Abstract
The preparation of copper manganite (hopcalite, Cu1.5Mn1.5O4), as a single phase, was achieved by using a sustainable method of green synthesis. This method is based on the replacement of the conventional “brute force” ceramic preparation by the recent “soft force” green synthesis via the egg white assisted one-step method. In other words, we present a facile and rapid methodology to prepare the nanocrystalline Cu1.5Mn1.5O4 spinel as a single phase, compared to our previous work using ceramic and glycine-assisted combustion methods. The as-synthesized copper manganite was characterized using X-ray diffraction (XRD), Fourier-transform infrared (FTIR), energy-dispersive spectroscopy (EDS), and scanning electron microscope (SEM). We used a vibrating sample magnetometer to determine the magnetic properties of the prepared sample (VSM). XRD, FTIR, SEM, EDS and transmittance electron micrograph (TEM) resulted in synthesis of a successful cubic spinel Cu1.5Mn1.5O4 system with a sponge crystal structure. The particles of the prepared materials are polycrystalline in their nature and the sizes ranged between 50 and 100 nm. The magnetic measurement demonstrated that the generated nanostructure has been found to exhibit ferromagnetism at room temperature with an optimum saturation magnetization value (0.2944 emu/g).
1. Introduction
In several important applications such as electronics, optics, magnetism, catalysis to energy storage and conversion, spinel type materials have long attracted scientific and technological consideration [1]. Versatile applications for manganese-based spinels (MMn2O4) were determined in the case of lithium insertion electrodes, magnetic solids and catalysts [2,3,4]. For medical applications, including bio imaging, target drug development and hyperthermia, certain materials were being used [5,6]. The different benefits of manganese, such as low cost, easy implementation, low toxicity, multiple valence and a prominent Jahn–Teller effect, could be related to these behaviors [7].
Among the numerous varieties of manganese-based spinels, one of the most incredibly interesting composite oxides is hopcalite (CuMn2O4 or Cu1.5Mn1.5O4) since it is very appropriate to several catalytic processes such as water-gas transfer, the reduction of nitrogen oxide with carbon monoxide, the direct decomposition of NO, selective ammonia oxidation to nitrogen gas and, more recently, in steam reforming and oxidative steam reforming of methanol, and the selective oxidation of carbon monoxide, benzyl alcohol and toluene [8,9,10,11]. Towards the production of copper-manganese mixed oxides, different preparation techniques have been employed. Co-precipitation, deposition-precipitation, impregnation, sol-gel, solvothermal and combustion methods include such techniques [12,13,14,15]. It is reported that due to Jahn–Teller distortion, the crystal structure of manganese-based spinels, such as spinel lithium manganese oxide (LiMn2O4), often has a tetragonal unit cell, but under certain preparation conditions it can become cubic [16].
The processing of single phase containing hopcalite nanoparticles is complicated, depending upon if the secondary phase of manganese and copper is more likely to be implemented during the synthesis process. In our previous work, the solid state reaction between both copper nitrate and manganese carbonate resulted in the production of a Cu1.5Mn1.5O4 spinel compound with the subsequent formation of both Mn2O3 and CuO. In the same way, the use of copper chloride as a CuO source brought about the formation of both CuMn2O4 and Mn2O3 [15]. Researchers have focused on the synthesis of such a compound by the fusion of copper with manganese metals or by the coprecipitation technique of certain modifications to avoid such a challenge [15,16,17,18]. The high concentration of Cu+ and Mn4+ cations in Cu1.5Mn1.5O4 solid could activate O2 and NOx in the diesel exhaust and convert these gases to NO2, so this solid is expected to be quite favorable for diesel soot combustion [18]. Consequently, a few disadvantages, such as the complication of preparation methods, remain. The rapid and reasonable synthesis of crystalline spinels at room temperature and ambient atmosphere, however, represents a problem, despite continuous efforts.
In this research, to prepare copper maganite nanoparticles (Cu1.5Mn1.5O4 NPs) through egg white assisted green synthesis, a facial, scalable and environmentally friendly method was used. Here, we demonstrate the experimental method of preparing Cu1.5Mn1.5O4 nanoparticles through using egg white as an auxiliary bio material, which provides the dispersion of nanoscale oxides in one phase. The single-step process is straightforward, environmentally friendly, inexpensive and reproducible, generating high-quality nanoparticles of Cu1.5Mn1.5O4 that can be regulated in terms of structure, morphology and distribution of particle size. The characterization of the Cu1.5Mn1.5O4 NPs and magnetic properties developed has been determined.
2. Materials and Methods
2.1. Production of Cu1.5Mn1.5O4 Nanoparticles
An egg white-based precursor containing copper nitrate trihydrate (2.41 g) and manganese nitrate tetrahydrate (2.51 g) was used to prepare the Cu1.5Mn1.5O4 nanocomposite. These reagents were dissolved in distilled water with an equimolar ratio of Cu and Mn until a clear solution was obtained; egg white (0, 2.5 and 5 mL) was added into the mixture. To evaporate water and increase the viscosity, the obtained mixture was first stirred at 60 °C, and then the solution was heated at 120 °C to create a gel. The resulting precursor gel was calcined for 15 min at 300 °C in a furnace. Several more foams were created; then, a spark appeared at one corner when a crucible temperature was achieved. The spreading of this spark through the mass led to the creation of a voluminous and fluffy substance in the container, with the subsequent production of black powder by grinding the end product. The produced solids prepared by using 0, 2.5 and 5 mL egg white are labeled as S1, S2 and S3, respectively. The chemicals used in the present work were of analytical grade delivered by Prolabo Company (Highlands North, South Africa). Figure 1 represents a clear schematic of the synthesis process.
