1. Introduction
The current model of industrial development considers not only the economic principles, but also that the parameters associated with the environment becoming more relevant. In accordance with European Environmental Policy, the European Commission is promoting the circular economy [
1], which in this document it is sought with two main objectives: the recovery of waste [
2] and avoiding its disposal in landfills [
3].
The concept of waste recovery or a circular economy is not new, considering its enunciation in the last century [
4], where some characteristics of the circular economy were introduced. The current concept has evolved, considering the complete life cycle of the product [
5], ecological legislation [
4], and the blue economy [
6]. The circular economy has become popular since it was introduced by policy makers from China and the European Union (EU) as a solution to contribute to reducing the environmental impact of industries [
7]. The scientific interest in developing new productive processes considering zero waste has increased in the last decade, going from just 10 indexed publications in 2008 to more than 100 in 2016 [
8].
In this sense, Circular Economy focuses, among other actions [
9], on reducing the exploitation of certain materials (rare earths, critical materials and bio-ingredients) and fossil energy sources [
10]. The product can be divided into three main categories: making products more energy efficient, banning dangerous substances, and ensuring that the product is disposed of in an appropriate way at its end-of-life stage [
11]. For these reasons, the economy and the environment should coexist in balance [
8]. In addition, the circular economy offers substantial opportunities for reducing CO
2 emissions [
12], there being an urgent need for a transition towards a more sustainable society. It includes the replacement of fossil fuels with more sustainable alternatives [
13]. Moreover, European Policies focus on the use of sustainable energy [
14], including biomass and waste-derived fuels [
15].
In recent decades, growth and industrial activity have caused contamination of the soil by the spillage of polluting effluents, both liquid and soil. In the industrial sector, ore processing and waste disposal in landfills are global sources of heavy elements in the surface environment [
16], and one of the options for the use of these wastes is to use them as environmentally friendly fuel [
17]. Another important factor at present is that there is a dependence on oil, so it is necessary to explore alternative fuels, as well as to carry out a strict regulation of emissions [
18]. In order to reduce landfilling to favor increased recycling of energy and materials, the environmental impact, consumption of energy resources, and economic cost are lowered [
19].
The cement sector is responsible for 12–15% of the primary energy consumed—75% of this energy is provided by the fuel used in the manufacture of cement [
20]. Overall, CO
2 emissions from this sector are approximately 5–7% of anthropogenic emissions [
21,
22]. In the last few decades of the cement industry, there has been an enormous amount of research to reduce the energy and environmental cost by using alternative fuels and raw material [
23], since CO
2 is produced in the calcination process itself [
24]. The cement sector is one of the industries with the highest rate of CO
2 emissions [
25] that is regulated by European directives [
26]. Different alternatives are being considered to reduce its carbon footprint, one of them being the use of waste as fuel. The use of this wastes is carried out through different technologies, some of which are listed below.
Within liquid waste fuels is the process of dried sewage sludge to generate heat and electricity, which is currently practiced on an industrial scale in many countries [
27]; it has been found that up to 14% of the raw materials used in cement manufacture could be replaced by sewage sludge [
28].
Another process is the use of organic waste solvents that cannot be regenerated, are generally incinerated, and have a high energy content, which is usually recovered in the incineration process and used for stream and electricity production [
29].
As solid waste, scrap tires with high calorific value can be used as fuel in the cement sector [
30] with environmental benefits, as natural rubber reduces CO
2 emissions [
31].
The energy balance is the first step in chemical, thermal, fluid and energy–environmental calculations, as well as the introduction to thermodynamic calculation [
27,
32]. As an effective method for adjusting the accumulation of thermal energy, the research on energy balance is quite necessary when it comes to reducing the thermal effect on dry hobbing [
33].
