Calcium Release from Different Toothpastes after the Incorporation of Tricalcium Phosphate and Amorphous Calcium Phosphate
Abstract
1. Introduction
2. Materials and Methods
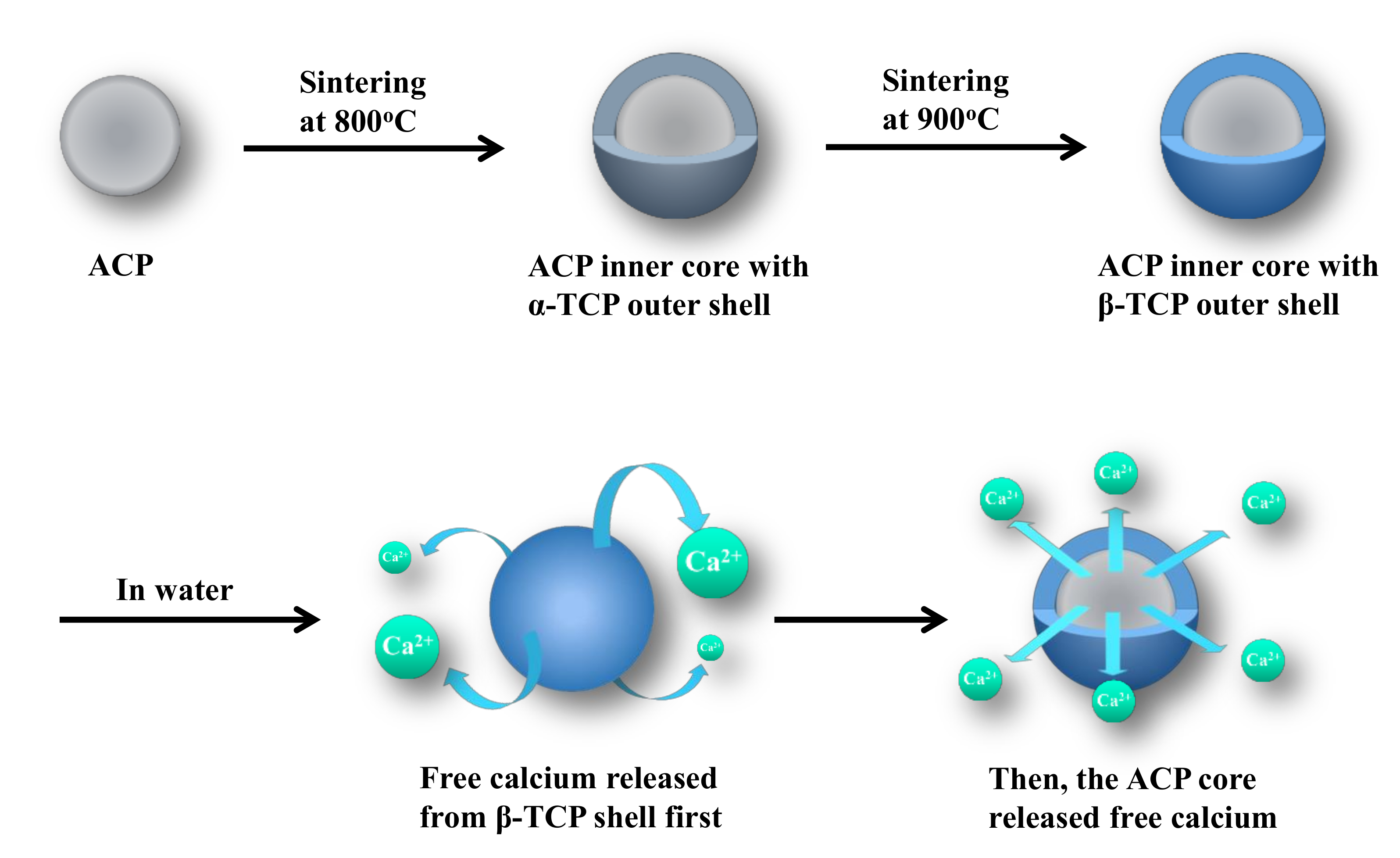
2.1. Sample Sintering
2.2. Characteristics of the Sintered TCP and ACP Powders
2.3. Preparation of Samples for pH Measurements
2.4. Preparation of TCP/ACP Powders Mixed Toothpaste
2.5. Measurement of Calcium Release
2.6. Statistical Analysis
3. Results
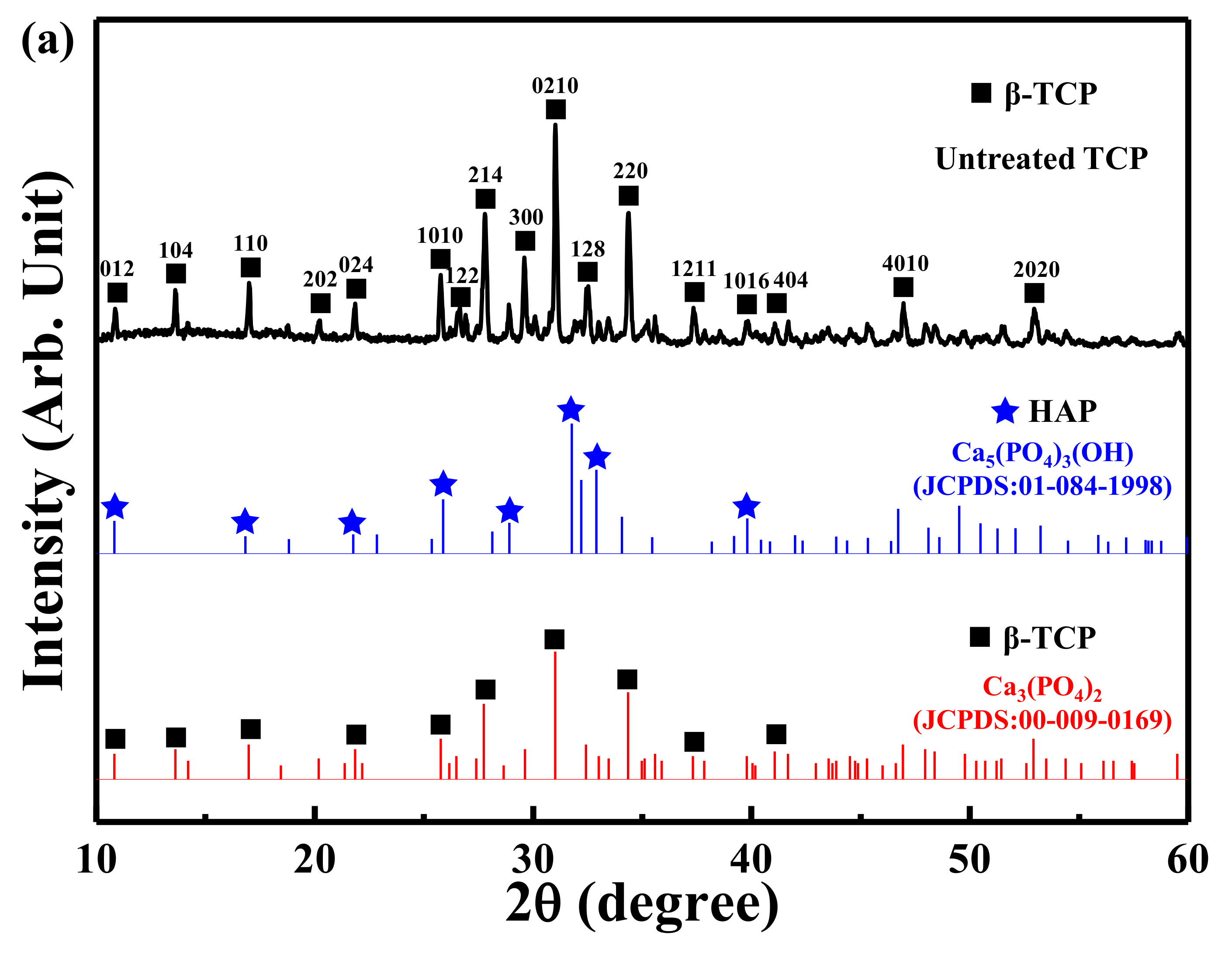
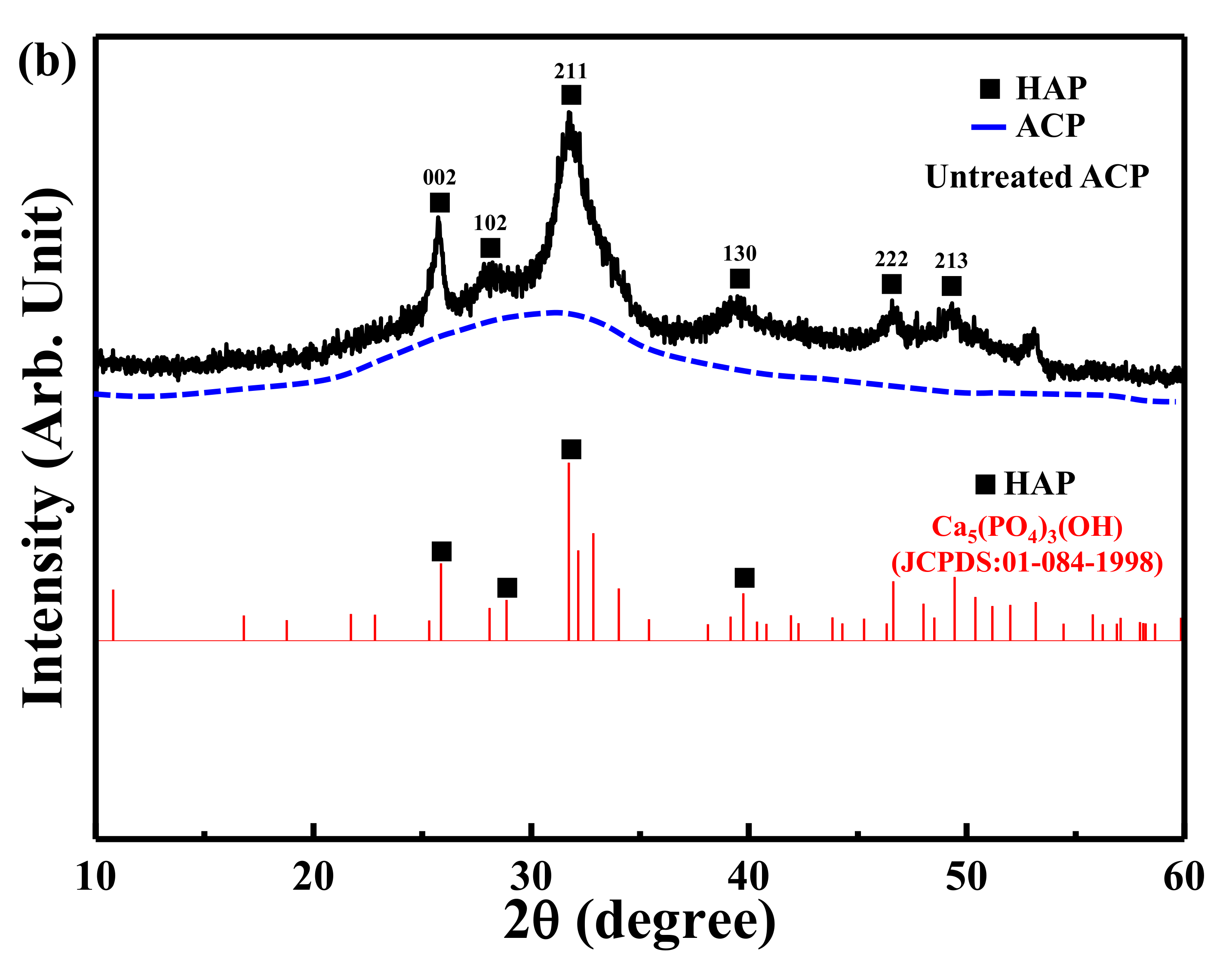
3.1. XRD Analysis of the Untreated TCP and ACP Samples
3.2. FTIR Analysis of the Sintered TCP and ACP Samples
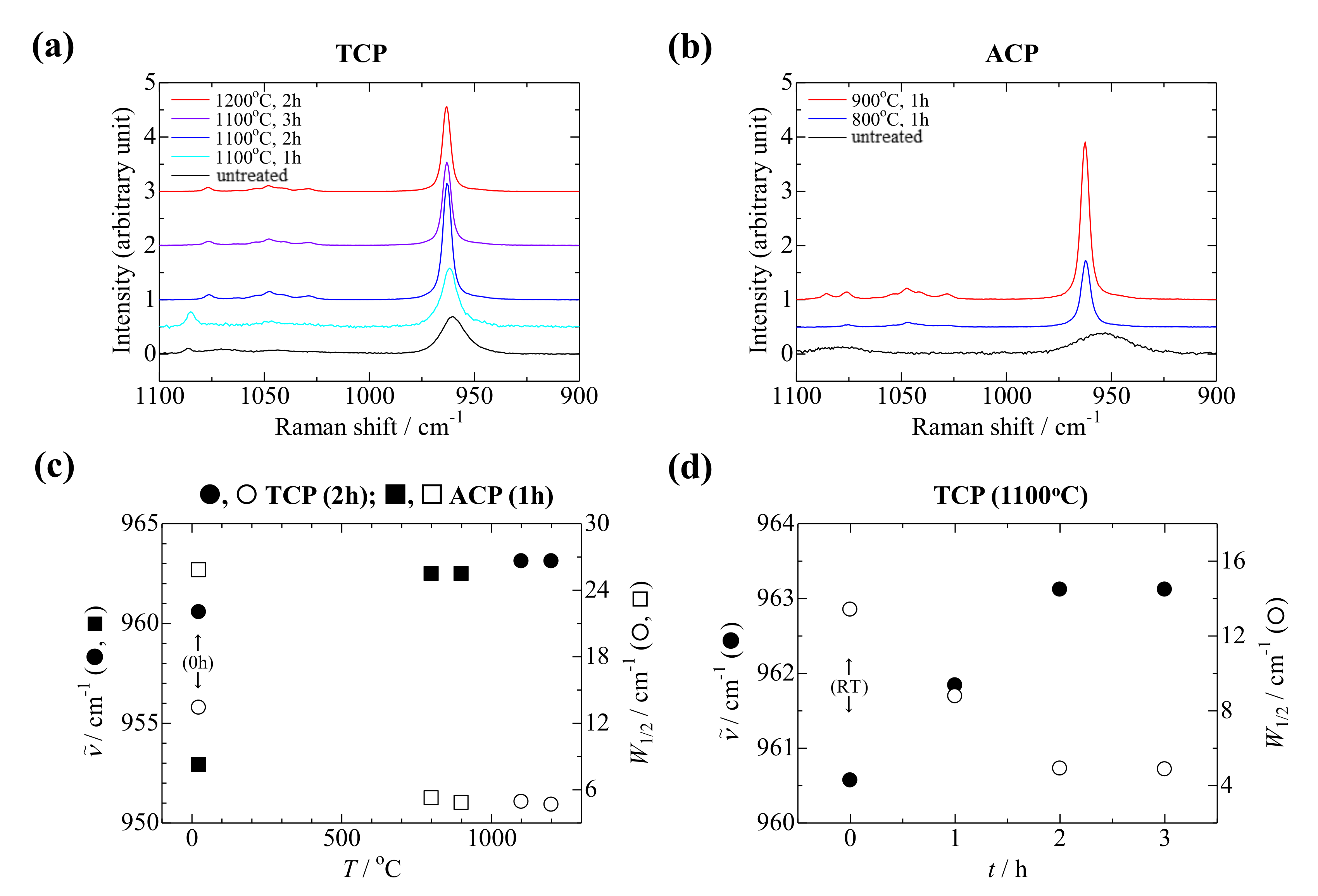
3.3. Raman Analysis of the Sintered TCP and ACP Samples
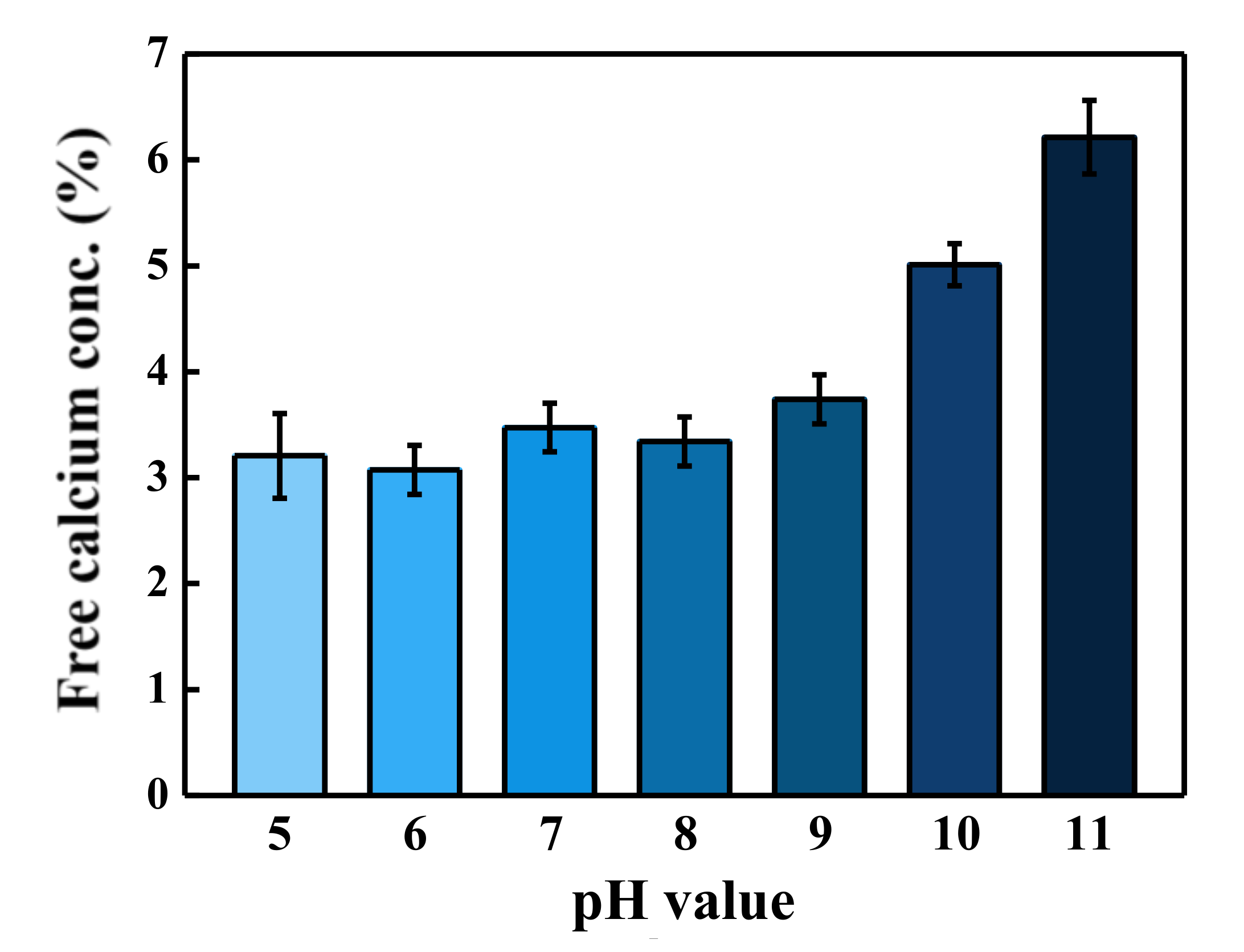
3.4. Comparison of Calcium Concentration at Various pH
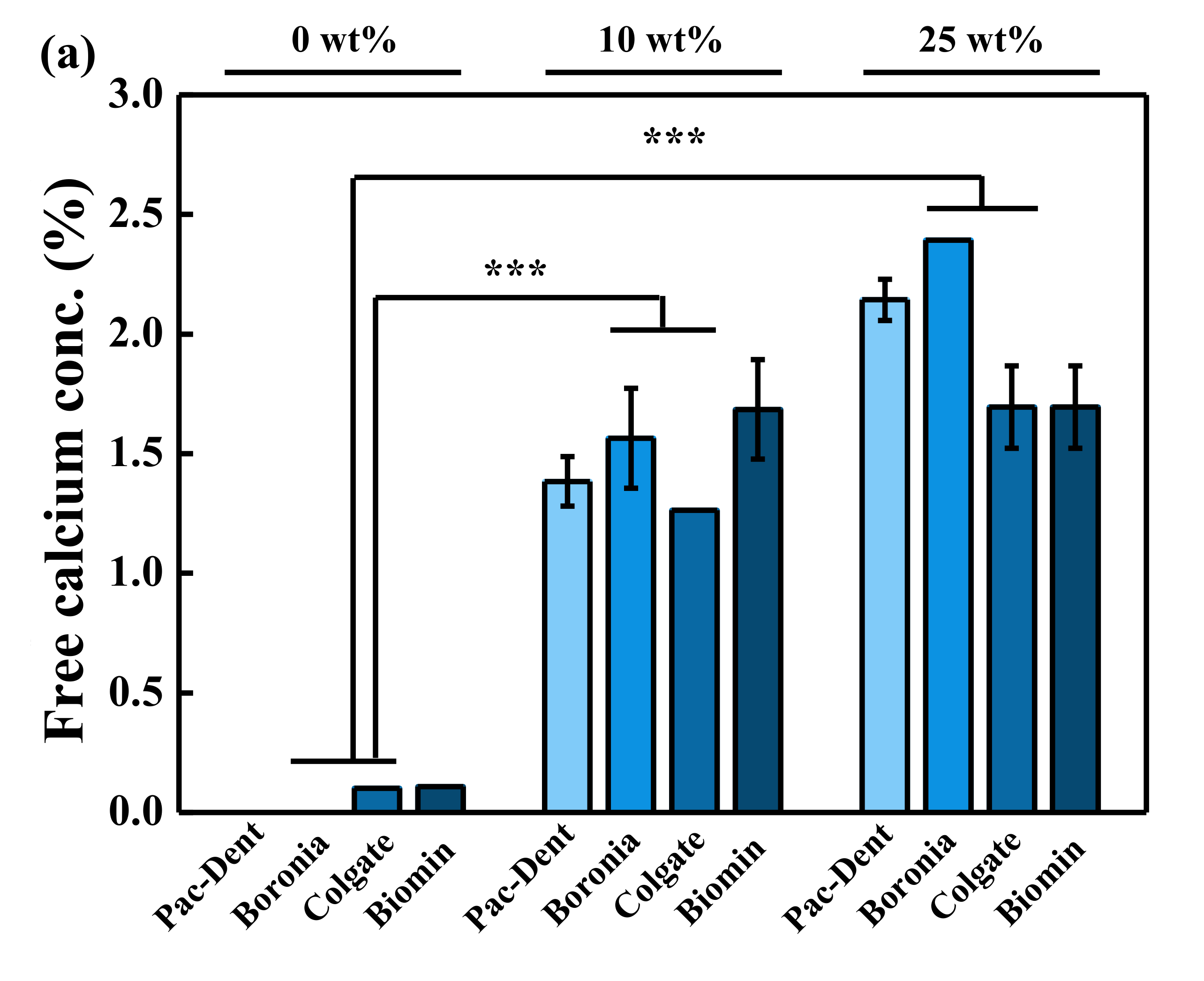
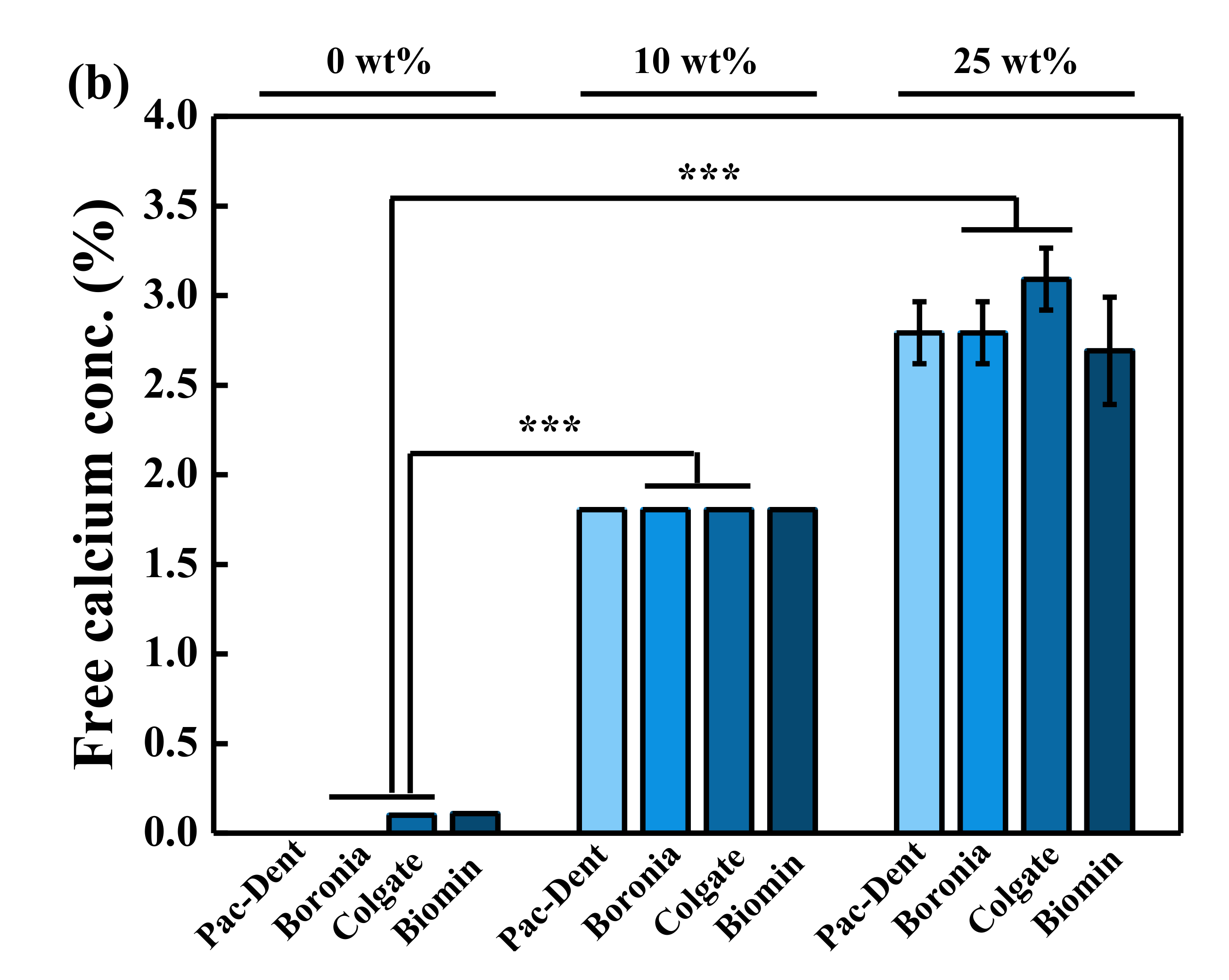
3.5. Comparison of Calcium Concentration of Normal Toothpastes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Featherstone, J.D. Dental caries: A dynamic disease process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Paes Leme, A.F.; Dalcico, R.; Tabchoury, C.P.; Del Bel Cury, A.A.; Rosalen, P.L.; Cury, J.A. In