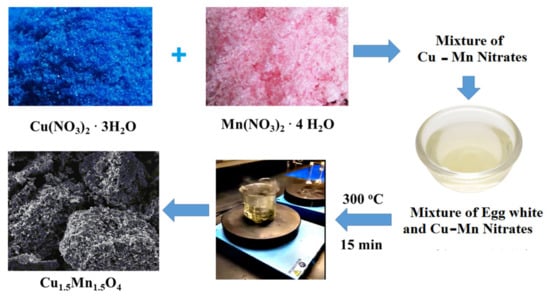
Figure 1.
Experimental flow chart for the synthesis of Cu1.5Mn1.5O4 nanoparticles (NPs).
2.2. Characterization Techniques
The BRUKER D8 (Bruker, Karlsruhe, Germany) advance diffractometer was used for the X-ray study of various mixed solids. The patterns were run at 40 kV with Cu Kαradiation and 40 mA with 2 theta 2° min−1 scanning speeds. The average crystallite size (D), dislocation density (δ) and strain (ε) of Cu1.5Mn1.5O4 present in the solid investigated were determined using Equations (1)–(3) and based on X-ray diffraction line broadening as follows [19]:
D = 0.89 λ/β cosθ
δ = 1/D2
ε = β cosθ/4
Where D is the average crystallite size of the phase under examination, 0.89 is the Scherrer constant, β is the full width at half the height of the peaks in radians, θ is the diffraction angle in radians, and λ is the wavelength of the X rays in nanometers.
The Fourier-transform infrared spectrum (FTIR) of the investigated solid was calculated using a PerkinElmer Spectrophotometer (type 1430, PerkinElmer, Unit a Llantrisant CF72 United Kingdom). The IR spectrum was collected at wavelengths from 4000 to 400 cm−1. A total of 200 mg of potassium bromide (KBr) grade vacuum-dried IR was blended with 2 mg of solid samples. Dispersed for 3 min by grinding in a vibratory ball mill, the mixture was inserted as a disk with 13 mm diameter into a steel die and subjected to 12 ton pressure. In the double grating IR spectrometer holder, the sample disk was inserted.
Scanning electron microscope (SEM) and transmittance electron micrograph (TEM) were recorded with the help of JEOL JAX-840A and JEOL Model 1230 (JEOL, Tokyo, Japan), respectively, and in order to disperse individual particles over mount setup and Cupper grids, the samples were dispersed in ethanol and then treated ultrasonically for a few minutes.
Energy dispersive X-ray analysis (EDS with Mapping) was performed with a Delta kevex device attached to an electron microscope, JED- 2200 Series (JEOL, Tokyo, Japan). The following parameters were used: 20 kV accelerating voltage, 120 s accumulation time, and 6 μm window width. Using the Asa procedure, Zaf-correction, and Gaussian approximation, the surface molar composition was calculated.
The vibrating sample magnetometer (VSM) (9600-1 LDJ, Weistron Co., Ltd., West Hollywood, CA, USA), was used to investigate the magnetic properties of the solids evaluated in a maximum field of 20 kOe. Loops of hysteresis, magnetization of saturation (Ms), magnetization of remanence (Mr) and coercivity (Hc) were established.
3. Results
3.1. XRD Investigation
The XRD patterns of the as-synthesized samples are shown in Figure 2. The XRD patterns showed an amorphous state for samples prepared without and with 2.5 mL egg white, while the sample treated with 5 mL egg white is a very good crystalline system with 10 peaks, two of which are very strong peaks at degree 2 theta equal to 37,634 and 43,862 degrees, encoding plane parameters (222) and (004), respectively. The miller planes, two observed theta, two calculated theta and the difference between them are listed in Table 1. These peaks are consistent with the values recorded for Cu1.5Mn1.5O4 NPs Cubic Spinel (PDF file No. 35-1171) [20]. The Fulprof program is used to calculate the lattice parameters, unit cell dimensions, crystal system, space group, and other crystallographic parameters [21]. The mean crystallite size of the Cu1.5Mn1.5O4 particles was calculated depending upon Scherer’s formula. The values of the crystallite size (D), X-ray density (Dx), unit cell volume (V), lattice constant (a), dislocation density (δ), micro-strain (ε), the distance between the reacting ions (LA and LB) and bond lengths (A–O and B–O) on tetrahedral (A) sites and octahedral (B) sites in the spinel Cu1.5Mn1.5O4 NPs are shown in Table 2 [22,23,24,25].
