Abstract
Unpaved roads could be a significant source of dust emission. A common and effective practice to suppress this emission is the application of brine solution on these roads. However, this application could increase the risk of water source salinization in arid and semiarid regions, such as Israel. The general objective of the present study was to investigate the potential effects of treated wastewater (TWW), fresh water (FW), and brine applications as anti-dust emission solutions on water source salinization in these regions. A rainfall simulator experiment and a mass balance model were used for this goal. The TWW loaded the highest amounts of Cl, Na, and Ca+Mg on the unpaved roads, while the brine loaded higher amounts of Cl and Ca+Mg than the FW, and ~0 Na. In the rainfall experiment, runoff was not formed, and ~100% of the loaded amounts were leached downwards by rain, indicating a negligible salinization risk to surface water. We estimated that the average increases in the Cl concentrations in the modeled aquifer, following TWW, brine, and FW applications, were low: 1.2–1.6, 0.58–0.8, and 0.32–0.4 mg L−1, respectively. Thus, the solution selection for preventing dust emission should be based on the total cost of the solution application.
1. Introduction
Dust emission to the atmosphere, caused by wind erosion, could have significant effects on the environment and human health [1,2,3]. This depends on the characteristics of the winds and the eroded surface area [4,5]. Anthropogenic activities, such as mining, quarrying, excavating, and the use of unpaved roads, increase the terrain’s sensitivity to dust emission. For example, heavy vehicles traveling on unpaved roads could grind the roads’ surfaces to fine particles that become available to wind erosion [6,7,8].
Environmental regulators, such as the U.S. Environmental Protection Agency (EPA), recommend and enforce the use of dust control techniques. A common practice to prevent dust emission from unpaved roads is the application of anti-dust emission solutions. The two main types of such solutions are as follows: (i) Natural or synthetic polymers, e.g., lignin, resin, bitumen, and polyvinyl acetate (PVA) [9]. These materials contain long organic chains with functional groups that could adsorb and adhere to dust particles, forming bigger and heavier aggregates [10]. (ii) Brines with high concentrations of hydrous calcium–magnesium chloride solution, which decreases the dust emission by a hygroscopic mechanism [11]. For both cases, the increased weights of these aggregates limit their lifting by the erosive forces of the wind, leading to a reduction in dust emission.
Katra [12] tested the effectiveness of the lignin, resin, bitumen, PVA, and brine application in preventing dust emission by wind from unpaved roads. These tests were conducted under controlled laboratory and field conditions, using wind tunnels with varied wind velocities and time durations. It was found in this experiment that among the tested anti-dust emission solutions, brine from the Dead Sea was the most effective solution in preventing the dust emission; the brine application decreased the dust emission by >90% of the dust emissions under the control treatment [12].
Arid and semiarid regions are characterized by a long dry season and a short wet season with limited precipitation. Thus, natural water resources, such as groundwater and non-salty surface water (fresh water, FW) in these regions, are scarce [13]. Moreover, the climate conditions and the high population growth in these regions lead to an escalation of irrigation with low quality water, such as treated wastewater (TWW) and saline water [13], which increases the salinization of the water sources in the region [13].
Most of the haul roads in mines and quarries are unpaved roads covered with a layer (usually 20–30 cm thick) of calcareous granules (granular material) that sensitive to dust emission. A common practice to prevent the dust emission from these unpaved roads in arid and semiarid regions, such as Israel, is the application of hyper-saline solution (brine) on the unpaved roads. In recent years, concerns have been raised regarding the application of the brine solution on unpaved roads for controlling dust emission. It was claimed that the application of the brine solution with very high salinity will further increase the salinization of the water resources. Indeed, some quarries use FW instead of brine solution in their anti-dust practices. However, since FW is scarce in arid and semiarid regions, TWW is also used for the purpose of anti-dust emission on unpaved roads.
The TWW is characterized by moderate salt concentration, particularly Cl and Na ions; high concentrations of toxic, inorganic macro- and micro-elements, such as NO3, boron and heavy metals; and high contents of suspended and soluble organic matters, including organic pollutants and pathogens [13]. The brine solution, however, is characterized by very high salt concentration, particularly of Cl, Ca, and Mg ions, and a high concentration of toxic heavy metals, such as Ni, Co, Cd, and Pb. Therefore, during the rainfall season, high concentrations of salts and toxic elements and compounds could be leached from the unpaved roads treated with TWW or brine solutions and salt, and contaminate the water sources.
The present study is focused on the salinization of water sources following the application of anti-dust emission solutions on unpaved roads. The trend of using FW or TWW as anti-dust emission solutions instead of brine solution in arid and semiarid regions is based on the assumption that a decrease in the salinity of the anti-dust emission solution will necessarily decrease the salinization of the water sources in the region. However, the salinization of the water sources, under the application of anti-dust emission solutions, is dependent on varied factors other than the salinity of the anti-dust emission solutions. Therefore, the general objective of the present study was to investigate the effects of the application of TWW, FW, and brine solutions on unpaved roads as anti-dust emission solutions on the salinity of the water resources in arid and semiarid regions, such as Israel. The specific objectives were to determine or estimate the effects of the application of TWW, FW and brine solutions on the following: (i) The accumulated amounts of the solutes on the unpaved roads. (ii) The leaching rates of the ions, Cl, Na, and Ca+Mg, from the treated granular material by consecutive rainstorms. (iii) The distribution movement of the rainwater and solutes during the rainstorm via lateral movement on the terrine surface as surface runoff, or vertical movement with the infiltrating water; (iv) the long-term average of the salinization rates of the water sources.
2. Materials and Methods
The study comprises two main parts: (i) a rainfall simulator experiment to study the leaching rate of the ions, Cl, Na, and Ca+Mg, from the treated granular material during consecutive rainstorms, and (ii) an estimation of the water sources’ salinization following the application of the anti-dust emission solutions, brine, TWW, and FW, on the unpaved roads.
