B.; Madhavi, S.; Hng, H.H.; Lou, X.W. Formation of Fe 2O 3 microboxes with hierarchical shell structures from metal-organic frameworks and their lithium storage properties. J. Am. Chem. Soc. 2012, 134, 17388–17391. [Google Scholar] [CrossRef]
- Han, D.; Zhang, J.; Weng, Z.; Kong, D.; Tao, Y.; Ding, F.; Ruan, D.; Yang, Q.H. Two-dimensional materials for lithium/sodium-ion capacitors. Mater. Today Energy 2019, 11, 30–45. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 2016, 1. [Google Scholar] [CrossRef]
- Pramudita, J.C.; Sehrawat, D.; Goonetilleke, D.; Sharma, N. An Initial Review of the Status of Electrode Materials for Potassium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602911. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Zhou, Z. MXene-based materials for electrochemical energy storage. J. Energy Chem. 2018, 27, 73–85. [Google Scholar] [CrossRef]
- Su, D.S.; Centi, G. A perspective on carbon materials for future energy application. J. Energy Chem. 2013, 22, 151–173. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Y.; Lian, R.; Yang, D.; Zhang, D.; Meng, X.; Liu, Y.; Wei, Y.; Chen, G. Atomic insight into the structural transformation and anionic/cationic redox reactions of VS2 nanosheets in sodium-ion batteries. J. Mater. Chem. A 2018, 6, 15985–15992. [Google Scholar] [CrossRef]
- Xie, Y.; Dall’Agnese, Y.; Naguib, M.; Gogotsi, Y.; Barsoum, M.W.; Zhuang, H.L.; Kent, P.R.C. Prediction and characterization of mxene nanosheet anodes for non-lithium-ion batteries. ACS Nano 2014, 8, 9606–9615. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhao, M.Q.; Anasori, B.; Maleski, K.; Ren, C.E.; Li, J.; Byles, B.W.; Pomerantseva, E.; Wang, G.; Gogotsi, Y. Porous heterostructured MXene/carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices. Nano Energy 2016, 26, 513–523. [Google Scholar] [CrossRef]
- Salavati, M.; Rabczuk, T. Application of highly stretchable and conductive two-dimensional 1T VS2 and VSe2 as anode materials for Li-, Na- and Ca-ion storage. Comput. Mater. Sci. 2019, 160, 360–367. [Google Scholar] [CrossRef]
- Wu, Y.; Nie, P.; Jiang, J.; Ding, B.; Dou, H.; Zhang, X. MoS 2 -Nanosheet-Decorated 2D Titanium Carbide (MXene) as High-Performance Anodes for Sodium-Ion Batteries. ChemElectroChem 2017, 4, 1560–1565. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, D.; Yang, D.; Wei, L.; Liu, B.; Wang, X.; Chen, G.; Wei, Y. Superior Mg2+ storage properties of VS2 nanosheets by using an APC-PP14Cl/THF electrolyte. Energy Storage Mater. 2019, 23, 749–756. [Google Scholar] [CrossRef]
- Mortazavi, B.; Makaremi, M.; Shahrokhi, M.; Raeisi, M.; Singh, C.V.; Rabczuk, T.; Pereira, L.F.C. Borophene hydride: A stiff 2D material with high thermal conductivity and attractive optical and electronic properties. Nanoscale 2018, 10, 3759–3768. [Google Scholar] [CrossRef] [PubMed]
- Salavati, M.; Alajlan, N.; Rabczuk, T. Super-stretchability in two-dimensional RuCl3 and RuBr3 confirmed by first-principles simulations. Phys. E Low-Dimens. Syst. Nanostruct. 2019, 113. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, S.; Liu, H.; Li, Y.; Cui, G.; Li, Y. Graphdiyne for high capacity and long-life lithium storage. Nano Energy 2015, 11, 481–489. [Google Scholar] [CrossRef]
- Mortazavi, B.; Dianat, A.; Rahaman, O.; Cuniberti, G.; Rabczuk, T. Borophene as an anode material for Ca, Mg, Na or Li ion storage: A first-principle study. J. Power Sources 2016, 329, 456–461. [Google Scholar] [CrossRef]
- Li, L.; Zuo, Z.; Shang, H.; Wang, F.; Li, Y. In-situ constructing 3D graphdiyne as all-carbon binder for high-performance silicon anode. Nano Energy 2018, 53, 135–143. [Google Scholar] [CrossRef]
- Kuang, P.; Zhu, B.; Li, Y.; Liu, H.; Yu, J.; Fan, K. Graphdiyne: A superior carbon additive to boost the activity of water oxidation catalysts. Nanoscale Horiz. 2018, 3, 317–326. [Google Scholar] [CrossRef]