situ effect of frequent sucrose exposure on enamel demineralization and on plaque composition after APF application and F dentifrice use. J. Dent. Res. 2004, 83, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Matsunaga, K.; Kadoma, Y. Correlation in inorganic ion concentration between saliva and plaque fluid. J. Med. Dent. Sci. 2000, 47, 55–59. [Google Scholar] [PubMed]
- Meyer, F.; Amaechi, B.T.; Fabritius, H.O.; Enax, J. Overview of Calcium Phosphates used in Biomimetic Oral Care. Open Dent. J. 2018, 12, 406–423. [Google Scholar] [CrossRef] [PubMed]
- Enax, J.; Epple, M. Synthetic hydroxyapatite as a biomimetic oral care agent. Oral Health Prev. Dent. 2018, 16, 7–19. [Google Scholar] [PubMed]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. Engl. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Al-Maula, B.H. Calcium oxide nanoparticles effect on the initial caries remineralization. Aip Conf. Proc. 2020, 2213, 020120. [Google Scholar]
- Van Loveren, C. (Ed.) Toothpastes: Monographs in Oral Science; Karger: Basel, Switzerland, 2013; Volume 23. [Google Scholar]
- Amaechi, B.T.; van Loveren, C. Fluorides and non-fluoride remineralization systems. Monogr. Oral. Sci. 2013, 23, 15–26. [Google Scholar]