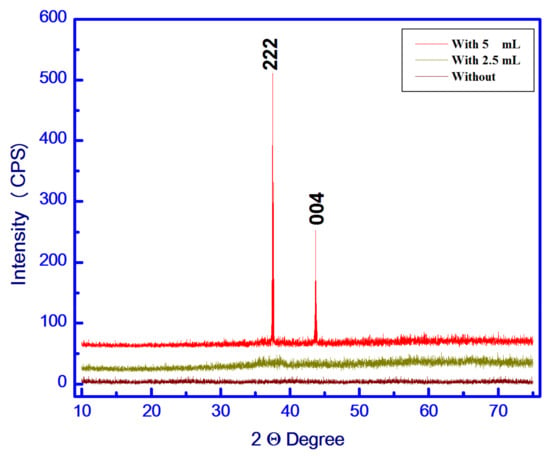
Figure 2.
X-ray diffraction (XRD) pattern of the S1, S2 and S3 samples.

Table 1.
Structural data of the as-synthesized Cu1.5Mn1.5O4 NPs.

Table 2.
Some structural properties of the as-synthesized Cu1.5Mn1.5O4 NPs.
3.2. FTIR Study
The chemical structural nature of the S3 sample containing a Cu1.5Mn1.5O4 lattice was identified by FTIR analysis. The FTIR spectrum measured in the range of 4000 to 400 cm−1 is shown in Figure 3. (i) An extreme broad band detected in the 3650–3100 cm−1 region and a small band of around 1677 cm−1 may due to the stretching and bending vibrations of the adsorbed water molecules of the O-H surface hydroxyl groups [26]. Consequently, these bands validate which high humidity is absorbed by the nanocrystalline system with high surface-to-volume ratio [27]. (ii) The stretching vibration of O=C=O resulted in an IR band observed at 2352 cm−1 [28]. During KBr palletization, the adsorption of CO2 from the air on the surface of the as-synthesized material resulted in the presence of an IR band at 1411 cm−1 [28]. Depending on the presence of carbon traces, the transmittance peak was observed due to C-OH stretching vibration at about 1147 cm−1 [28]. (iii) It is possible to attribute the characteristic bands observed around 500 and 600 cm−1 to the octahedral (B) and tetrahedral modes, respectively, in the spinel system [29]. Indeed, the 500 and 618 cm−1 bands are related to the vibration of trivalent metal ions in B-sites and divalent metal ions in A-sites of the spinel Cu1.5Mn1.5O4 lattice, respectively [30,31].
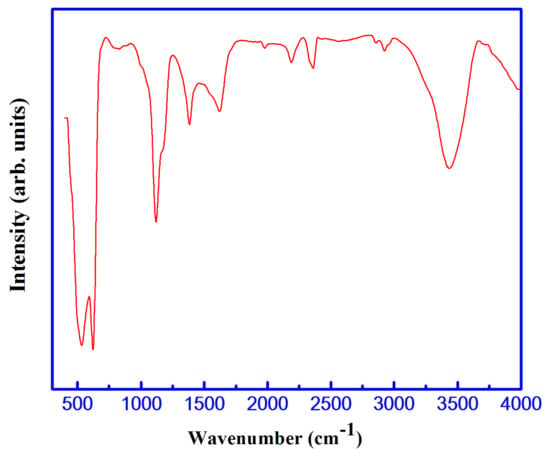
Figure 3.
Fourier-transform infrared spectrum (FTIR) spectrum of the S3 sample.
3.3. Energy Dispersive X-ray (EDX) Analysis
Figure 4 shows the EDS pattern of the S3 sample. From the whole figure, the existence of the signal characteristic elements of copper (Cu), manganese (Mn) and oxygen (O) was recorded. In comparison, the percentage weights of Cu, Mn, and O measured from EDS were 49 percent, 29 percent, and 21 percent, respectively. The signal observed at 0.27 Kev in the spectrum is due to the presence of carbon (C, 1 percent) as an impurity, which is confirmed from the obtained FTIR analysis. However, EDS mapping findings indicate that copper, manganese, and oxygen atoms are very well distributed.
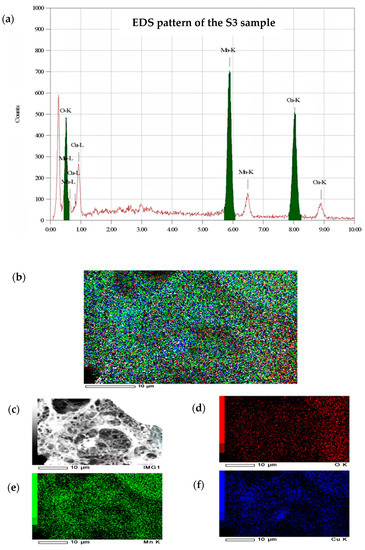
Figure 4.
(a) EDS pattern and mapping of the S3 sample, (b) Cu, Mn, and O K, (c) SEM, (d) O K, (e) Mn K and (f) Cu K.
3.4. Scanning Electron Microscopic (SEM) Analysis
The surface morphology based study of the S3 sample was determined using SEM analysis. The SEM images with different magnifications were collected in Figure 5. Figure 5a–f represents the SEM images of solid Cu1.5Mn1.5O4 obtained by using egg white assisted green synthesis. From the SEM images of this composite, it was observed that the particles have a spongy structure containing pores and voids. However, these particles have a tendency of agglomerations with a higher homogeneity. The synthesized nanoparticles had sizes ranging between 50 and 100 nm, indicating the formation of a polycrystalline solid. These observations are in agreement with the XRD measurement.