2.1. Rainfall Simulator Study
Chemical analysis of the studied materials: Granules of calcareous material (granular material), containing mainly particles with 5–10 mm sizes, were obtained from Modiim Quarry Ltd, Israel. These calcareous granules are used as a cover layer on the unpaved roads. The brine solution and secondary TWW were obtained from the Dead Sea Works and the Taoz treated wastewater plant near the city of Beit-Shemes in Israel, respectively. These solutions were used in the rainfall simulator experiment. A sample of the brine solution was obtained a couple of days before the beginning of the experiment, and the TWW samples were taken <48 h before their uses in the experiment. Filtrated samples of <0.45 µm of the brine and TWW solutions were analyzed using the following standard methods: (i) Electrical conductivity (EC) and pH values by standard EC and pH meters, respectively. (ii) Cl concentration by Digital Chloridometer, 442500, Labconco, Kansas City, MO. (iii) Concentrations of Na by flame photometer model 420 Clinical Flame Photometer, Sherwood Scientific Ltd, Cambridge, UK. (iv) Concentration of various elements by Dual-View High-Resolution ICP-OES Plasma Quant 9000 Elite, Analytik Jena, Germany. The chemical properties of the brine and TWW solutions that were used in the rainfall simulator study are presented in Table 1.

Table 1.
Electrical conductivity (EC), pH values, and the concentrations of various ions in the studied treatment solutions: brine from the Dead Sea and treated wastewater (TWW) from the Taoz wastewater treatment plant.
The treatments: The granular material was packed and leveled in metal trays with a perforated bottom and dimensions of 0.3 × 0.5 m2 area and 0.02 m depth for each tray. The trays with the granular material were treated by the following treatments, with 4 replicates (4 trays) for each treatment:
- Control: four trays with untreated granular material were subjected to 3 consecutive rainstorms by a rainfall simulator, 1 storm a day, as described below. After each rainstorm, the trays were left for 3 days in a net house for drying to air-dry.
- TWW treatment: four trays with the granular material were sprayed 10 times with 4 mm of TWW in each time. After each spray, the trays were left for 3 days in a net house for drying to air-dry. Three days after the last TWW application, the trays were subjected to 3 consecutive rainstorms, with no further TWW addition between the rainstorms, as described above for the control treatment.
- Brine treatment: four trays with the granular material were sprayed 1 time with 4 mm of brine solution, and then were left for 3 days at a net house for drying to air-dry. After the drying period, the trays were subjected to 6 consecutive rainstorms, with 3 days of drying between the rainstorms, as described above for the control treatment, and with no further brine addition between the consecutive rainstorms.
Simulated rainstorms runs: A laboratory rotary disc-type rainfall simulator [14] was used to determine the leakage of the ions, Cl, Na, and Ca+Mg (the studied ions), from the pretreated granular materials during consecutive rainstorms. Before each rainstorm, 4 trays with the pretreated granular materials were placed in a carousel below the rainfall rotary disc. Each tray was placed at a 9% gradient on an 8 cm layer of coarse gravel in a box. The trays were exposed to a rainstorm of 50 mm of deionized water, with the following mechanical parameters: rain intensity = 48 mm h−1, raindrop mean diameter = 1.9 mm, drop velocity = 6.02 m s−1, and kinetic energy of 18.1 J mm−1 m−2. During the rainstorm, the water volumes percolating through the granular material in each tray (outflow leachate) were recorded at different times, and the infiltration rates were calculated with respect to the cumulative rainfall. In addition, the outflow leachates from each tray were collected in 5 separated fractions (every 10 mm of rainfall) along each rainstorm, and their volumes were measured. Subsamples were taken from each leached fraction, filtered through a <0.45 µm filter, and the EC values and concentrations of Cl, Na, Ca, and Mg were measured as described above. Surface runoff was not formed during the rainstorms, and therefore was not measured.
2.2. Estimation of the Water Sources Salinization
The effects of the anti-dust emission solutions (brine, TWW, and FW) on water sources’ salinization were estimated based on the following assumptions and measurements:
- A mass balance model, under long-term and steady state conditions, was used to estimate the effects of the application of the treatments on the groundwater salinization.
- Because many quarries in Israel are located above the western mountain aquifer (Yarqon-Taninim aquifer), the mass balance model was based on the characteristics of this aquifer and on the activities and the environmental conditions at its basin. The dominant rocks in the Yarqon-Taninim aquifer are cracked limestone with heterogeneous paths for water and solutes movement. The recharge of the aquifer occurs through annual average rainfall ranging from 200 to 600 mm. This rainfall falls in the short winter (4 months), while the rest of the months are completely dry.
- The leaching rates of the studied ions from the treated granular materials during consecutive rainstorms were determined by the rainfall simulator experiment.
- The chemical compositions of the TWW, brine, and FW solutions, which were used in the rainfall simulator experiment and in the model, are presented in Table 1 and Table 2.
 Table 2. Typical electrical conductivity (EC), pH values, and concentrations of various ions in the treated wastewater (TWW) and fresh water (FW) that were used in the region of the Yarkon-Taninim aquifer [15].
Table 2. Typical electrical conductivity (EC), pH values, and concentrations of various ions in the treated wastewater (TWW) and fresh water (FW) that were used in the region of the Yarkon-Taninim aquifer [15].
3. Results and Discussion
The evaluation of the salinization risks of the water sources following the application of the anti-dust emission solutions (brine, TWW, and FW) was based on two main processes: (i) The annual loaded amounts of the solutes and their accumulations on the unpaved roads following the applications of the treatment solutions in the dry season (summer). (ii) Leaching rates by rainfall of the studied ions (Cl, Na, and Ca+Mg) from the granular material, which was pretreated with the treatment solutions.
The leaching rates of the Cl, Na, and Ca+Mg ions from the pretreated granular materials were determined using a rainfall simulator. Runoff was not formed in the different treated granular materials during the consecutive rainstorms. The packaging of the granular materials in the surface of the unpaved roads and in the rainfall simulator trays was similar in general. Thus, the infiltration rates of the granular materials are >48 mm h−1 (the rainstorms’ intensity), and most of the movement of the solutes with the rainwater is vertical. Therefore, the salinization risk to the surface water sources near the treated unpaved roads by the treatment solutions is likely negligible, unless the infiltration rate of the surface area of the unpaved roads below the granular material layer is lower than the rainfall intensity. Otherwise, on the sublayer, the lateral movement of water with soluble ions could occur. However, this movement is very slow, and usually infiltrates through the cracks into the rocks, so would not be a predominant flow route.
