Deciphering the Antitussive, Expectorant, and Anti-Inflammatory Potentials of ShashamKyeongok-Go and Their Phytochemical Attributes: In Vivo Appraisal in ICR Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Analysis of Specific Ingredients of AR, KOG, and SKOG
2.2.1. Instrument and Reagents
2.2.2. Preparation of Standard Solutions
2.2.3. Preparation of Test Samples for UPLC Analysis
2.2.4. Phytochemical Quantification by UPLC
2.3. Antitussive Assay
2.3.1. Rodents and Husbandry
2.3.2. Treatment and Grouping
2.3.3. Body Weight Measurement
2.3.4. Coughing Induction and Monitoring
2.3.5. Histopathology
2.4. Expectorant Assay
2.4.1. Rodents and Husbandry
2.4.2. Treatment and Grouping
2.4.3. BW Measurement
2.4.4. Measurement of Mucous Secretion
2.4.5. Histopathology
2.5. Anti-Inflammatory Assay
2.5.1. Rodents and Husbandry
2.5.2. Treatment and Grouping
2.5.3. BW Measurement
2.5.4. Ear Weight Measurement
2.5.5. Histopathology
2.6. Statistical Analyses
3. Results
3.1. Contents of Specific Compounds of SKOG
3.2. Antitussive Assay
3.2.1. Changes in BW and BW Gain
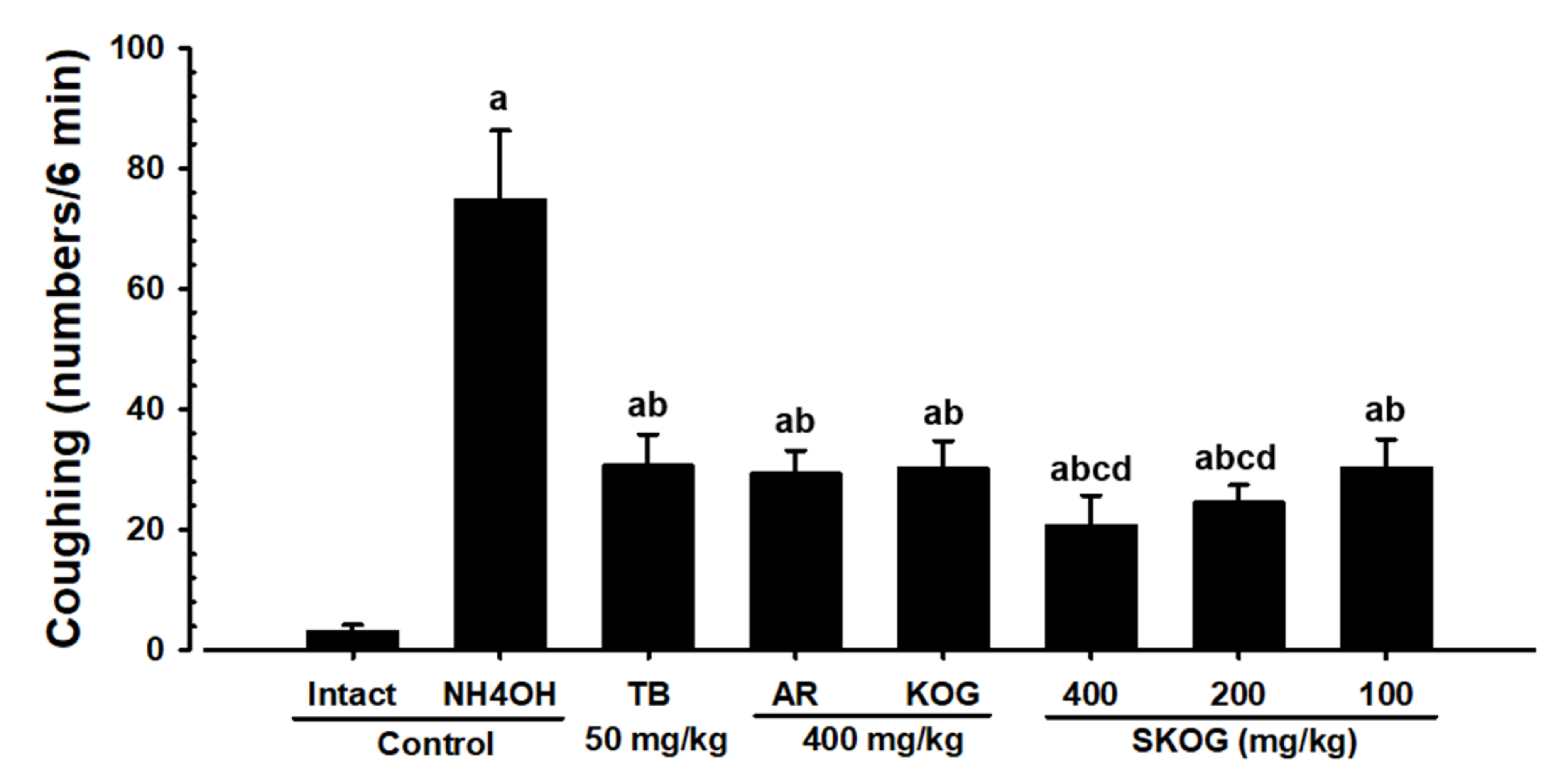
3.2.2. Changes in the Coughing Frequency
3.2.3. Histopathological Findings of the Trachea and Lung
3.3. Expectorant Assay
3.3.1. Changes in BW and BW Gain
3.3.2. Body-Surface Gross Findings
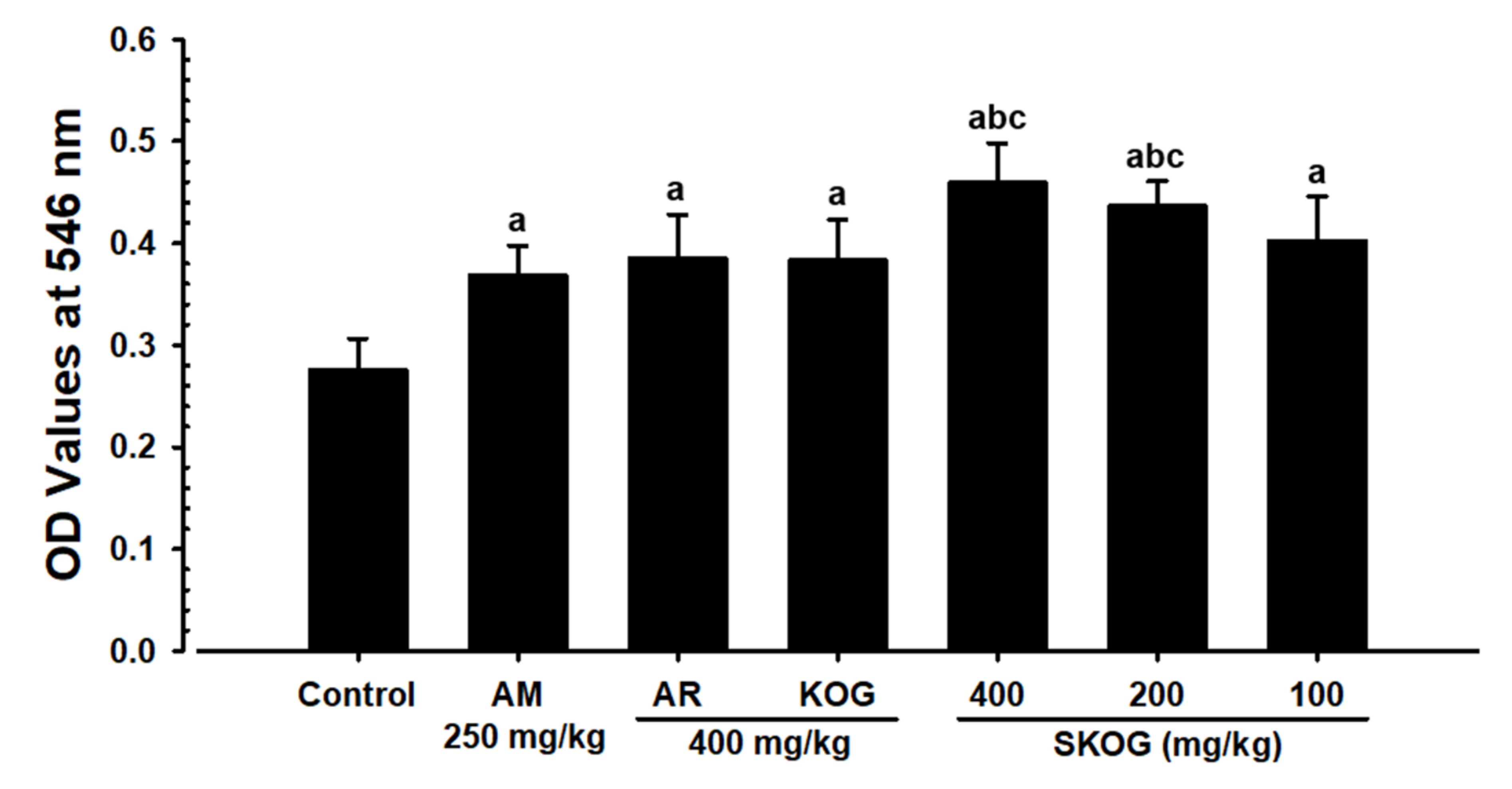
3.3.3. Changes in Mucous Secretion
3.3.4. Histopathological Findings in the Intrapulmonary Secondary Bronchus
3.4. Anti-Inflammatory Assay
3.4.1. Changes in BW and BW Gain
3.4.2. Ear Gross-Findings
3.4.3. Changes in Ear Weight
3.4.4. Histopathological Findings on the Ear
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Periods | Body Weights (g) at Test Material Administration | Body Weight Gains (g) [B – A] | ||
|---|---|---|---|---|
| Groups | First [A] | Last [B] | ||
| Controls | ||||
| Intact | 29.34 ± 1.29 | 30.98 ± 1.39 | 1.64 ± 0.74 | |
| NH4OH | 29.44 ± 1.65 | 31.11 ± 2.22 | 1.67 ± 0.84 | |
| Reference | ||||
| TB 50 mg/kg | 29.23 ± 1.49 | 30.94 ± 1.77 | 1.71 ± 0.97 | |
| AR 400 mg/kg | 29.41 ± 0.96 | 31.12 ± 1.74 | 1.71 ± 1.18 | |
| KOG 400 mg/kg | 29.58 ± 1.24 | 31.10 ± 1.56 | 1.52 ± 0.61 | |
| SKOG | ||||
| 400 mg/kg | 29.66 ± 1.07 | 31.35 ± 1.34 | 1.69 ± 0.70 | |
| 200 mg/kg | 29.27 ± 0.75 | 31.04 ± 0.62 | 1.77 ± 0.32 | |
| 100 mg/kg | 29.41 ± 0.86 | 30.92 ± 1.17 | 1.51 ± 0.88 | |
| Periods | Body Weights (g) at Test Material Administration | Body Weight Gains (g) [B – A] | ||
|---|---|---|---|---|
| Groups | First [A] | Last [B] | ||
| Controls | ||||
| Intact | 29.09 ± 1.23 | 30.96 ± 1.01 | 1.87 ± 0.60 | |
| Reference | ||||
| AM 250 mg/kg | 29.23 ± 1.16 | 31.22 ± 1.65 | 1.99 ± 0.73 | |
| AR 400 mg/kg | 29.40 ± 0.81 | 31.11 ± 1.25 | 1.71 ± 0.85 | |
| KOG 400 mg/kg | 29.00 ± 1.60 | 30.69 ± 1.77 | 1.69 ± 0.90 | |
| SKOG | ||||
| 400 mg/kg | 29.26 ± 1.12 | 31.01 ± 1.76 | 1.75 ± 1.02 | |
| 200 mg/kg | 29.20 ± 1.26 | 31.25 ± 1.83 | 2.05 ± 0.92 | |
| 100 mg/kg | 29.18 ± 1.04 | 31.23 ± 1.76 | 2.05 ± 1.03 | |