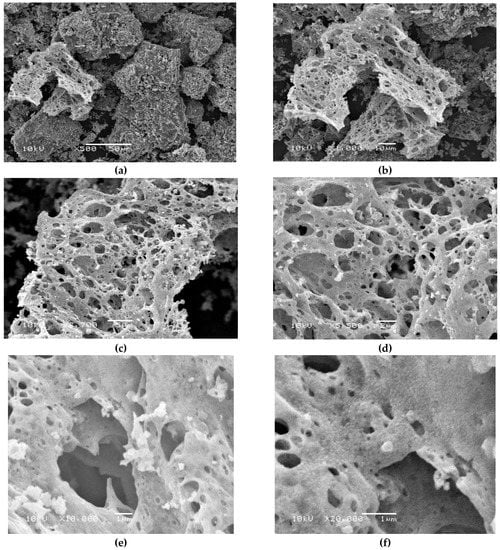
Figure 5.
Scanning electron microscope (SEM) of the S3 sample with different magnifications. (a) 500×, (b) 1000×, (c) 2700×, (d) 5500×, (e) 10,000× and (f) 20,000×.
3.5. Transmission Electron Microscopic (TEM) Analysis
We used TEM analysis as an additional surface morphology of the S3 sample. The TEM images are shown in Figure 6 with different magnifications, 50 KX and 80 KX. From these figures, we can observe that there is an agglomeration of particles in the resulting Cu-Mn mixed oxides. Our method of preparing the studied mixed solids resulted in the creation of particles with homogeneous, uniform size and diameters varying from 50 nm to 100 nm. Indeed, the contrast observed in TEM images could be related to phase contrast. During the evaluation of the promoted sample, the distinct carbon particles were observed from the strong contrast in TEM images that would be predicted from the large difference in the atomic number and density of Cu1.5Mn1.5O4 nanoparticles and that of carbon. This observation could be attributed to the sensitivity of the TEM technique to the atomic number and density of the investigated material. However, the TEM morphology supports the results obtained from SEM and XRD measurements.
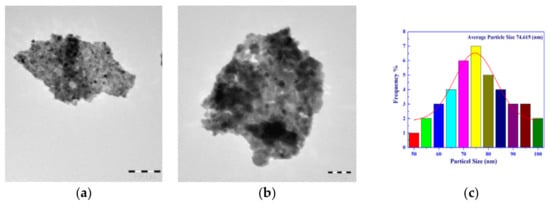
Figure 6.
Transmittance electron micrograph (TEM) of the S3 sample with different magnifications. (a) is 50 k×, (b) is 80 k× and (c) particle distribution histogram.
3.6. Magnetic Study
A vibrating sample magnetometer (VSM) measured the magnetic properties of the S3 sample at room temperature. Magnetization plots (M) as a function of the applied magnetic field (H) are shown in Figure 7. This figure shows the resulting cycle of hysteresis at 300 K. The values of saturation magnetization (Ms), remenant magnetization (Mr), squareness or the ratio of remenant magnetization to saturation magnetization (Mr/Ms), coercive force or coercive force (Hc) and anisotropic constant (Ka) for the S3 sample containing Cu1.5Mn1.5O4 NPs are reported in Table 3 [25].
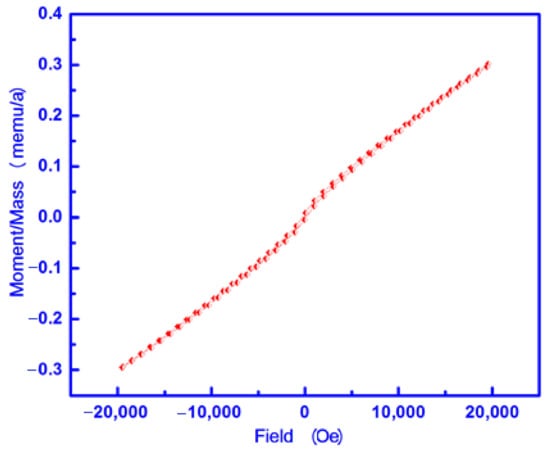
Figure 7.
M-H curve of the S3 sample.

Table 3.
Magnetic properties of the S3 sample.