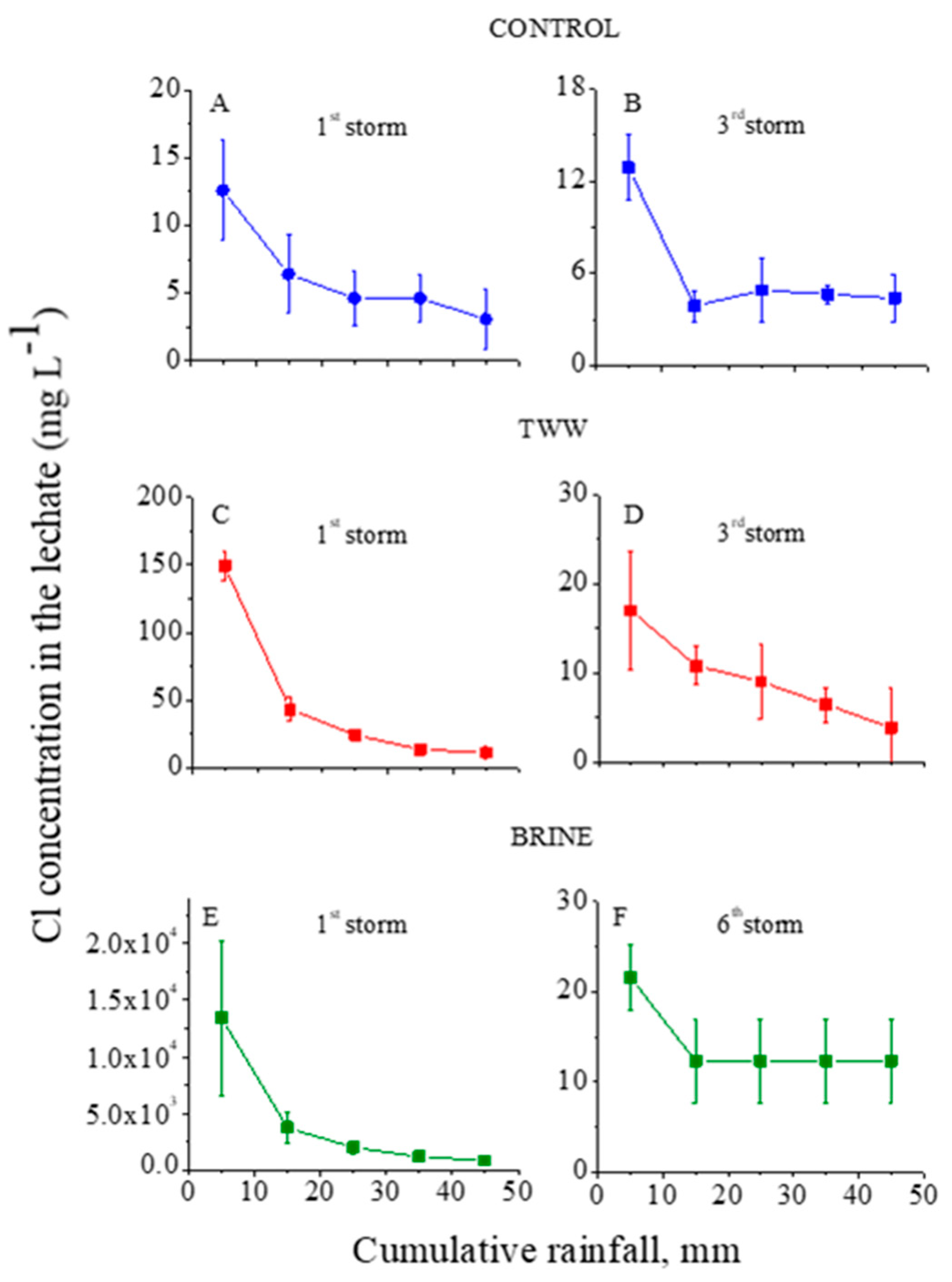
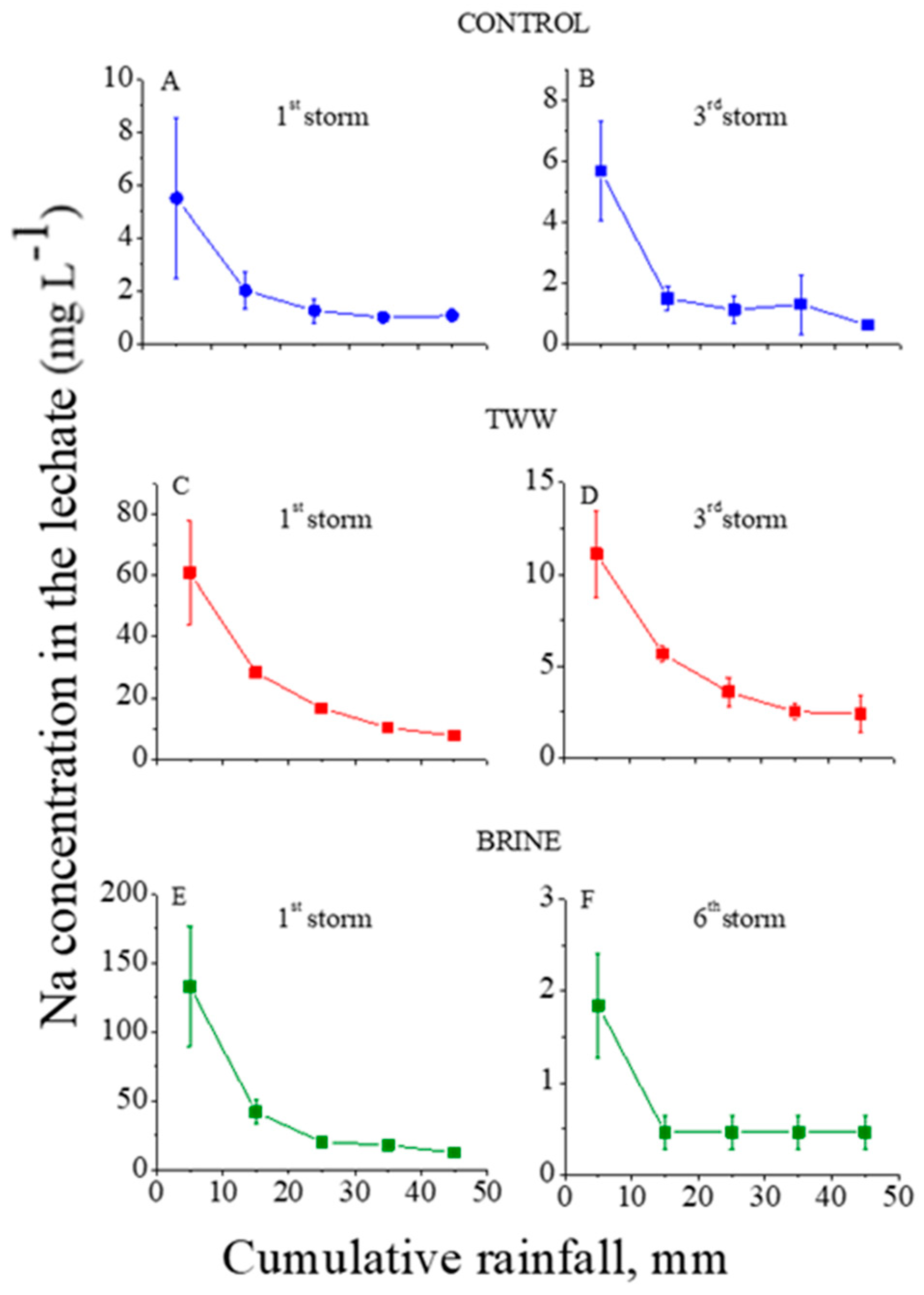
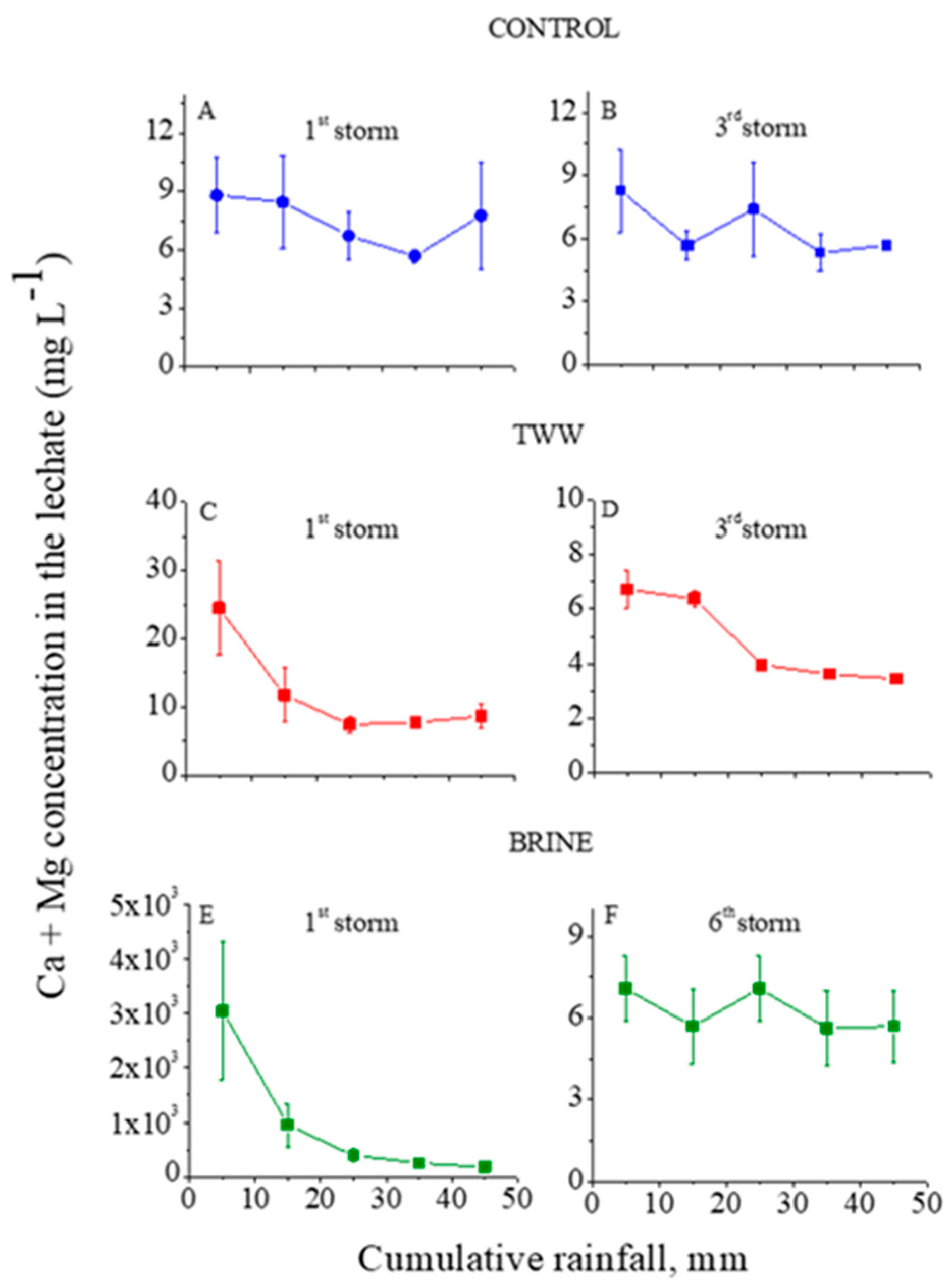
The ion concentrations in the outflow (leachate) from the trays with the treated granular material as functions of the cumulative rainfall are presented in Figure 1, Figure 2 and Figure 3 for Cl, Na, and Ca+Mg, respectively. In these figures, the first and third (last) consecutive rainstorms for the control and TWW treatments, and the first and sixth (last) for the brine treatment are presented. Because the concentrations of each ion in the leachate in the second and the last consecutive rainstorms were fairly similar, only the first and the last consecutive rainstorm are presented in the figures. In addition, in the control treatment, the concentrations of the analyzed ions in the leachate reached steady state values quite fast, and no later than the end of the third consecutive rainstorm.
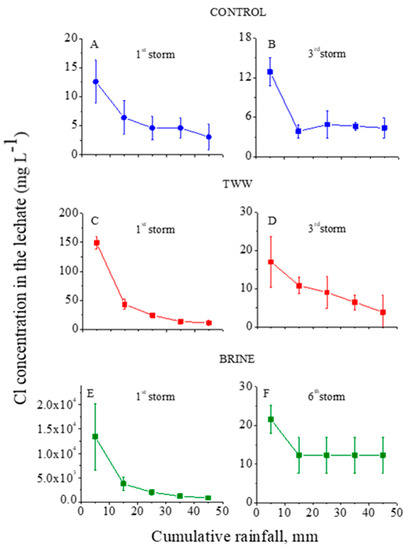
Figure 1.