| Periods | Body Weights (g) at Test Material Administration | Body Weight Gains (g) [B – A] | ||
|---|---|---|---|---|
| Groups | First [A] | Last [B] | ||
| Controls | ||||
| Intact | 28.94 ± 1.06 | 31.04 ± 1.49 | 2.10 ± 0.90 | |
| Xylene | 28.93 ± 1.01 | 30.97 ± 1.62 | 2.04 ± 0.79 | |
| Reference | ||||
| DEXA 1 mg/kg | 28.72 ± 1.10 | 27.80 ± 1.66 ab | −0.92 ± 0.76 ab | |
| AR 400 mg/kg | 28.99 ± 1.21 | 31.12 ± 2.51 | 2.13 ± 1.63 | |
| KOG 400 mg/kg | 29.20 ± 1.11 | 31.02 ± 1.78 | 1.82 ± 1.01 | |
| SKOG | ||||
| 400 mg/kg | 29.11 ± 1.27 | 31.29 ± 2.19 | 2.18 ± 1.34 | |
| 200 mg/kg | 28.95 ± 1.26 | 31.29 ± 1.66 | 2.34 ± 0.70 | |
| 100 mg/kg | 29.07 ± 0.72 | 31.08 ± 1.10 | 2.01 ± 0.86 | |
References
- Pavord, I.D. Cough and asthma. Pulm. Pharmacol. Ther. 2004, 17, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Dapaah, G.; Koffuor, G.A.; Mante, P.K.; Ben, I.O. Antitussive, expectorant and analgesic effects of the ethanol seed extract of Picralima nitida (Stapf) Th. & H. Durand. Res. Pharm. Sci. 2016, 11, 100. [Google Scholar] [PubMed]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [Green Version]
- Sanak, M. Eicosanoid mediators in the airway inflammation of asthmatic patients: What is new? Allergy Asthma Immunol. Res. 2016, 8, 481–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woloski, J.R.; Heston, S.; Calderon, S.P.E. Respiratory Allergic Disorders. Prim. Care Clin. Off. Pract. 2016, 43, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Konno, C.; Saito, T.; Oshima, Y.; Hikino, H.; Kabuto, C. Structure of methyl adenophorate and triphyllol, triterpenoids of Adenophora triphylla var. japonica roots. Planta Med. 1981, 42, 268–274. [Google Scholar] [CrossRef]
- Kim, J.B.; Song, H.N. Effects of Kyeongok-go and its two added precriptions on hyperlipidemic rats induced by high-fat diet. J. Physiol. Pathol. Korean Med. 2014, 28, 371–378. [Google Scholar] [CrossRef]
- Lee, D.-R.; Lee, Y.-S.; Choi, B.-K.; Lee, H.J.; Park, S.-B.; Kim, T.-M.; Oh, H.J.; Yang, S.H.; Suh, J.-W. Roots extracts of Adenophora triphylla var. japonica improve obesity in 3T3-L1 adipocytes and high-fat diet-induced obese mice. Asian Pac. J. Trop. Med. 2015, 8, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Asano, N.; Nishida, M.; Miyauchi, M.; Ikeda, K.; Yamamoto, M.; Kizu, H.; Kameda, Y.; Watson, A.A.; Nash, R.J.; Fleet, G.W.J. Polyhydroxylated pyrrolidine and piperidine alkaloids from Adenophora triphylla var. japonica (Campanulaceae). Phytochemistry 2000, 53, 379–382. [Google Scholar] [CrossRef]
- Ahn, E.K.; Oh, J.S. Lupenone isolated from Adenophora triphylla var. japonica extract inhibits adipogenic differentiation through the downregulation of PPARγ in 3T3-L1 cells. Phytother. Res. 2013, 27, 761–766. [Google Scholar] [CrossRef]