4. Discussion
In this investigation, the egg white mediated green synthesis brought about the formation of Cu1.5Mn1.5O4 NPs as a single phase, and the mean average crystallite was approximately 75 nm. However, it can be seen from Table 1 that there is micro-strain and dislocation in the crystal lattice of Cu1.5Mn1.5O4 NPs. A dislocation inside a crystal structure is a crystallographic defect (slight misalignment), which greatly affects the properties of materials. The standard atomic array of the perfect crystals is skewed by this defect [22,23,24,25,32,33,34]. Within the crystal lattices, the strain confirms the existence of defects. In addition, the XRD and FTIR studies confirm that Cu1.5Mn1.5O4 NPs have a single-spinel structure. Furthermore, Cu1.5Mn1.5O4 NPs can be prepared via a solid state reaction between Cu and Mn oxides via a ceramic path [15,17,18]. At the beginning of the solid state relation between unreacted Cu and Mn oxides, a thin layer of the copper manganite is formed to cover the unreacted oxides and prevent these oxides from interacting together. This thin layer acts as an energy barrier that must be overcome by the diffusion process to achieve the complete conversion of the unreacted oxides into Cu1.5Mn1.5O4 NPs. In other words, the counter-diffusion of copper and manganese ions through a relatively rigid manganite film about the surface of Cu and Mn oxides led to the formation of particles of Cu1.5Mn1.5O4. Furthermore, by reacting with manganese ions, oxygen passes through the reacted region to be added to the CuO interface and form spinel. Indeed, techniques such as the route of preparation can be used to facilitate this diffusion [15,17]. Copper manganite (Cu1.5Mn1.5O4) formation occurs according to the following stoichiometric reaction:
6CuO + 3Mn2O3 + 0.5O2 → 4Cu1.5Mn1.5O4
Copper manganite crystallizes in an inverse cubic spinel structure, where manganese ions are distributed among the tetrahedral and octahedral sites while copper ions are located at the octahedral sites [15]. In fact, the presence of the Mnn+1/Mnn+ redox couple located in B-sites of the semiconductor spinel has been considered as the responsible factor of the controlled-valence conduction mechanism proposed by Verwey [35]. According to the preparation conditions, the copper manganite crystallizes in a random cubic spinel structure via the distribution of both Cu and Mn ions among the tetrahedral and octahedral sites. This structure could be attributed to the presence of Cu+ ions at the sites [18]. Indeed, Cu+ ions have a strong preference for tetrahedral interstices [36,37,38]. However, if Mn3+ ions in octahedral sites are disproportionate to Mn2+ and Mn4+, then Mn2+ ions will move to tetrahedral sites [35]. In this regard, some researchers pointed out the stabilization of Cu+ and Mn4+ cations in the tetrahedral and octahedral sites, respectively, without the presence of a stabilizing agent [18,36,37,38]. These researchers confirmed abundant Cu+ and Mn4+ cations according to the following reaction:
Cu2+ + Mn3+ → Cu+ + Mn4+
It was recently confirmed that it was not possible to prepare a pure cubic spinel Cu1.5Mn1.5O4 phase [39,40,41,42,43]. Many other authors mentioned that during the sintering phase of Cu-containing spinel materials, Cu+ can be produced. Any oxidation of Cu+ to Cu2+ on cooling in air does not allow the residual CuO to be incorporated into the spinel material [15,42]. This action led to the coexistence of Cu1.5Mn1.5O4 and CuO as a second phase. Similar results were observed in our previous works, which indicate the formation of Cu1.5Mn1.5O4 with both CuO or Mn2O3 by using the ceramic method with and without glycine via the thermal treatment of calculated amounts of copper nitrate and different manganese precursors [14,15]. The glycine assisted combustion route followed by heating treatment at 900 °C for a mixture of copper and manganese nitrates resulted in the formation of Cu1.5Mn1.5O4 with Mn2O3 as a second phase [14]. Additionally, the heat treatment at 800 °C for a mixture of copper nitrate and manganese carbonate brought about the production of Cu1.5Mn1.5O4 and Mn2O3 crystallites [15]. However, in this study, the presence of 5 mL egg white inhibited the oxidation of Cu+ to Cu2+, yielding Cu1.5Mn1.5O4 without the appearance of any secondary phase. This does not negate the presence of Cu2+ ions, especially at octahedral sites. The comparison between this investigation and our previous works confirms the role of the metal precursor and preparation method in the change of the crystallographic of the product. In other words, this study resulted in the preparation of cubic spinel Cu1.5Mn1.5O4 as a single phase.
The nano regime range of the synthesized Cu1.5Mn1.5O4 NPs with certain agglomerations has been verified by SEM results. Figure 5f indicates that the nanoparticles were clustered or very aggregated. The TEM results gave the same results that were obtained using the SEM technique.
From the EDS spectrum, it was evident that the Cu1.5Mn1.5O4 NPs were successfully synthesized in accordance with the main components of the sample prepared as Cu, Mn, and O elements with carbon traces. Nevertheless, the EDS mapping results were compatible with that of the XRD of the as-synthesized material.
The biosynthesized Cu1.5Mn1.5O4NPs exhibit magnetic hysteresis loops with coercivity (215.16 Oe) and saturation magnetization (0.2986 emu/g). In order to understand the mechanism responsible for ferromagnetism in Cu1.5Mn1.5O4 NPs at room temperature, the origin of ferromagnetism is analyzed by different possibilities that can be present in this system. In other words, Cu1.5Mn1.5O4 NPs have a room temperature ferromagnetic-like state (RTFM) depending upon the competition between ferromagnetic and antiferromagnetic sublattices [44]. This competition could be attributed to the presence of a complex magnetic structure with the existence of several magnetic sublattices in the oxides. Some authors observed the Mn2+-Mn3+ antiferromagnetic interactions at low temperatures [41]. These authors reported Mn3+ and Mn4+ ferromagnetic interactions at a higher temperature close to the magnetic transition. The interaction between Cu2+ and Mn3+ ions has been suggested to be ferromagnetic [45]. From this point, we can add that the ferromagnetic behavior of the prepared sample with 5 mL egg white is also due to the interaction between Cu+ and Mn2+ ions at the tetrahedral site involved in the Cu1.5Mn1.5O4 lattice. It is reasonable to suspect that the indirect coupling between Mn ions mediated by O and Cu ions leads to the stabilization of ferromagnetism [46,47]. Additionally, Cu-O-Cu interactions may also play a role. In addition, the investigated system has superparamagnetism properties depending on its nano particle nature. Moreover, the squareness of the prepared Cu1.5Mn1.5O4 NPs was below 0.5, indicating a decrease in anisotropy in the crystal lattice [27]. This behavior could be attributed to magnetostatic interaction between these particles [27]. Therefore, the prepared Cu1.5Mn1.5O4 system can be used in the manufacture of spintronic devices depending upon its room temperature ferromagnetic behavior.