Cl− concentrations in the outflow leachate from the trays with treated granular materials as functions of the cumulative rainfall of the 1st (A) and 3rd (B) consecutive rainstorms for the control, of the 1st (C) and 3rd (D) consecutive rainstorms for the treated wastewater (TWW) treatment, and of the 1st (E) and 6th (F) consecutive rainstorms for the brine treatment. Vertical lines near the symbols are standard deviations (the Y-axes’ scales are different).
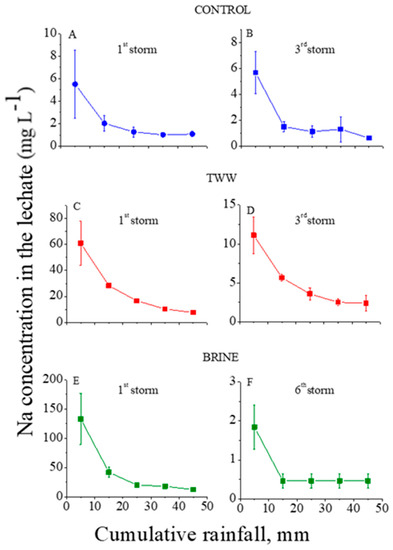
Figure 2.
Na+ concentrations in the outflow leachate from the trays with treated granular materials as functions of the cumulative rainfall of the 1st (A) and 3rd (B) consecutive rainstorms for the control, of the 1st (C) and 3rd (D) consecutive rainstorms for the treated wastewater (TWW) treatments, and of the 1st (E) and 6th (F) consecutive rainstorms for the brine treatment. Vertical lines near the symbols are standard deviations (the Y-axes’ scales are different).
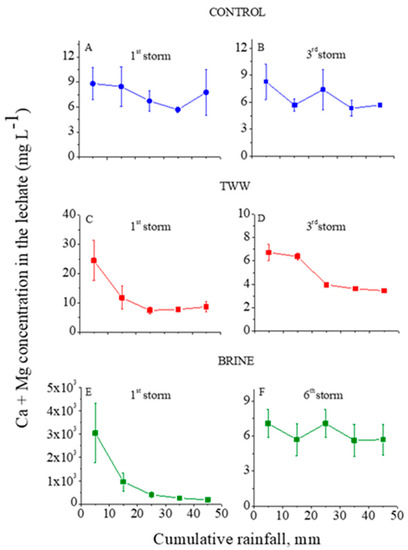
Figure 3.
Ca+Mg concentrations in the outflow leachate from the trays with treated granular materials as functions of the cumulative rainfall of the 1st (A) and 3rd (B) consecutive rainstorms for the control, of the 1st (C) and 3rd (D) consecutive rainstorms for the treated wastewater (TWW) treatment, and of the 1st (E) and 6th (F) consecutive rainstorms for the brine treatment. Vertical lines near the symbols are standard deviations (the Y-axes’ scales are different).
In the control treatment, the concentrations of Cl and Na in the leachate at the first and third consecutive rainstorms were relatively low: <13 and <6 mg L−1, respectively (Figure 1A and Figure 2A). These low concentrations suggest that the amounts of highly soluble minerals with Na and Cl in the granular material were low. In contrast, the dissolution of the lime in the calcareous granular material in the control treatment kept the concentrations of the Ca+Mg in the leachate relatively high and quite constant, with an average value of ~7.5 mg L−1 along the consecutive rainstorms (Figure 3A,B). For the TWW and brine treatments, in the beginning of the first rainstorms (Figure 1, Figure 2 and Figure 3) the concentrations of the ions (Cl, Na, and Ca+Mg) in the leachate were higher than in the control treatment. These high concentrations were a result of the previous applications of the TWW and brine solutions on the granular materials. However, these high concentrations of Cl, Na, and Ca+Mg decreased sharply with the cumulative rainfall, and near the end of the last rainstorm, the concentrations of these ions were similar to their corresponding concentrations in the control treatment (Figure 1, Figure 2 and Figure 3).
According to the rainfall simulator results (Figure 1, Figure 2 and Figure 3), for each studied ion and treatment solution the total leaching amounts (£j) of ion j (Cl, Na, or Ca+Mg) from the treated granular material during the consecutive rainstorms, as a percentage of the total added amount of ion j by the treatment solution, were calculated by Equation (1):
where k is the consecutive serial number of the consecutive rainstorm; Vdi and Cdi are the leachate volumes (L m−2) and concentrations (gr L−1) of ion j, respectively, for brine or TWW applications; and the Vci and Cci are the leachate volumes (L m−2) and concentrations (gr L−1) of ion j, respectively, for the control treatment, when I is the consecutive serial number of the rainfall fractions in each consecutive rainstorm. Sj is the amount of ion j (mg m−2) added to the granular material with each application event of the brine or TWW, when L is the total number of the application events. For determining the £j values for brine treatment by Equation (1), the values of Vci and Cci of the fourth, fifth and sixth consecutive rainstorms in the control treatment were, most likely, equal, corresponding with the values of the third rainstorm in the control treatment (Figure 1A, Figure 2A, and Figure 3A). This is because the values of Vci and Cci at the third rainstorm in the control treatment reached steady state values (Figure 1A, Figure 2A, and Figure 3A).
The £j values for Cl, Na, and Ca+Mg ions indicated that >99.5% of the amounts of these ions added onto the granular material by the WWT or brine solutions were leached down during the rainstorms. These results strengthen the previous observation that the calcareous granular material has a negligible capacity to adsorb the studied ions, and to prevent their vertical movement.
The total loaded amounts of the solutes on the unpaved roads by the treatment solutions could be leached down by the rainstorm and increase the salinity of the groundwater. Therefore, the load of each studied ion by each solution treatment should be determined. The total annual load amount (Pj) (kg m−2) of ion j, where j could be Cl, Na, or Ca+Mg, on 1 m2 of the surface area of the unpaved road by the application of brine, TWW, or FW, was determined by Equation (2):
where Cji is the concentration (mg L−1) of the applied ion j (Cl, Na, or Ca+Mg), and Vji is the volume (L m−2) of the brine, TWW, or FW solution containing the ion j, which is applied in the application event i on a specific application day. β is the number of application days in a year. According to the Israeli regulation, for the three treatment solutions (brine, TWW, and FW), the Vji equals 4 L m−2; for the FW and TWW treatment solutions, the N equals 10 and β equals 192; and for the brine solution the N equals 1 and β equals 1.