- Yoon, Y.P.; Lee, H.J.; Lee, D.-U.; Lee, S.K.; Hong, J.-H.; Lee, C.J. Effects of lupenone, lupeol, and taraxerol derived from Adenophora triphylla on the gene expression and production of airway MUC5AC mucin. Tuberc. Respir. Dis. 2015, 78, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Ha, I.J.; Chun, J.; Kang, S.S.; Kim, Y.S. Separation of two cytotoxic saponins from the roots of Adenophora triphylla var. japonica by High-speed Counter-current Chromatography. Phytochem. Anal. 2013, 24, 148–154. [Google Scholar] [CrossRef]
- Chun, J.; Kang, M.; Kim, Y.S. A triterpenoid saponin from Adenophora triphylla var. japonica suppresses the growth of human gastric cancer cells via regulation of apoptosis and autophagy. Tumor Biol. 2014, 35, 12021–12030. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.R.; Jung, C.J.; Ku, S.M.; Jung, D.H.; Ku, S.K.; Choi, J.S. Antitussive, expectorant, and anti-inflammatory effects of Adenophorae Radix powder in ICR mice. J. Ethnopharmacol. 2019, 239, 111915. [Google Scholar] [CrossRef] [PubMed]
- Na, C.-S.; Shin, W.; Lee, Y.-M.; Moon, Y.-S.; Noh, H.-k.; Seo, S.-H.; Son, H.-S. Effect of original kyungokgo & iksuyongjingo plus Sparassis crispa on antioxidant, immunity improvement and sensory evaluation. Korea J. Herbol. 2016, 31, 43–51. [Google Scholar]
- Lee, K.-S.; Kim, G.-H.; Kim, H.-H.; Seong, B.-J.; Kim, S.-I.; Han, S.-H.; Kang, E.J.; Yoo, Y.C. Qualities and anti-inflammatory activity of Kyungokgos sold in local markets. J. Korean Soc. Food Sci. Nutr. 2013, 42, 335–341. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Oh, J.-M.; Kim, Y.-K. Inhibitory effects on bone resorption and osteoblast proliferation of Kyungok-go. Herb. Formula Sci. 2011, 19, 61–71. [Google Scholar]
- Cha, Y.-Y. A Comparative Study on Effects of Kyungohkgo and Kyungohkgo Ga Nokyong on growth in growth deficiency rat with insufficient nutrition diet. J. Korean Med. Obes. Res. 2009, 9, 59–69. [Google Scholar]
- Zhang, J.-L.; Wang, H.; Pi, H.-F.; Ruan, H.-L.; Zhang, P.; Wu, J.-Z. Structural analysis and antitussive evaluation of five novel esters of verticinone and bile acids. Steroids 2009, 74, 424–434. [Google Scholar] [CrossRef]
- Wanga, D.; Wanga, S.; Chena, X.; Xua, X.; Zhub, J.; Nieb, L.; Longa, X. Antitussive, expectorant and anti-inflammatory activities of four alkaloids isolated from Bulbus of Fritillaria wabuensis. J. Ethnopharmacol. 2012, 139, 189–193. [Google Scholar] [CrossRef]
- Engler, H.; Szelenyi, I. Tracheal phenol red secretion, a new method for screening mucosecretolytic compounds. J. Pharmacol. Methods 1984, 11, 151–157. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, H.-D.; Lee, B.-W.; Lim, M.-K.; Ku, S.K. Effects of magnetic infrared laser on xylene-induced acute inflammation in mice. J. Phys. Ther. Sci. 2008, 20, 255–259. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-S.; Ku, S.-K. Effects of Picrorrhiza Rhizoma on acute inflammation in mice. Biomol. Ther. 2008, 16, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Food and Drug Safety. The Korean Pharmacopoeia (The Korea Food and Drug Administration Notification 2012-9); Ministry of Food and Drug Safety: Cheongju, Korea, 2012.