- Lata, S.; Varghese, N.; Varughese, J.M. Remineralization potential of fluoride and amorphous calcium phosphate-casein phospho peptide on enamel lesions: An in vitro comparative evaluation. J. Conserv. Dent. JCD 2010, 13, 42. [Google Scholar] [CrossRef]
- Walsh, L. Contemporary technologies for remineralisation therapies: A review. Int. Dent. SA 2009, 11, 6–16. [Google Scholar]
- Hemagaran, G.; Neelakantan, P. Remineralization of the tooth structure—The future of dentistry. Int. J. Pharmtech. Res. 2014, 6, 487–493. [Google Scholar]
- Li, Y.B.; Li, D.X.; Weng, W.J. Amorphous calcium phosphates and its biomedical application. J. Inorgan. Mater. 2007, 22, 775–782. [Google Scholar]
- Sun, W.; Zhang, F.; Guo, J.; Wu, J.; Wu, W. Effects of Amorphous Calcium Phosphate on Periodontal Ligament Cell Adhesion and Proliferation in vitro. J. Med. Biol. Eng. 2008, 28, 31–37. [Google Scholar]
- Zhao, J.; Liu, Y.; Sun, W.B.; Zhang, H. Amorphous calcium phosphate and its application in dentistry. Chem. Cent. J. 2011, 5, 40. [Google Scholar] [CrossRef]
- Xu, H.H.; Moreau, J.L.; Sun, L.; Chow, L.C. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent. Mater. 2011, 27, 762–769. [Google Scholar] [CrossRef]
- Shetty, N.; Kundabala, M. Biominerals in restorative dentistry. J. Interdiscip. Dent. 2013, 3, 64–70. [Google Scholar] [CrossRef]