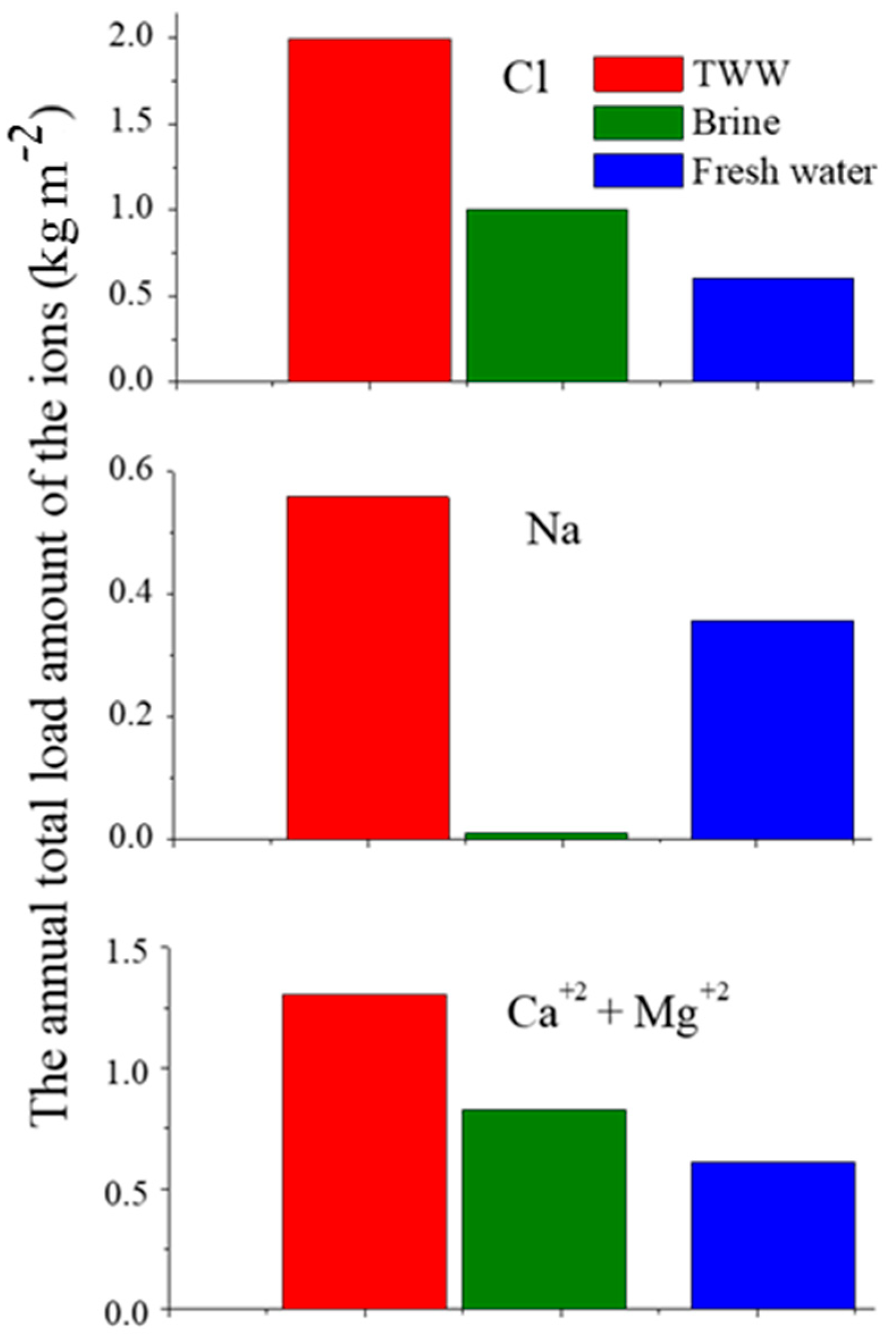
The Pj values of Cl, Na, and Ca+Mg on the unpaved roads by the TWW, brine, and FW applications are presented in Figure 4. It can be seen from this figure that (i) the TWW application loaded the highest annual amounts of Cl, Na, and Ca+Mg on the unpaved roads, and (ii) the brine application loaded higher annual amounts of Cl and Ca+Mg than the FW application, and the lowest annual amount of Na (~0). In addition, different mechanisms were responsible for the dust emission control in the various studied solutions (brine, TWW, and FW). The mechanism of the brine solution controlling dust emission is based on the high hygroscopic properties of the Ca and Mg elements. The concentrations of the Ca and Mg ions in the brine solution are very high, 23.2 and 62.2 g L−1, respectively (Table 1). The application of the brine solution causes the accumulation of high contents of Ca and Mg on the unpaved road, which absorb large amounts of H2O molecules from the air. These H2O molecules are adsorbed on the dust particles by adhesion forces, forming big and heavy aggregates which are beyond the lifting capability of the wind. Consequently, the dust emission under the brine application decreases.
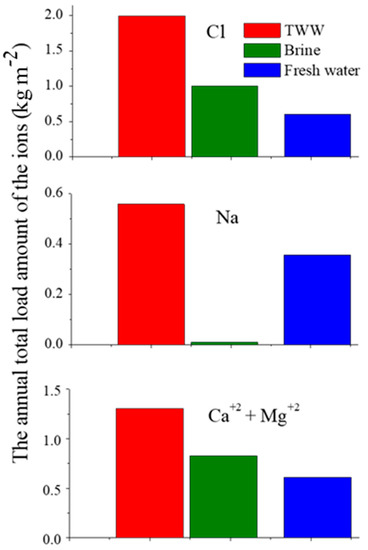
Figure 4.
The annual loaded amounts (Pj) of Cl (upper part), Na (middle part), or Ca+Mg (bottom part), on the unpaved roads following the treated wastewater (TWW), brine and fresh water (FW) applications (the Y-axes’ scales are different).
In contrast, the application of the TWW and FW solutions with relatively low concentrations of Ca and Mg, <127.5 and <59.8 mg L−1, respectively (Table 2), leads to the lower accumulation of these ions on the unpaved roads. Furthermore, the frequent applications of the TWW and FW on the unpaved roads, 10 times in each day during the dry season, leach down the Ca and Mg ions from the unpaved roads’ surfaces, which in turn decreases their accumulations on the unpaved roads. In this case, the hygroscopic mechanism is negligible. Therefore, the mechanism of the WWT and FW application in controlling dust emission is based on the “active wetting” mechanism, i.e., repetitive and frequent wetting of the unpaved roads throughout the day to keep the roads wet during the quarries’ activities.