- Lebargy, F.; Lenormand, E.; Pariente, R.; Fournier, M. Morphological changes in rat tracheal mucosa immediately after antigen challenge. Bull. Eur. Physiopathol. Respir. 1987, 23, 417–421. [Google Scholar] [PubMed]
- Ku, S.K.; Kim, J.W.; Cho, H.R.; Kim, K.Y.; Min, Y.H.; Park, J.H.; Kim, J.S.; Park, J.H.; Seo, B.I.; Roh, S.S. Effect of β-glucan originated from Aureobasidium pullulans on asthma induced by ovalbumin in mouse. Arch. Pharmacal Res. 2012, 35, 1073–1081. [Google Scholar] [CrossRef]
- Choi, H.-Y.; Jung, T.-Y.; Ku, S.-K.; Yang, H.-B.; Lee, H.-S. Toxico-pathological study p, p-DDE after experimental aerosol exposed to ICR mouse. Toxicol. Res. 2005, 21, 151–160. [Google Scholar]
- Honda, H.; Fujimoto, M.; Miyamoto, S.; Ishikawa, N.; Serada, S.; Hattori, N.; Nomura, S.; Kohno, N.; Yokoyama, A.; Naka, T. Sputum leucine-rich alpha-2 glycoprotein as a marker of airway inflammation in asthma. PLoS ONE 2016, 11, e0162672. [Google Scholar] [CrossRef]
- Lee, C.W.; Park, S.M.; Kim, Y.S.; Jegal, K.H.; Lee, J.R.; Cho, I.J.; Ku, S.K.; Lee, J.Y.; Ahn, Y.-T.; Son, Y. Biomolecular evidence of anti-inflammatory effects by Clematis mandshurica Ruprecht root extract in rodent cells. J. Ethnopharmacol. 2014, 155, 1141–1155. [Google Scholar] [CrossRef]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.N.M.; Balakrishnan, R.B.S.; Shamsudeen, S.; Bahwani, S.A.; Adam, F. A concise review of the natural existance, synthesis, properties, and applications of syringaldehyde. Bioresources 2012, 7, 4377–4399. [Google Scholar]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethyl furfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Chao-Hsiang, C.; Yin-Shiou, L.I.N.; Chien, M.-Y.; Wen-Chi, H.O.U.; Miao-Lin, H.U. Antioxidant and antihypertensive activities of acteoside and its analogs. Bot. Stud. 2012, 53, 421–429. [Google Scholar]