- Melo, M.A.; Weir, M.D.; Passos, V.F.; Powers, M.; Xu, H.H. Ph-activated nano-amorphous calcium phosphate-based cement to reduce dental enamel demineralization. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1778–1785. [Google Scholar] [CrossRef]
- Babu, N.R.; Rao, K.P.; Kumar, T.S.S. Effect of coralline derived biphasic calcium phosphate shot blasting on titanium surfaces. Trans. Indian Inst. Met. 2004, 57, 85–89. [Google Scholar]
- Urist, M.R.; O’Connor, B.T. Bone Graft, Derivatives and Substitutes; Butterworth-Heinemann Ltd.: Boston, MA, USA, 1994. [Google Scholar]
- Roy, D.M.; Linnehan, S.K. Hydroxyapatite formed from coral skeletal carbonate by hydrothermal exchange. Nature 1974, 247, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef]
- Vecstaudza, J.; Locs, J. Novel preparation route of stable amorphous calcium phosphate nanoparticles with high specific surface area. J. Alloy. Compd. 2017, 700, 215–222. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Multiphasic calcium orthophosphate (CaPO4) bioceramics and their biomedical applications. Ceram. Int. 2016, 42, 6529–6554. [Google Scholar] [CrossRef]
- Zou, C.; Cheng, K.; Weng, W.; Song, C.; Du, P.; Shen, G.; Han, G. Characterization and dissolution–reprecipitation behavior of biphasic tricalcium phosphate powders. J. Alloy. Compd. 2011, 509, 6852–6858. [Google Scholar] [CrossRef]
- Leitão, T.J.; Cury, J.A.; Tenuta, L.M.A. Kinetics of calcium binding to dental biofilm bacteria. PLoS ONE 2018, 13, e0191284. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Prutskij, T.; Ippolitov, Y. Phase Transformations in a Human Tooth Tissue at the Initial Stage of Caries. PLoS ONE 2015, 10, e0124008. [Google Scholar] [CrossRef]
- Usha, C.; Sathyanarayanan, R. Dental caries—A complete changeover (Part I). J. Conserv. Dent. 2009, 12, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Lijima, M.; Hayashi, K.; Moriwaki, Y. Effects of the Ca2+ and PO43− ion flow on the lengthwise growth of octacalcium phosphate in a model system of enamel crystal formation with controlled ionic diffusion. J. Cryst. Growth 2002, 234, 539–544. [Google Scholar]
- Dorozhkin, S.V. Amorphous calcium (ortho)phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Pan, H.; Zeng, Y.; Xu, X.; Tang, R. Roles of amorphous calcium phosphate and biological additives in the assembly of hydroxyapatite nanoparticles. J. Phys. Chem. B 2007, 111, 13410–13418. [Google Scholar] [CrossRef] [PubMed]