5. Conclusions
Egg white assisted green synthesis resulted in spinel Cu1.5Mn1.5O4 NPs’ formation in a few minutes, without the appearance of any second phase. This process has many advantages for the synthesis of Cu1.5Mn1.5O4 NPs with the presence of small carbon additives, such as no toxicity, economic feasibility, ease to scale up, being less time consuming and being an environmentally friendly approach. On the other side, in our previous work, both the ceramic method and glycine assisted combustion route followed by heating at 900 °C and 800 °C, respectively, led to the formation of Cu1.5Mn1.5O4 NPs with both Mn2O3 and CuO as second phases, respectively. The as-synthesized material consisted almost entirely of Cu1.5Mn1.5O4 NPs, as approved with XRD and FTIR data. Using SEM, EDS mapping and TEM techniques, the elements and morphology of the prepared sample were characterized. Characterization techniques enabled us to calculate the crystallite size, X-ray density, unit cell volume, lattice constant, dislocation density, micro-strain, and the distance between the reacting ions and bond lengths on tetra-hedral (A) sites and octahedral (B) sites for the spinel Cu1.5Mn1.5O4 NPs. The morphological analysis of the prepared system displays a spongy, fragile structure containing pores and voids. The synthesized system has room temperature ferromagnetic behavior with an optimum value, 0.2986 emu/g, of saturation magnetism. This green process of synthesizing Cu1.5Mn1.5O4 NPs may also be applied in the future to the production of industrial single phases of inorganic functional materials.
Author Contributions
Conceptualizing, N.M.D., G.M.A.-S., as well as O.H.A.-E.; technique, N.M.D., G.M.A.-S. and O.H.A.-E.; program, O.H.A.-E.; validation N.M.D., G.M.A.-S., and O.H.A.-E.; formal analysis, N.M.D., G.M.A.-S., and O.H.A.-E.; systematic evaluation, N.M.D., G.M.A.-S., and O.H.A.-E.; inquiry, N.M.D., G.M.A.-S.; resources, N.M.D., G.M.A.-S. and O.H.A.-E.; curation of results, N.M.D., G.M.A.-S., and O.H.A.-E.; writing—original preparation of the draft, N.M.D. and O.H.A.-E.; writing—review and editing, N.M.D., G.M.A.-S., and O.H.A.-E.; visualization, N.M.D. and O.H.A.-E.; supervision, N.M.D. and O.H.A.-E.; project management, N.M.D., G.M.A.-S., and O.H.A.-E.; procurement of financing, G.M.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at Princes Nourah bint Abdulrahman University through the fast-track research-funding program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moyer, G.; Kukuruznyak, D.A.; Nguyen, N.; Prowse, M.S.; Ohuchi, F.S. Thermopower and electrical conductivity of Mn1.68 − XCu0.6 + X + Y + ZCo0.24 − YNi0.48 − Z thin film oxides obtained through metal organic decomposition processing. J. Appl. Phys. 2006, 100, 083504. [Google Scholar] [CrossRef]
- Thackeray, M.M. Manganese oxides for lithium batteries. Prog. Solid State Chem. 1997, 25, 1–71. [Google Scholar] [CrossRef]
- Choi, H.C.; Shim, J.H.; Min, B.I. Electronic structures and magnetic properties of spinel ZnMn2O4 under high pressure. Phys. Rev. B 2006, 74, 172103. [Google Scholar] [CrossRef]
- Clarke, T.J.; Davies, T.E.; Kondrat, S.A.; Taylor, S.H. Mechanochemical synthesis of copper manganese oxide for the ambient temperature oxidation of carbon monoxide. Appl. Catal. B 2015, 165, 222–231. [Google Scholar] [CrossRef]
- Singh, G.; Vladimir, S.; Myasnichenko, V.S.; Glomm, W.R. New insights into size-controlled reproducible synthesis of anisotropic Fe3O4 nanoparticles: The importance of the reaction environment. Mater. Adv. 2020, 1, 1077–1082. [Google Scholar] [CrossRef]
- McDonagh, B.H.; Hak, G.S.S.; Bandyopadhyay, S.; Augestad, I.L.; Peddis, D.; Sandvig, I.; Sandvig, A.; Glomm, W.R. L-DOPA-coated manganese oxide nanoparticles as dual MRI contrast agents and drug-delivery vehicles. Small 2016, 12, 301–306. [Google Scholar] [CrossRef]
- Shoemaker, D.P.; Li, J.; Seshadri, R. Unraveling atomic positions in an oxide spinel with two Jahn–teller ions: Local structure investigation of CuMn2O4. J. Am. Chem. Soc. 2009, 131, 11450–11457. [Google Scholar] [CrossRef] [PubMed]