The effects of the vertical leaching of the solutes, which accumulated on the unpaved roads following the application of the treatment solutions, on the groundwater salinization could be indicated by the Cl− concentration increase in the groundwater. The Cl− is a conservative ion, very mobile in soil/rock systems, and is the most dominant anion in groundwater. Therefore, the concentration of Cl− is used as a parameter of water salinity; the higher the Cl− concentration, the higher the water salinity is [16].
Many quarries in Israel are located above the Yarkon-Taninim aquifer, where the dominant rocks in this aquifer are cracked limestone. The paths for water and solutes movement in this aquifer are heterogeneous, complicated, and difficult to describe and predict. Nevertheless, the comparative effects of the treatment solutions (brine, TWW, and FW) on groundwater salinity could be fairly estimated on the basis of the following assumptions and simplifications: (1) Most of the added solutes on the unpaved roads during the dry season reach the groundwater. (2) Under long-term and steady state conditions, the average amounts of the Cl− that were added on the unpaved roads or pumped with the water from the aquifer are equal. (3) The annual average volume of the water that is pumped from the aquifer and that which recharges the aquifer are equal.
According to the above conditions, a mass balance model (Equation (3)) was used to predict the effects of brine, TWW, or FW applications on the long-term annual average salinity increase (ΔCd) in the pumped water from the Yarkon-Taninim aquifer.
where A is the long-term average area (m2) of all the unpaved roads located above the Yarkon-Taninim aquifer and treated with the treatment solutions (brine, TWW, or FW). The Pcl is the long-term average amount (mg m−2/yr) of the Cl− that was added on the A area, and is calculated by Equation (2). WR is the long-term average of the water volume (L/yr) that was pumped from the Yarkon-Taninim aquifer.
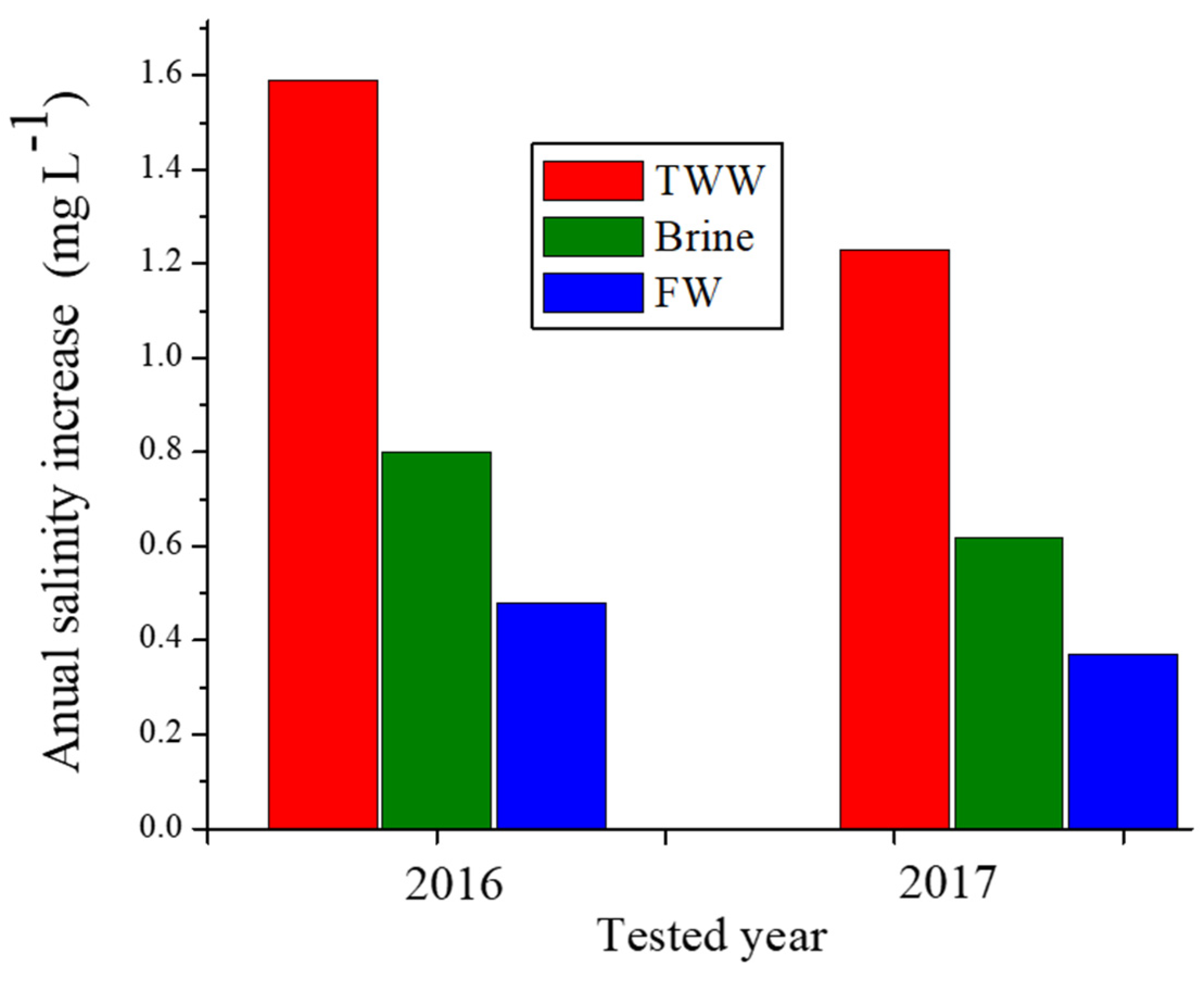
The ΔCcl values of the pumped water from the Yarkon-Taninim aquifer, which were calculated by Equation (3), are presented in Figure 5 for the various treatment solutions (brine, TWW, FW), and two different A values. For these calculations, a WR value of 400 × 109 L per a year was used (a long-term annual average of the pumped water from this aquifer), as were A values in the years 2016 and 2017, which were used as two examples. The long-term annual average values of the ΔCcl in the pumped water from the Yarkan-Taninim aquifer following the TWW, brine, and FW applications were 1.6, 0.8, and 0.4 mg/L, respectively, in the first example (2016), and 1.2, 0.58, and 0.32 mg/L, respectively, in the second example (2017) (Figure 5). These ΔCcl values in the first and second examples under the application of TWW were approximately two and four times higher than under brine and FW applications, respectively. In spite of these large differences in the ΔCcl values between the various treatments (Figure 5), the long-term annual average additions of Cl− concentration in the groundwater following the three treatment solutions were relatively low and ranged between 0.32 to 1.6 mg L−1 in the two examples (Figure 5).