- Posner, A.S.; Betts, F. Synthetic amorphous calcium phosphate and its relation to bone mineral structure. Acc. Chem. Res. 1975, 8, 273–281. [Google Scholar] [CrossRef]
- Jepsen, S.; Deschner, J.; Braun, A.; Schwarz, F.; Eberhard, J. Calculus removal and the prevention of its formation. Periodontol. 2000 2011, 55, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yip, H.K. Supragingival calculus: Formation and control. Crit. Rev. Oral Biol. Med. 2002, 13, 426–441. [Google Scholar] [CrossRef] [PubMed]
- Mandel, I.D. Calculus update: Prevalence, pathogenicity and prevention. J. Am. Dent. Assoc. 1995, 126, 573–580. [Google Scholar] [CrossRef]
- White, D.J. Dental calculus: Recent insights into occurrence, formation, prevention, removal and oral health effects of supragingival and subgingival deposits. Eur. J. Oral. Sci. 1997, 105, 508–522. [Google Scholar] [CrossRef] [PubMed]

| Product | Brand | Active Ingredients | Inactive Ingredients |
|---|---|---|---|
| iBrite Nano-Bio Activation Toothpaste | Pac-Dent Inc., USA | sodium fluoride, potassium nitrate | saccharin sodium, FD&C blue #1, FD&C green #3, glycerin, hydrated silica, polyethylene glycol 400, water, sodium lauryl sulfate, sodium pyrophosphate, sodium citrate, sodium phosphate, tribasic, carboxy methylcellulose sodium |