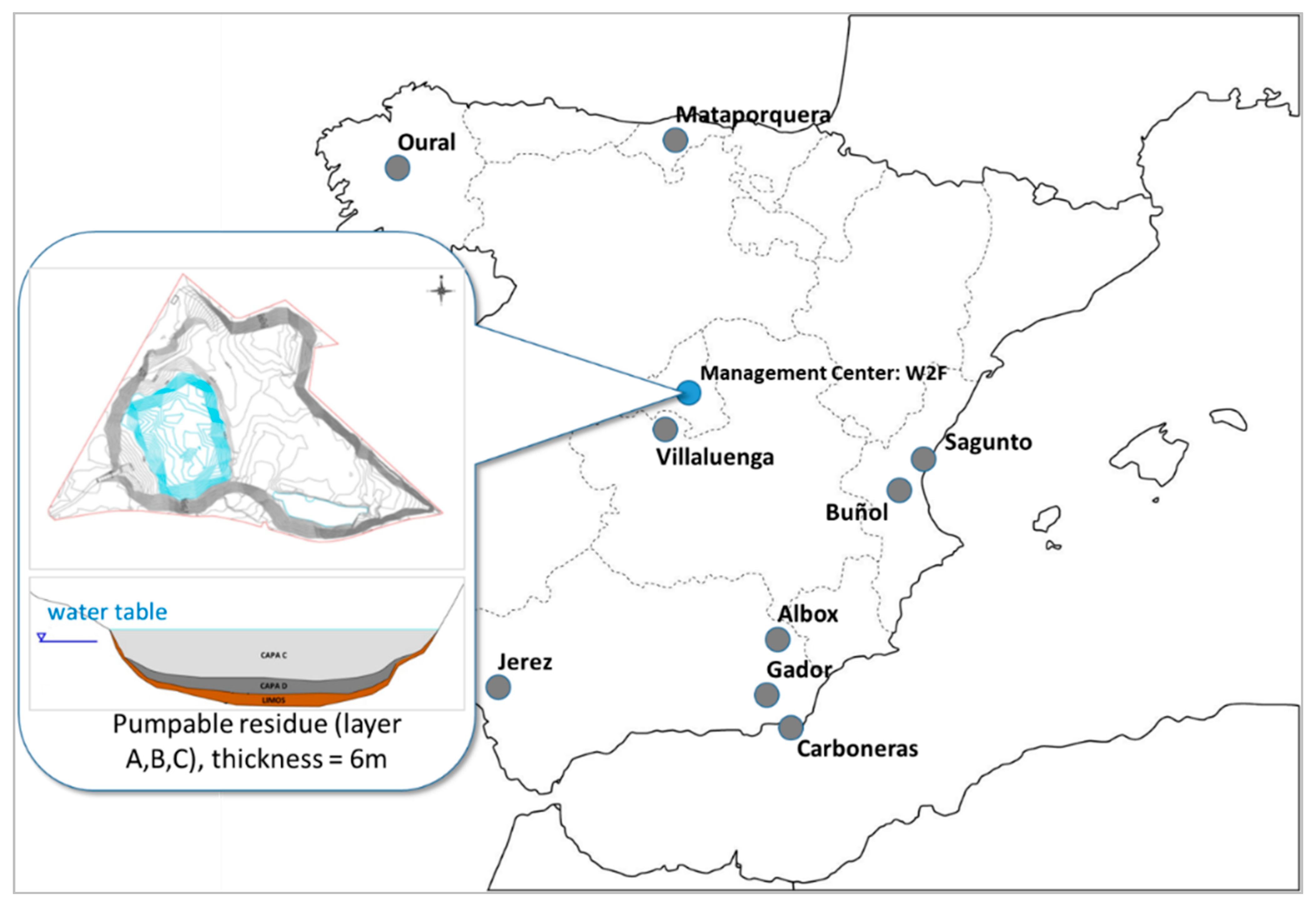
The present study describes the results achieved in a real waste-to-fuel process and how it contributes to reducing greenhouse emissions from large stationary emitters, instead of conventional treatment where this dangerous waste is incinerated. The biggest challenge for the research team was waste extraction and its transformation into usable fuel in cement plants; the problems in managing this residue were due to its consideration as dangerous waste (
Figure 1).
2. Materials and Methods
This section includes the waste lagoon location and description, as well as the characterization of hydrocarbon waste in the laboratory. Two alternatives are described, and waste-to-fuel characterization is considered to be used in cement factories. The final subsection explains energy balance methodology for both alternatives considered in this study.