- Zhi, K.-D.; Liu, Q.-S.; Zhang, Y.-G.; Shuang, H.E.; He, R.-X. Effect of precipitator on the texture and activity of copper-manganese mixed oxide catalysts for the water gas shift reaction. J. Fuel Chem. Technol. 2010, 38, 445–451. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.C.; Chen, M.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Waste-free soft reactive grinding synthesis of high-surface-area copper–manganese spinel oxide catalysts highly effective for methanol steam reforming. Catal. Lett. 2008, 121, 144–150. [Google Scholar] [CrossRef]
- Solis, T.V.; Lopez, I.; Marban, G. Copper manganite as a catalyst for the PROX reaction. Deactivation studies. Int. J. Hydrog. Energy 2010, 35, 1879–1887. [Google Scholar] [CrossRef]
- Tang, Q.; Gong, X.; Zhao, P.; Chen, Y.; Yang, Y. Copper–manganese oxide catalysts supported on alumina: Physicochemical features and catalytic performances in the aerobic oxidation of benzyl alcohol. Appl. Catal. A 2010, 389, 101–107. [Google Scholar] [CrossRef]
- Mirzaei, A.A.; Shaterian, H.R.; Habibi, M.; Hutchings, G.J.; Taylor, S.H. Characterisation of copper-manganese oxide catalysts: Effect of precipitate ageing upon the structure and morphology of precursors and catalysts. Appl. Catal. A 2003, 253, 499–508. [Google Scholar] [CrossRef]
- Jobbágy, M.; Mariño, F.; Schönbrod, B.; Baronetti, G.; Laborde, M. Synthesis of copper-promoted CeO2 catalysts. Chem. Mater. 2006, 18, 1945–1950. [Google Scholar] [CrossRef]
- Deraz, N.M.; Abd-Elkader, O.H. Synthesis and characterization of nano-crystalline bixbyite-hopcalite solids. Int. J. Electrochem. Sci. 2013, 8, 10112–10120. [Google Scholar]
- Deraz, N.M.; Abd-Elkader, O.H. Effects of precursor on preparation and properties of nano-crystalline hopcalite particles. Asian J. Chem. 2014, 26, 2133–2137. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, G.; Jeong, M.; Lee, M.-J.; Kang, K.; Cho, J. Hierarchical surface atomic structure of a manganese-based spinel cathode for lithium-ion batteries. Angew. Chem. 2015, 127, 1169–1174. [Google Scholar] [CrossRef]
- Parida, S.K.; Mohapatra, J.; Mishra, D.K. Structural and magnetic behavior of spinel CuMn2O4 synthesized by co-melting technique. Mater. Lett. 2016, 181, 116–118. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, X.X.; Pan, L.Y.; Wang, M.; Chen, H.R.; Shi, J.L. Facile synthesis of spinel Cu1.5Mn1.5O4 microspheres with high activity for the catalytic combustion of diesel soot. RSC Adv. 2017, 7, 20451–20459. [Google Scholar] [CrossRef]
- Shaltout, A.A.; Abd-Elkader, O.H. The role of gas direction in a modified Grimm-type glow discharge for controlling the degree of crystallinity in brass alloy thin films. Vacuum 2015, 121, 105–112. [Google Scholar] [CrossRef]
- Vandenberghe, R.E.; Legrand, E.; Scheerlinck, D.; Brabers, V.A.M. Neutron diffraction study of the cation ordering in Cu1.5Mn1.5O4 and CuMg0.5Mn1.5O4. Acta Cryst. 1976, B32, 2796–2798. [Google Scholar]
- Boultif, A.; Louer, D. Powder pattern indexing with the dichotomy method. J. Appl. Cryst. 2004, 37, 724–731. [Google Scholar] [CrossRef]
- Rendale, M.K.; Mathad, S.N.; Puri, V. Thick films of magnesium zinc ferrite with lithium substitution: Structural characteristics. Int. J. Self-Propag. High-Temp. Synth. 2015, 24, 112–117. [Google Scholar] [CrossRef]
- Pathan, A.T.; Mathad, S.N.; Shaikh, A.M. Infrared spectral studies of Co2+ substituted Li-Ni-Zn nano-structured ferrites. J. Self-Propag. High-Temp. Synth. 2014, 23, 112–117. [Google Scholar] [CrossRef]
- Shedam, R.M.; Gadkari, A.B.; Mathad, S.N.; Shedam, M.R. Synthesis and structural investigation of nano-sized cadmium ferrite. J. Mod. Mater. 2016, 2, 7–12. [Google Scholar] [CrossRef]
- Patange, S.M.; Shirsath, S.E.; Toksha, B.G.; Jadhav, S.S.; Jadhav, K.M. Electrical and magnetic properties of Cr3+ substituted nanocrystalline nickel ferrite. J. Appl. Phys. 2009, 106, 023914. [Google Scholar] [CrossRef]
- Nie, L.; Yu, J.; Li, X.; Cheng, B.; Liu, G.; Jaroniec, M. Enhanced performance of Na OH-modified Pt/TiO2 toward room temperature selective oxidation of formaldehyde. Environ. Sci. Technol. 2013, 47, 2777–2783. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Deraz, N.M.; Abd-Elkader, O.H. Magnetic and characterization studies of Co/Co3O4 nanocomposite. Processes 2020, 8, 844. [Google Scholar] [CrossRef]