- Shang, H.; Zuo, Z.; Li, L.; Wang, F.; Liu, H.; Li, Y.; Li, Y. Ultrathin Graphdiyne Nanosheets Grown In Situ on Copper Nanowires and Their Performance as Lithium-Ion Battery Anodes. Angew. Chemie Int. Ed. 2017, 57, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Zuo, Z.; Yu, L.; Wang, F.; He, F.; Li, Y. Low-Temperature Growth of All-Carbon Graphdiyne on a Silicon Anode for High-Performance Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1801459. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, R.; Toyoda, R.; Shiotsuki, R.; Fukui, N.; Wada, K.; Maeda, H.; Sakamoto, R.; Sasaki, S.; Masunaga, H.; Nagashio, K.; et al. Expansion of the Graphdiyne Family: A Triphenylene-Cored Analogue. ACS Appl. Mater. Interfaces 2019, 11, 2730–2733. [Google Scholar] [CrossRef]
- Matsuoka, R.; Sakamoto, R.; Hoshiko, K.; Sasaki, S.; Masunaga, H.; Nagashio, K.; Nishihara, H. Crystalline Graphdiyne Nanosheets Produced at a Gas/Liquid or Liquid/Liquid Interface. J. Am. Chem. Soc. 2017, 139, 3145–3152. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Lee, S.; Park, S.; Lee, C.Y. Graphene chemiresistors modified with functionalized triphenylene for highly sensitive and selective detection of dimethyl methylphosphonate. RSC Adv. 2019, 9, 33976–33980. [Google Scholar] [CrossRef]
- Taghilou, H.; Fathi, D. Spin related transport in two pyrene and Triphenylene graphene nanodisks using NEGF method. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 101, 208–211. [Google Scholar] [CrossRef]
- Mortazavi, B.; Shahrokhi, M.; Madjet, M.E.; Makaremi, M.; Ahzi, S.; Rabczuk, T. N-, P-, As-triphenylene-graphdiyne: Strong and stable 2D semiconductors with outstanding capacities as anodes for Li-ion batteries. Carbon 2019, 141, 291–303. [Google Scholar] [CrossRef]
- Srinivasu, K.; Ghosh, S.K. Graphyne and graphdiyne: Promising materials for nanoelectronics and energy storage applications. J. Phys. Chem. C 2012, 116, 5951–5956. [Google Scholar] [CrossRef]
- Li, X.; Wang, N.; He, J.; Yang, Z.; Tu, Z.; Zhao, F.; Wang, K.; Yi, Y.; Huang, C. Designing the efficient lithium diffusion and storage channels based on graphdiyne. Carbon N. Y. 2020, 162, 579–585. [Google Scholar] [CrossRef]
- Jia, Z.; Li, Y.; Zuo, Z.; Liu, H.; Huang, C.; Li, Y. Synthesis and Properties of 2D Carbon - Graphdiyne. Acc. Chem. Res. 2017, 50, 2470–2478. [Google Scholar] [CrossRef]
- Sun, C.; Searles, D.J. Lithium storage on graphdiyne predicted by DFT calculations. J. Phys. Chem. C 2012, 116, 26222–26226. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Liu, H.; Guo, Y.; Li, Y.; Zhu, D. Architecture of graphdiyne nanoscale films. Chem. Commun. 2010, 46, 3256–3258. [Google Scholar] [CrossRef]
- Baughman, R.H.; Eckhardt, H.; Kertesz, M. Structure-property predictions for new planar forms of carbon: Layered phases containing sp 2 and sp atoms. J. Chem. Phys. 1987, 87, 6687–6699. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Liu, H.; Li, Y. Graphdiyne and graphyne: From theoretical predictions to practical construction. Chem. Soc. Rev. 2014, 43, 2572–2586. [Google Scholar] [CrossRef]
- Long, M.; Tang, L.; Wang, D.; Li, Y.; Shuai, Z. Electronic structure and carrier mobility in graphdiyne sheet and nanoribbons: Theoretical predictions. ACS Nano 2011, 5, 2593–2600. [Google Scholar] [CrossRef]
- Pan, L.D.; Zhang, L.Z.; Song, B.Q.; Du, S.X.; Gao, H.J. Graphyne- and graphdiyne-based nanoribbons: Density functional theory calculations of electronic structures. Appl. Phys. Lett. 2011, 98, 173102. [Google Scholar] [CrossRef]
- Mortazavi, B.; Makaremi, M.; Shahrokhi, M.; Fan, Z.; Rabczuk, T. N-graphdiyne two-dimensional nanomaterials: Semiconductors with low thermal conductivity and high stretchability. Carbon N. Y. 2018, 137, 57–67. [Google Scholar] [CrossRef]