- Kim, S.-W.; Choi, S.-C.; Choi, E.-Y.; Kim, K.-S.; Oh, J.-M.; Lee, H.-J.; Oh, H.-M.; Kim, S.; Oh, B.-S.; Kimm, K.-C. Catalposide, a compound isolated from Catalpa ovata, attenuates induction of intestinal epithelial proinflammatory gene expression and reduces the severity of trinitrobenzene sulfonic acid-induced colitis in mice. Inflamm. Bowel Dis. 2004, 10, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Z.; Chen, Z.; Zhong, Z.; Li, Z. Ginsenoside Rg3 exerts anti-depressive effect on an NMDA-treated cell model and a chronic mild stress animal model. J. Pharmacol. Sci. 2017, 134, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Koshimizu, K. Biological Reference Data Book on Experimental Animals; Soft Science: Tokyo, Japan, 1989; p. 158. [Google Scholar]
- Kim, H.-D.; Cho, K.-H.; Lee, B.-W.; Kwon, Y.-S.; Lee, H.-S.; Choi, S.-H.; Ku, S.-K. Effects of magnetic infrared laser irradiation on formalin-induced chronic paw inflammation of mice. J. Phys. Ther. Sci. 2010, 22, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Davey, M.G.; Hedrick, H.L.; Mendoza, J.M.; Kanai, M.; Adzick, N.S.; Flake, A.W. Pulmonary epithelial liquid absorption, expressed in relation to alveolar surface area, is reduced in fetal lambs following in utero tracheal occlusion. Pediatric Pulmonol. 2002, 34, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.; Wadsworth, S.; Dorscheid, D.; Man, S.-F.P.; Sin, D.D. Estradiol increases. mucus synthesis in bronchial epithelial cells. PLoS ONE 2014, 9, e100633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.G.; Kang, M.; Lee, Y.