- Halder, M.; Islam, M.D.M.; Ansari, Z.; Ahammed, S.; Sen, K.; Islam, S.K.M. Biogenic nano-CuO-catalyzed facile CN cross-Coupling reactions: Scope and mechanism. ACS Sustain. Chem. Eng. 2017, 5, 648–657. [Google Scholar] [CrossRef]
- Sundar, S.; Venkatachalam, G.; Kwon, S.J. Biosynthesis of copper oxide (CuO) nanowires and their use for the electrochemical sensing of dopamine. Nanomaterials 2018, 8, 823. [Google Scholar] [CrossRef] [PubMed]
- Waldron, R.D. Infrared spectra of ferrites. Phys. Rev. 1955, 99, 1725–1727. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Ge, B.; Zhou, H.; Yuan, A.; Shen, X. Concave Co3O4 octahedral mesocrystal: Polymer-mediated synthesis and sensing properties. Cryst. Eng. Comm. 2012, 14, 6264–6270. [Google Scholar] [CrossRef]
- Kung, C.Y.; Young, S.L.; Chen, H.Z.; Kao, M.C.; Horng, L.; Shih, Y.T.; Lin, C.C.; Lin, T.T. Influence of Y-doped induced defects on the optical and magnetic properties of ZnO nanorod arrays prepared by low-temperature hydrothermal process. Nanoscale Res. Lett. 2012, 7, 372. [Google Scholar] [CrossRef]
- Mathad, S.N.; Jadhav, R.N.; Patil, N.D.; Puri, V. Structural and mechanical properties of Sr+2-doped bismuth manganite thick films. Int. J. Self-Propag. High-Temp. Synth. 2013, 22, 180–184. [Google Scholar] [CrossRef]
- Mathad, S.N.; Jadhav, R.N.; Phadtare, V.; Puri, V. Structural and mechanical properties of Sr-doped barium niobate thick films. Int. J. Self-Propag. High-Temp. Synth. 2014, 23, 145–150. [Google Scholar] [CrossRef]
- Verwey, E.J.W. Oxidic Semiconductors in Semi-Conducting Materials; Henisch, H.K., Ed.; Butterworths: London, UK, 1951; p. 151. [Google Scholar]
- Decyk, P.; Więckowski, A.B.; Najder-Kozdrowska, L.; Bilkova, I.; Ziółek, M. Copper and manganese mixed oxides in zeolites: Study of EMR spectra. In Proceedings of the III Forum EMR, Kraków, Poland, 23–25 May 2014; Volume 1, pp. 23–25. [Google Scholar]
- Vandenberghe, R.E.; Legrand, E.; Scheerlinck, D.; Brabers, V.A.M. On the stability of the cubic spinel structure in the systemCuxMn3-xO. Acta Cryst. Sect B Struct. Sci. Cryst. Eng. Mater. 1976, 32, 2796–2798. [Google Scholar]
- Bayon, R.; Vicente, G.S.; Maffiotte, C.; Morales, A. Preparation of selective absorbers based on Cu MN spinel’s by dip-coating method. Renew. Energy 2008, 33, 348–353. [Google Scholar] [CrossRef]
- Blasse, G. Ferromagnetism and ferrimagnetism of oxygen spinels containing tetravalent manganese. J. Phys. Chem. Solids 1966, 27, 383–389. [Google Scholar] [CrossRef]
- Buhl, R. Manganites spinelles purs d’elements de transition preparations et structures cristallographiques. J. Phys. Chem. Solids 1969, 30, 805–812. [Google Scholar] [CrossRef]
- Robbrecht, G.G.; Henriet-Iserentant, C.M. On the lattice parameters and the tetragonal distortion of the copper and cadmium manganite systems. Phys. Status Solidi 1970, 41, K43–K46. [Google Scholar] [CrossRef]
- Jung, H.-R.; Lee, S.-G.; Lee, D.-J.; Kim, K.-M. Characterization and site distributions of cation in nickel manganite thermistors. J. Ceram Process Res. 2016, 17, 758–762. [Google Scholar]
- Yang, S.G.; Li, T.; Gu, B.X.; Du, Y.W. Ferromagnetism in Mn-doped CuO. Appl. Phys. Lett. 2003, 83, 3746–3748. [Google Scholar] [CrossRef]
- Xiao, Z.R.; Guo, G.Y. Structural, electronic and magnetic properties of V2O5−x: An ab initio study. J. Chem. Phys. 2009, 130, 214704–214710. [Google Scholar] [CrossRef] [PubMed]
- Cue, X.Y.; Medvedeva, J.E.; Delley, B.; Freeman, A.J.; Newman, N.; Stampfl, C. Role of embedded clustering in dilute magnetic semiconductors: Cr doped GaN. Phys. Rev. Lett. 2005, 95, 256404. [Google Scholar] [CrossRef] [PubMed]
- Lisboa-Filho, P.N.; Bahout, M.; Barahona, M.; Moure, C.; Peña, O. Oxygen stoichiometry effects in spinel-type NiMn2O4−δ samples. J. Phys. Chem. Solids 2005, 66, 1206–1212. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, F.; Pan, L.; Zhang, Y.; Fan, C.; Zhang, Y.; Xiao, J.Q. Structural and magnetic properties of Mn-doped CuO thin films. J. Appl. Phys. 2007, 101, 09H111-4. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).