| Advanced Whitening Toothpaste | Colgate-Palmolive Company, USA | sodium fluoride, triclosan | water, hydrated silica, glycerin, sorbitol, butyl ester of methyl vinyl ether/maleic anhydride copolymer, sodium lauryl sulfate, sodium hydroxide, propylene glycol, saccharin sodium, carboxy methylcellulose sodium, titanium dioxide, carrageenan |
| Herbal Whitening Toothpaste | Boronia, Taiwan | sodium mono fluoro phosphate | water, butylene glycol, panax ginseng root extract, vinegar, pruns mume fruit extract, allium sativum (garlic) bulb extract, platycondon grandiflorum root extract, codonopsis lanceolata root extract, rubus fruticcsus (blackberry) fruit extract, acanthopanax senticosus (eleuthero) root extract, glycine soja (soybean) seed extract, oryza sativa (rice) extract, sesamum indicum (sesame) seed extract |
| BioMin F Toothpaste | BioMin Technologies Limited, UK | fluoro calcium phospho silicate | glycerin, silica, polyethylene glycol 400, sodium lauryl sulphate, titanium dioxide, aroma, carbomer, potassium acesulfame. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, P.-J.; Lee, C.-Y.; Ou, K.-L.; Lan, W.-C.; Chuo, Y.-C.; Lin, H.-Y.; Chao, H.-W.; Huang, B.-H.; Saito, T.; Tsai, H.