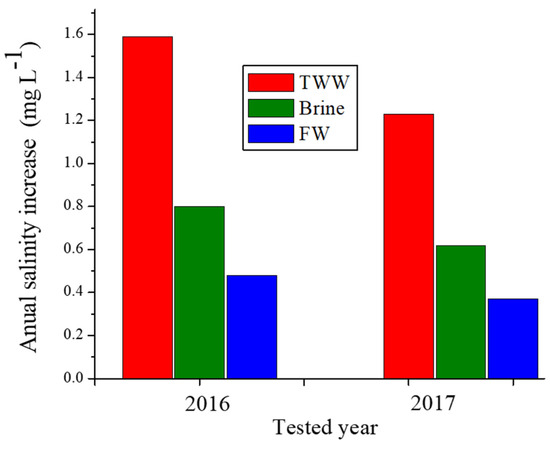
Figure 5.
The long-term average of the salinity increase (ΔCcl) of the pumped water from the Yarkon-Taninim aquifer following the treated wastewater (TWW), brine, or fresh water (FW) applications on the total area of the unpaved roads located above the aquifer in the years 2016 and 2017 as examples.
4. Conclusions
The estimation of the long-term average increases in Cl− concentrations in the pumped water from the groundwater, following the TWW, brine, or FW applications, expressed as percentages of the Cl concentration in the groundwater without the application of anti-dust emission, ranged between 0.5 and 2.2%. In such Cl concentration increases, using brine solutions with high concentrations of Ca and Mg ions could provide an environmentally sound solution for application on unpaved roads for dust control, and no regulatory restrictions are required on this use. Therefore, the selection of the solution to prevent dust emission should be basically based on the cost, including labor, transport and the solutions themselves.
Author Contributions
M.B.-H, original draft preparation; M.B.-H. and I.K., conceptualization, writing—review and editing; M.D. and U.N., conceptualization, review and editing; R.C., conceptualization, methodology, analyses. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by a grant from the Ministry of Energy, Israel (No. 215-17-014).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nenes, A.; Murray, B.; Bougiatioti, A. Mineral Dust and its Microphysical Interactions with Clouds. In Mineral Dust: A Key Player in the Earth System; Knippertz, P., Stuut, J.B., Eds.; Springer: New York, NY, USA, 2014; pp. 287–325. [Google Scholar] [CrossRef]
- Kok, J.F.; Ridley, D.A.; Zhou, Q.; Miller, R.L.; Zhao, C.; Heald, D.A.R.C.L.; Ward, D.S.; Albani, S.; Haustein, K. Smaller desert dust cooling effect estimated from analysis of dust size and abundance. Nat. Geosci. 2017, 10, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Jickells, T.D.; Anderson, K.; Andersen, K.K.; Baker, A.R.; Bergametti, G.; Brooks, N.; Cao, J.J.; Boyd, P.W.; Duce, R.A.; Hunter, K.A.; et al. Global Iron Connections Between Desert Dust, Ocean Biogeochemistry, and Climate. Science 2005, 308, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Kok, J.F.; Parteli, E.J.; Michaels, T.I.; Karam, D.B. The physics of wind-blown sand and dust. Rep. Prog. Phys. 2012, 75, 106901. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Raupach, M.R.; Findlater, P.A. Effect of saltation bombardment on the entrainment of dust by wind. J. Geophys. Res. Space Phys. 1993, 98, 12719–12726. [Google Scholar] [CrossRef]
- Gillies, J.; Etyemezian, V.; Kuhns, H.; Nikolic, D.; Gillette, D. Effect of vehicle characteristics on unpaved road dust emissions. Atmos. Environ. 2005, 39, 2341–2347. [Google Scholar] [CrossRef]
- Goossens, D.; Buck, B. Dust emission by off-road driving: Experiments on 17 arid soil types, Nevada, USA. Geomorphology 2009, 107, 118–138. [Google Scholar] [CrossRef]
- Yulevitch, G.; Danon, M.; Krasovitov, B.; Fominykh, A.; Swet, N.; Tsesarsky, M.; Katra, I. Evaluation of wind-induced dust-PM emission from unpaved roads varying in silt content by experimental results. Atmos. Pollut. Res. 2020, 11, 261–268. [Google Scholar] [CrossRef]
- Castellanos, A. The relationship between attractive interparticle forces and bulk behavior in dry and uncharged fine powders. Adv. Phys. 2005, 54, 263–376. [Google Scholar] [CrossRef]
- Ben-Hur, M. Using synthetic polymers as soil conditioners to control runoff and soil loss in arid and semiarid regions: A review. Aust. J. Soil Res. 2006, 44, 191–204. [Google Scholar] [CrossRef]
- Bustos, M.; Cordo, O.; Girardi, P.; Pereyra, M. Evaluation of the Use of Magnesium Chloride for Surface Stabilization and Dust Control on Unpaved Roads. Transp. Res. Rec. 2015, 2473, 13–22. [Google Scholar] [CrossRef]
- Katra, I. Comparison of various dust control products in wind-induced dust emission from unpaved roads. Appl. Sci. 2019, 9, 5204. [Google Scholar] [CrossRef]
- Ben-Hur, M. Sewage water treatments and reuse in Israel. In Water in the Middle East and in North Africa: Resources, Protection, and Management; Zereini, F., Jaeschke, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 167–180. [Google Scholar]
- Morin, J.; Goldberg, D.; Seginer, A.I. A Rainfall Simulator with a Rotating Disk. Trans. ASAE 1967, 10, 0074–0077. [Google Scholar] [CrossRef]
- Tarchitki, J.; Inbar, Y.; Shmoali, L. Environment Impact of Application of Plant Nutrient and Organic Wastes on Arable Land in Israel; Final Report; The Ministry of Environment Production: Israel, 2016; submitted. (In Hebrew) [Google Scholar]
- Edelstein, M.; Plaut, Z.; Ben-Hur, M. Water salinity and sodicity effects on soil structure and hydraulic properties—A review. Adv. Hort. Sci. 2010, 24, 154–160. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).