-H.; Min, W.G.; Kim, Y.H.; Kang, S.J.; Song, C.H.; Park, S.J.; Park, J.H.; Han, C.H. Bathing effects of various seawaters on allergic (atopic) dermatitis-like skin lesions induced by 2, 4-dinitrochlorobenzene in hairless mice. Evid.-Based Complement. Altern. Med. 2015, 2015, 179185. [Google Scholar] [CrossRef] [Green Version]
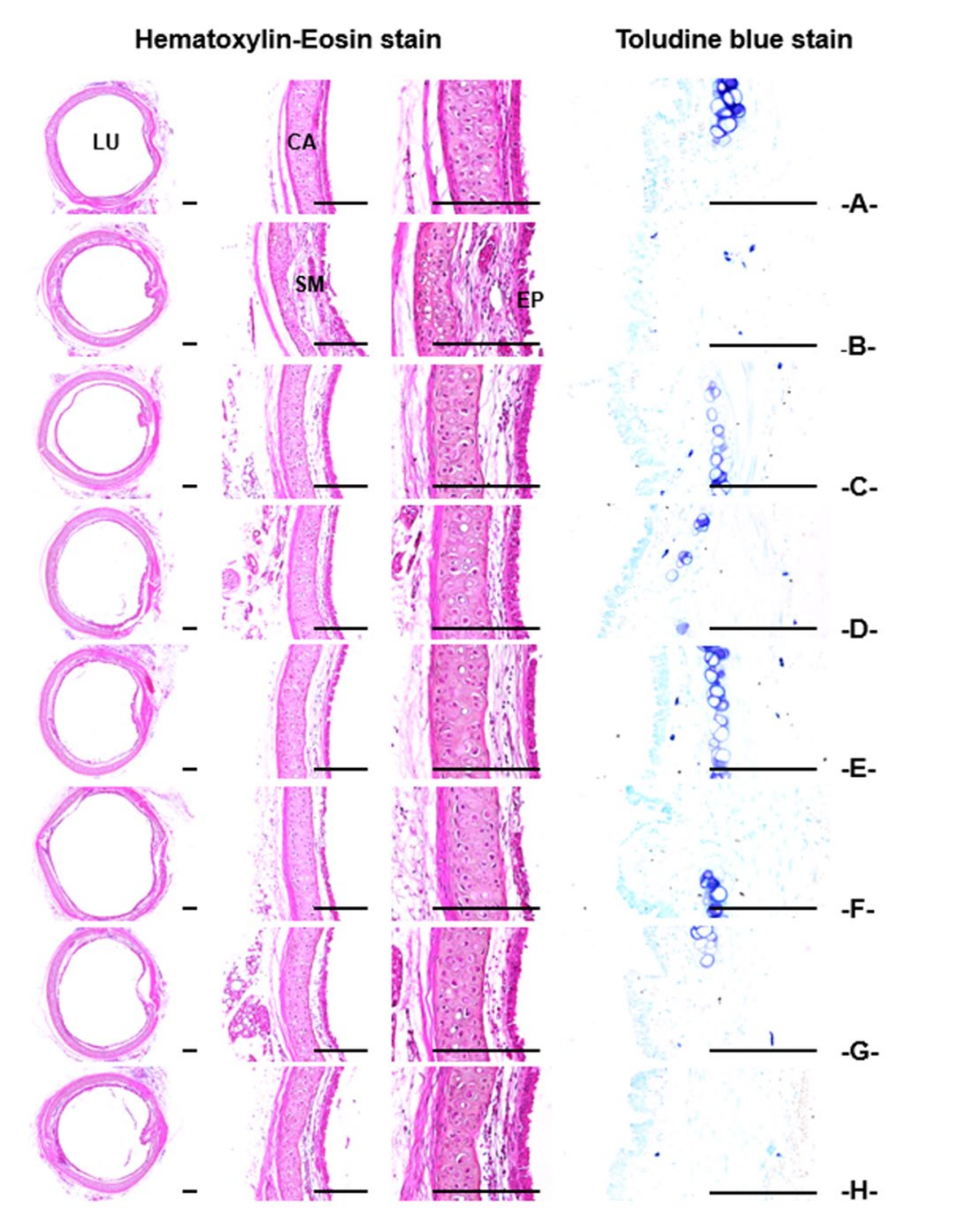
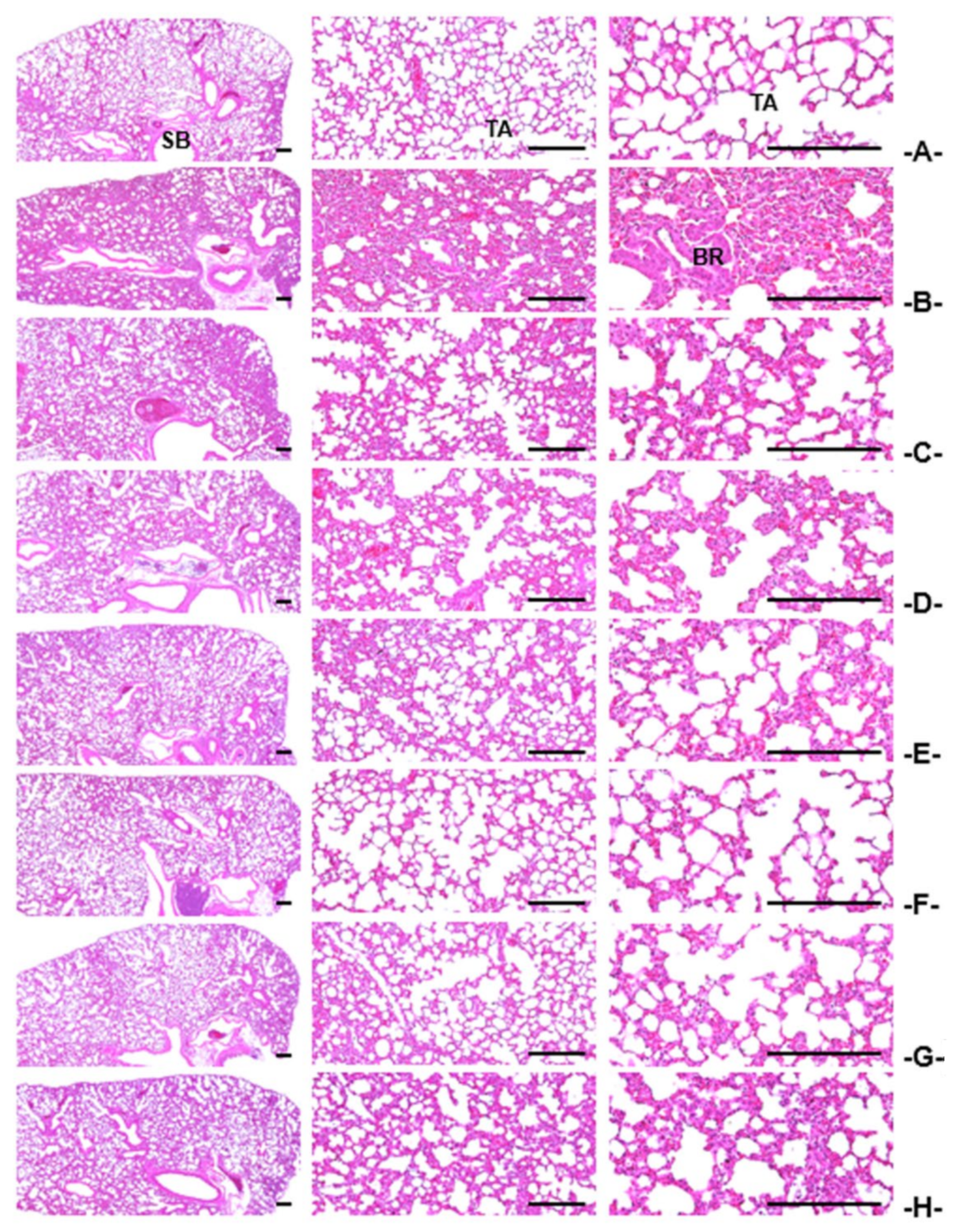
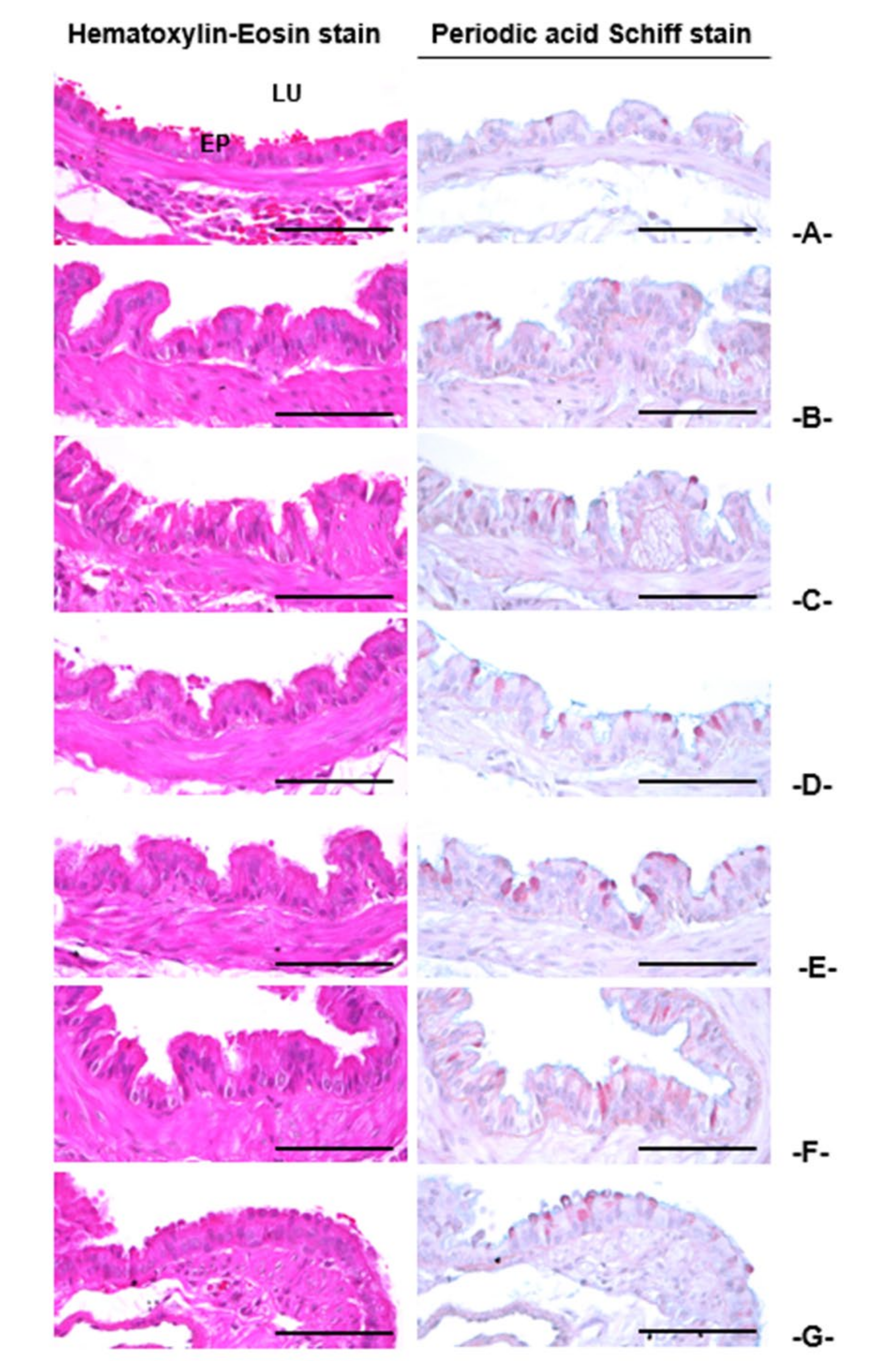
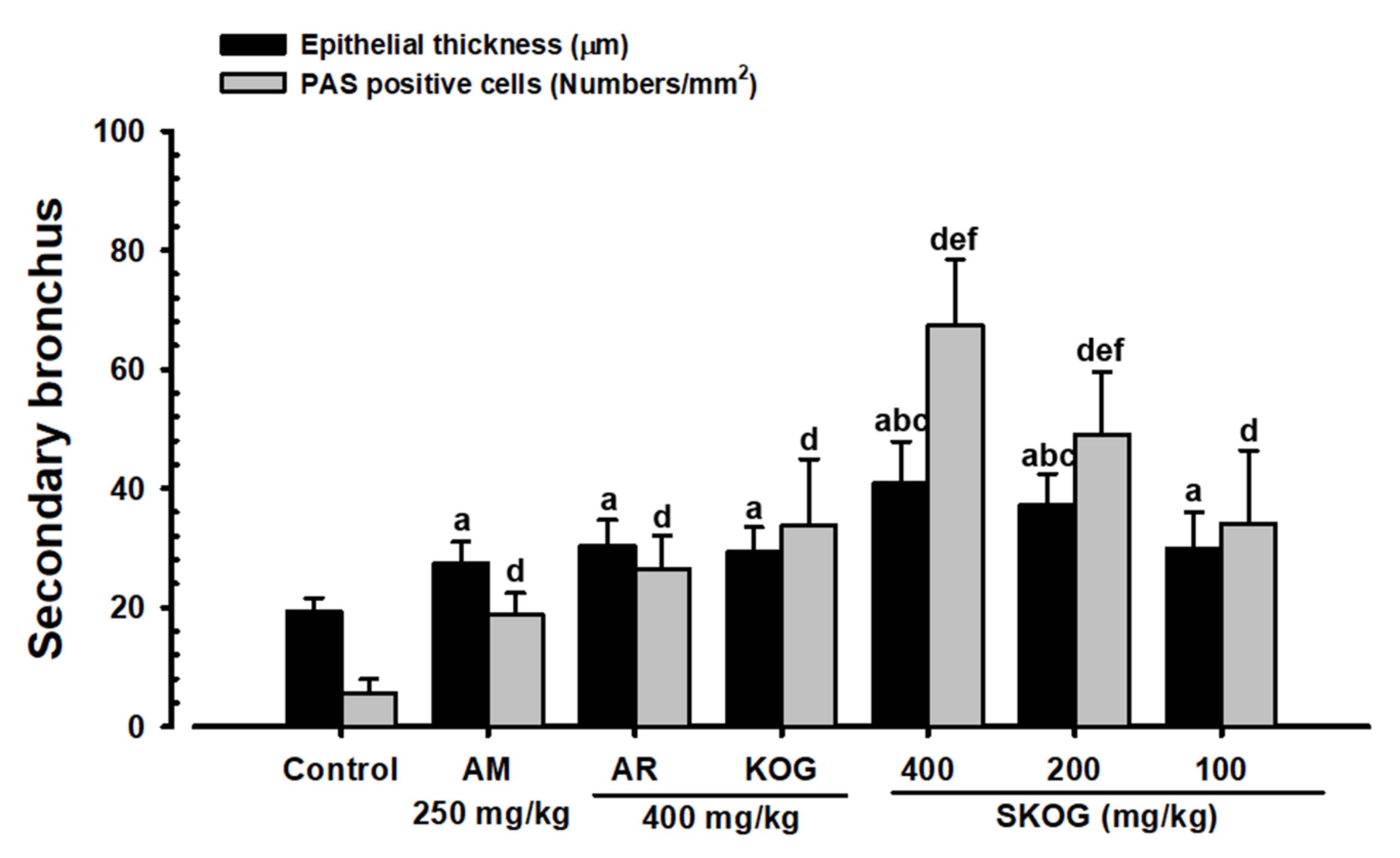
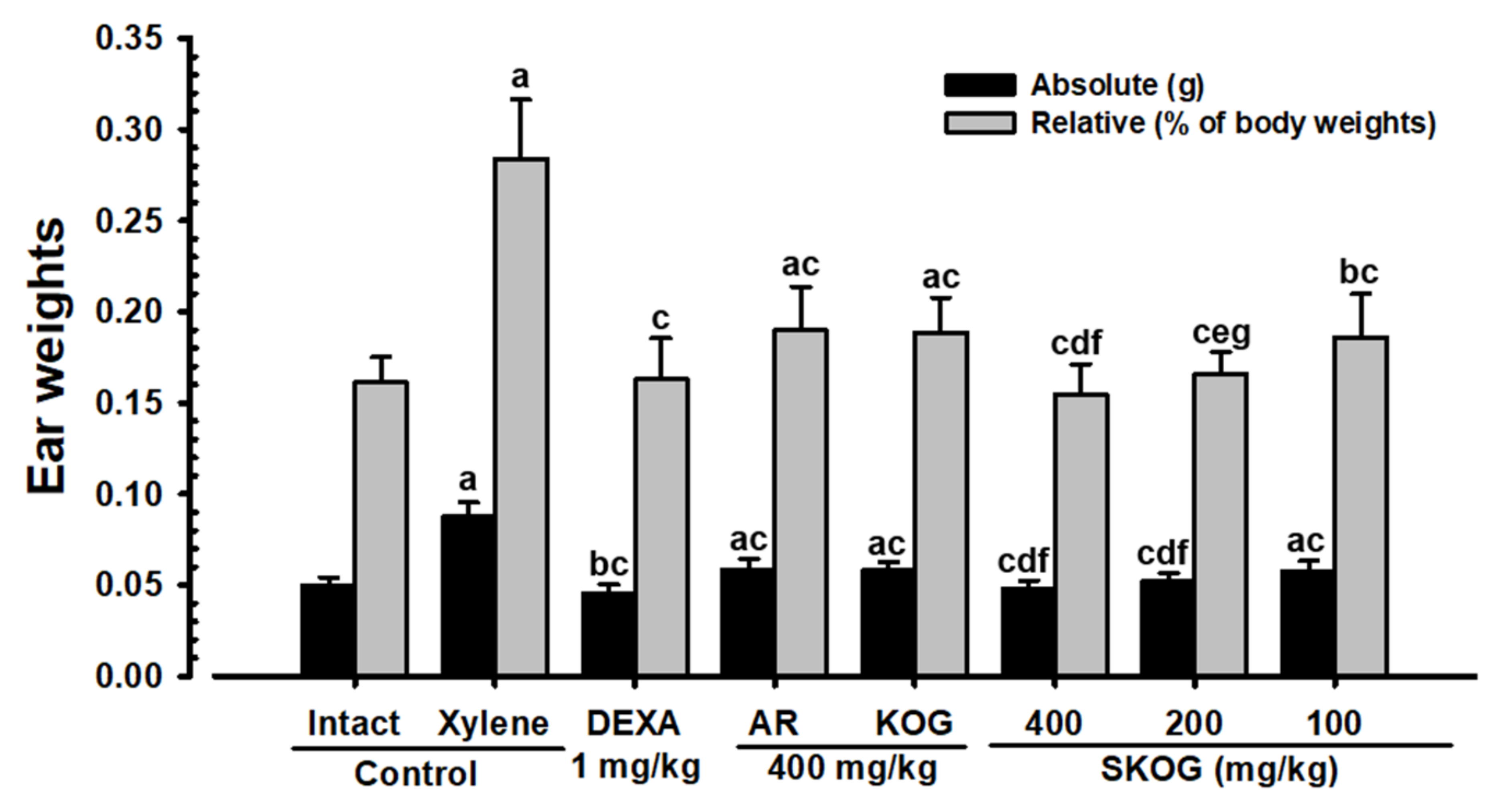
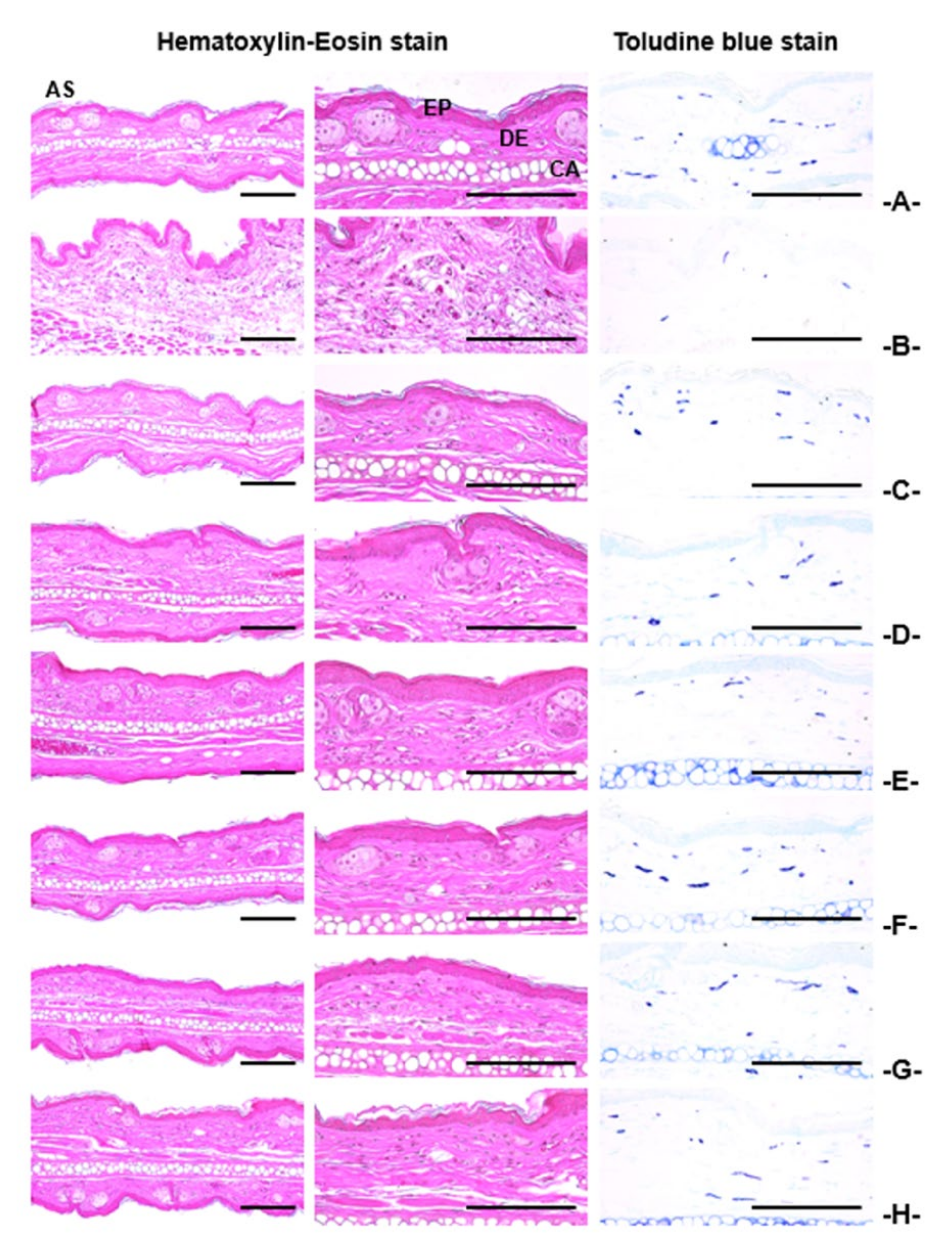
| Index Groups | Diameter of Lumen (μm) | Thickness (μm) | Cells (Numbers/mm2) | |||
|---|---|---|---|---|---|---|
| Total Wall | Epithelium | Submucosa | Inflammatory | Mast | ||
| Controls | ||||||
| Intact | 1177.86 ± 130.95 | 161.47 ± 15.34 | 14.38 ± 2.91 | 26.68 ± 4.85 | 21.40 ± 12.21 | 1.20 ± 0.79 |
| NH4OH | 658.57 ± 106.44 a | 220.38 ± 13.45 h | 37.53 ± 9.48 h | 89.73 ± 10.46 h | 449.30 ± 102.75 h | 38.70 ± 11.66 h |
| Reference | ||||||
| TB 50 mg/kg | 860.11 ± 100.75 ac | 191.23 ± 10.24 hi | 24.21 ± 4.36 hi | 47.31 ± 11.39 hi | 167.40 ± 40.26 hi | 14.30 ± 4.60 hi |
| AR 400 mg/kg | 934.14 ± 104.43 ac | 185.59 ± 10.94 hi | 23.07 ± 3.32 hi | 44.99 ± 6.09 hi | 62.70 ± 37.57 hi | 8.60 ± 2.27 hi |
| KOG 400 mg/kg | 934.89 ± 83.13 ac | 186.21 ± 11.10 hi | 23.87 ± 2.38 hi | 40.31 ± 6.64 hi | 184.10 ± 20.32 hi | 8.40 ± 1.51 hi |
| SKOG | ||||||
| 400 mg/kg | 1087.76 ± 118.72b bcdf | 171.56 ± 7.07 ikm | 18.92 ± 2.14 hikm | 30.30 ± 3.24 ikm | 85.50 ± 19.82 hikm | 1.80 ± 1.03 ikm |
| 200 mg/kg | 1036.55 ± 76.84 aceg | 175.45 ± 5.03 iln | 19.74 ± 1.37 hikm | 32.71 ± 3.79 hikm | 120.70 ± 20.94 hikm | 3.70 ± 1.34 hikm |