-Y.; et al. Calcium Release from Different Toothpastes after the Incorporation of Tricalcium Phosphate and Amorphous Calcium Phosphate. Appl. Sci. 2021, 11, 1848. https://doi.org/10.3390/app11041848
Hou P-J, Lee C-Y, Ou K-L, Lan W-C, Chuo Y-C, Lin H-Y, Chao H-W, Huang B-H, Saito T, Tsai H-Y, et al. Calcium Release from Different Toothpastes after the Incorporation of Tricalcium Phosphate and Amorphous Calcium Phosphate. Applied Sciences. 2021; 11(4):1848. https://doi.org/10.3390/app11041848
Chicago/Turabian StyleHou, Ping-Jen, Chang-Yu Lee, Keng-Liang Ou, Wen-Chien Lan, Yen-Chun Chuo, Hung-Yang Lin, Hsiao-Wei Chao, Bai-Hung Huang, Takashi Saito, Hsin-Yu Tsai, and et al. 2021. "Calcium Release from Different Toothpastes after the Incorporation of Tricalcium Phosphate and Amorphous Calcium Phosphate" Applied Sciences 11, no. 4: 1848. https://doi.org/10.3390/app11041848
APA StyleHou, P.-J., Lee, C.-Y., Ou, K.-L., Lan, W.-C., Chuo, Y.-C., Lin, H.-Y., Chao, H.-W., Huang, B.-H., Saito, T., Tsai, H.-Y., Yang, T.-S., Walinski, C. J., & Ruslin, M. (2021). Calcium Release from Different Toothpastes after the Incorporation of Tricalcium Phosphate and Amorphous Calcium Phosphate. Applied Sciences, 11(4), 1848. https://doi.org/10.3390/app11041848