- Mortazavi, B.; Shahrokhi, M.; Zhuang, X.; Rabczuk, T. Boron-graphdiyne: A superstretchable semiconductor with low thermal conductivity and ultrahigh capacity for Li, Na and Ca ion storage. J. Mater. Chem. A 2018, 6, 11022–11036. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Tu, Z.; Zhao, F.; He, J.; Guan, Z.; Huang, C.; Yi, Y.; Li, Y. Synthesis and Electronic Structure of Boron-Graphdiyne with an sp-Hybridized Carbon Skeleton and Its Application in Sodium Storage. Angew. Chemie Int. Ed. 2018, 57, 3968–3973. [Google Scholar] [CrossRef]
- Lu, C.; Yang, Y.; Wang, J.; Fu, R.; Zhao, X.; Zhao, L.; Ming, Y.; Hu, Y.; Lin, H.; Tao, X.; et al. High-performance graphdiyne-based electrochemical actuators. Nat. Commun. 2018, 9, 752. [Google Scholar] [CrossRef]
- Zhao, Y.; Wan, J.; Yao, H.; Zhang, L.; Lin, K.; Wang, L.; Yang, N.; Liu, D.; Song, L.; Zhu, J.; et al. Few-layer graphdiyne doped with sp-hybridized nitrogen atoms at acetylenic sites for oxygen reduction electrocatalysis. Nat. Chem. 2018, 10, 924–931. [Google Scholar] [CrossRef]
- Mortazavi, B.; Shahrokhi, M.; Madjet, M.E.; Hussain, T.; Zhuang, X.; Rabczuk, T. N-, B-, P-, Al-, As-, and Ga-graphdiyne/Graphyne Lattices: First-Principles Investigation of Mechanical, Optical and Electronic Properties. Available online: https://pubs.rsc.org/en/content/articlelanding/2019/tc/c9tc00082h/unauth#!divAbstract (accessed on 8 February 2019).
- Salavati, M.; Rabczuk, T. First-principles investigation of N-triphenylene-graphdiyne nanosheets as an anode material for Na, K, Mg and Ca storage. Comput. Mater. Sci. 2019, 169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Mortazavi, B.; Dianat, A.; Cuniberti, G.; Rabczuk, T. Application of silicene, germanene and stanene for Na or Li ion storage: A theoretical investigation. Electrochim. Acta 2016, 213, 865–870. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Scheffler, M.; Stampfl, C. Chapter 5 Theory of adsorption on metal substrates. Handb. Surf. Sci. 2000, 2, 285–356. [Google Scholar] [CrossRef]
- Aydinol, M.; Kohan, A.; Ceder, G.; Cho, K.; Joannopoulos, J. Ab initio study of lithium intercalation in metal oxides and metal dichalcogenides. Phys. Rev. B Condens. Matter Mater. Phys. 1997, 56, 1354–1365. [Google Scholar] [CrossRef]
- Lee, H.; Jang, B.; Koo, J.; Park, M.; Lee, H.; Nam, J.; Kwon, Y. Graphdiyne as a high-capacity lithium ion battery anode material. Appl. Phys. Lett. 2013, 103, 263904. [Google Scholar] [CrossRef]
- Li, J.; Jiu, T.; Chen, S.; Liu, L.; Yao, Q.; Bi, F.; Zhao, C.; Wang, Z.; Zhao, M.; Zhang, G.; et al. Graphdiyne as a Host Active Material for Perovskite Solar Cell Application. Nano Lett. 2018, 18, 6941–6947. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Pollak, E.; Geng, B.; Jeon, K.J.; Lucas, I.T.; Richardson, T.J.; Wang, F.; Kostecki, R. The interaction of Li+ with single-layer and few-layer graphene. Nano Lett. 2010, 10, 3386–3388. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hassanein, A. Kinetic Monte Carlo simulation of hydrogen diffusion on tungsten reconstructed (0 0 1) surface. Fusion Eng. Des. 2014, 89, 2545–2549. [Google Scholar] [CrossRef]
- Moon, J.; Lee, B.; Cho, M.; Cho, K. Ab initio and kinetic Monte Carlo simulation study of lithiation in crystalline and amorphous silicon. J. Power Sources 2014, 272, 1010–1017. [Google Scholar] [CrossRef]
- Zhong, K.; Hu, R.; Xu, G.; Yang, Y.; Zhang, J.M.; Huang, Z. Adsorption and ultrafast diffusion of lithium in bilayer graphene: Ab initio and kinetic Monte Carlo simulation study. Phys. Rev. B 2019, 99, 155403. [Google Scholar] [CrossRef]
- Hu, R.; Xu, G.; Yang, Y.; Zhang, J.M.; Zhong, K.; Huang, Z. Effect of stacking structure on lithium adsorption and diffusion in bilayer black phosphorene. Phys. Rev. B 2019, 100, 085422. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).