| 100 mg/kg | 936.74 ± 70.89 ac | 185.19 ± 7.57 hi | 23.76 ± 3.34 hi | 42.41 ± 8.60 hi | 177.60 ± 26.02 hi | 8.70 ± 2.16 hi |
| Index Groups | Alveolar Surface Area (%) | Septum Thickness (μm) | Inflammatory Cells (Numbers/mm2) |
|---|---|---|---|
| Controls | |||
| Intact | 78.87 ± 9.31 | 7.32 ± 1.45 | 60.20 ± 19.63 |
| NH4OH | 30.94 ± 9.58 a | 72.41 ± 10.80 g | 1886.70 ± 394.17 g |
| Reference | |||
| TB 50 mg/kg | 51.13 ± 5.95 ac | 30.53 ± 10.06 gh | 492.00 ± 114.18 gh |
| AR 400 mg/kg | 53.83 ± 7.88 ac | 27.22 ± 7.28 gh | 453.80 ± 104.35 gh |
| KOG 400 mg/kg | 52.34 ± 8.24 ac | 27.98 ± 3.75 gh | 492.60 ± 118.49 gh |
| SKOG | |||
| 400 mg/kg | 71.32 ± 5.65 bcdf | 12.56 ± 2.52 ghik | 234.20 ± 42.55 ghik |
| 200 mg/kg | 62.29 ± 6.45 acef | 20.26 ± 3.12 ghik | 347.60 ± 79.19 ghjk |
| 100 mg/kg | 53.36 ± 9.65 ac | 27.99 ± 6.85 gh | 481.90 ± 132.69 gh |
| Index Groups | Thickness (μm) | Cells (Numbers/mm2) | Collagen Fiber (%/mm2 of Dermis) | |||
|---|---|---|---|---|---|---|
| Total | Epidermis | Dermis | Inflammatory | Mast | ||
| Controls | ||||||
| Intact | 103.41 ± 11.47 | 8.98 ± 0.93 | 54.86 ± 11.97 | 15.20 ± 4.66 | 69.00 ± 15.48 | 78.31 ± 9.75 |
| Xylene | 264.48 ± 30.02 f | 9.07 ± 1.16 | 132.28 ± 22.16 f | 263.40 ± 55.50 f | 8.20 ± 4.16 a | 26.74 ± 6.58 f |
| Reference | ||||||
| DEXA 1 mg/kg | 99.55 ± 9.57 h | 8.50 ± 1.65 | 52.88 ± 13.72 h | 18.10 ± 10.54 h | 61.30 ± 12.65 c | 77.43 ± 12.79 h |
| AR 400 mg/kg | 166.51 ± 17.59 fh | 8.77 ± 0.71 | 75.41 ± 8.63 fh | 72.30 ± 14.17 fh | 45.20 ± 10.52 ac | 64.73 ± 10.85 gh |
| KOG 400 mg/kg | 158.83 ± 13.35 fh | 8.76 ± 0.82 | 71.55 ± 5.05 fh | 69.00 ± 12.00 fh | 42.40 ± 6.72 ac | 67.59 ± 4.70 gh |
| SKOG | ||||||
| 400 mg/kg | 105.94 ± 13.74 hij | 9.15 ± 1.09 | 51.58 ± 6.97 hij | 29.20 ± 8.26 fhij | 61.00 ± 10.92 cde | 81.51 ± 7.58 hij |
| 200 mg/kg | 128.25 ± 15.08 fhij | 8.28 ± 1.18 | 61.37 ± 6.03 hij | 48.40 ± 14.21 fhij | 58.20 ± 8.53 bcde | 76.84 ± 5.22 hij |
| 100 mg/kg | 160.57 ± 18.68 fh | 8.73 ± 0.92 | 71.69 ± 10.31 fh | 77.70 ± 21.78 fh | 42.80 ± 10.09 ac | 66.43 ± 10.37 gh |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.-R.; Jung, C.-J.; Ku, S.-M.; Jung, D.-H.; Ku, S.-K.; Mohibbullah, M.; Lee, H.-J.; Choi, J.-S. Deciphering the Antitussive, Expectorant, and Anti-Inflammatory Potentials of ShashamKyeongok-Go and Their Phytochemical Attributes: In Vivo Appraisal in ICR Mice. Appl. Sci. 2021, 11, 1349. https://doi.org/10.3390/app11031349
Hu J-R, Jung C-J, Ku S-M, Jung D-H, Ku S-K, Mohibbullah M, Lee H-J, Choi J-S. Deciphering the Antitussive, Expectorant, and Anti-Inflammatory Potentials of ShashamKyeongok-Go and Their Phytochemical Attributes: In Vivo Appraisal in ICR Mice. Applied Sciences. 2021; 11(3):1349. https://doi.org/10.3390/app11031349
Chicago/Turabian StyleHu, Jin-Ryul, Chul-Jong Jung, Seong-Min Ku, Dae-Hwa Jung, Sae-Kwang Ku, Md. Mohibbullah, Hae-Jeung Lee, and Jae-Suk Choi. 2021. "Deciphering the Antitussive, Expectorant, and Anti-Inflammatory Potentials of ShashamKyeongok-Go and Their Phytochemical Attributes: In Vivo Appraisal in ICR Mice" Applied Sciences 11, no. 3: 1349. https://doi.org/10.3390/app11031349
APA StyleHu, J.-R., Jung, C.-J., Ku, S.-M., Jung, D.-H., Ku, S.-K., Mohibbullah, M., Lee, H.-J., & Choi, J.-S. (2021). Deciphering the Antitussive, Expectorant, and Anti-Inflammatory Potentials of ShashamKyeongok-Go and Their Phytochemical Attributes: In Vivo Appraisal in ICR Mice. Applied Sciences, 11(3), 1349. https://doi.org/10.3390/app11031349