2.1. Description of Arganda Lagoon: Hydrocarbon Waste Disposal
The lagoon is located in the natural area of Boca Alta in the “Parque Regional del Sureste”, in the municipality of Arganda del Rey (Madrid Region). The approximate surface area of the lagoon is 12,750 m
2, with a shape similar to an irregular rectangle of 120 × 90 m. It consists of an estimated accumulation of 50,000 m
3 of oily petroleum sulfonate, which is the main component, known as “acid tar” (
Figure 2). The soil is contaminated with total petroleum hydrocarbons and heavy metals—mainly Pb and Zn, but to a lesser extent, Ba, Hg, Ni, and Va. The existing pollution in the lagoon leads to damage to plants and microorganisms, which originate due to the toxic, carcinogenic, and mutagenic potential of the hydrocarbons [
34].
In order to use all the material extracted from the lagoon and after its subsequent treatment, it has been decided to distribute it in nine cement industries in Spain. This distribution has been made throughout the entire extraction process, from 2015 to 2018, in a proportional manner and according to the consumption of each of the industries, as well as the geographical location and the availability of the waste at any time. This distribution is compared with the alternative of direct incineration of hazardous waste. The main residues have been treated: layers A, B, and C have already been extracted, and the extraction work of the non-pumpable layer (D) started at the beginning of 2020 (
Figure 3).
The geological location of the waste is in the alluvial plain of the Vega Baja of the Jarama River and is shaped by alluvial terraces. Its geological lithology presents alternations of conglomerates, gravels, sands, silts, and clays. Near the area where the lagoon is located, there are two large aquifers. The ancient mining activity that carried out excavations below the phreatic level led to the origin of this artificial lagoon. The content of the contaminating material is due to the discharge of petroleum sulfonate from the waste oil regeneration activity [
35]. These effluents are pumpable material that is extracted and managed through energy recovery. The lagoon effluents have been stratified in different phases, generating various layers:
Layer A: the most superficial oily phase, with a low density and a thickness of less than 0.10 m, formed by hydrocarbon compounds, therefore presenting a dark brown color.
Layer B: composed of water from stationary rains, which are contaminated by being in direct contact with A and C layers.
Layer C: with an approximate volume of 45,143 m3 and composed by oily petroleum sulphate, it is very viscous and has a black color. It is the most voluminous, so it is catalogued as the main nucleus of the lagoon. It does not have a constant thickness but varies according to the topography of the lagoon (5–7 m). Density, fluency, and viscosity vary with depth, so the deeper it is, the more viscous it is. This layer is considered in the present study.
Layer D: has an approximate volume of 4238 m3. This layer is the densest and deepest, occupying the bottom of the lagoon and part of the slopes. Black in color, it is composed of heavy hydrocarbons and cannot be pumped at room temperature. It has a very high viscosity but is fluid. Its thickness is variable, with an average of 0.5 m. This material comes from the thermal de-asphalting carried out for the recovery of oils. Also, in the deepest part of this layer, there are silts and sandy silts that provide additional waterproofing and with severe impact, because they contain heavy hydrocarbons, Polycyclic aromatic hydrocarbons (PAHs), and metals, followed by sands and gravel.
Moreover, in the lagoon there is not only the waste contained in these four layers, but also other polluting materials, such as drums, scrap tires, and other industrial waste.
2.2. Residue Characterization
During the extraction phase of the pumpable material, the extraction of the A, B, and most of C layers has been carried out. These layers present similar values for density, viscosity, and water content. While the D layer will be extracted in a second phase, because it has different parameters and so the density is higher, its viscosity is much higher, and the water content is much lower. For the first extraction, the work started in June 2015 and ended in December 2018. During this work, 1732 road tankers were used to transport the residue to an approved management center, where 42,246.83 tons of wastes were treated. The distance between the lagoon and the center is about 30 km, over which the road tankers consumed 33,774 L of fuel.
Table 1 shows the main characteristics of the polluting materials in the three layers.
2.3. Alternatives for the Use of the Residue
Once the waste has been extracted, there are two alternatives: (1) transporting it to a dangerous waste incinerator [
29,
36], or (2) transforming it to produce a fuel that can be used in cement plants.
The first alternative is the simplest one to carry out, but at the same time it is also the one that does not bring environmental benefits. This alternative consists of transporting the waste from the lagoon to the hazardous waste incinerator, where it would be burned directly without any type of treatment or change in the waste. The novel alternative considered in the present study is based on producing fuel from the waste in the lagoon and then distributing it to nine cement plants. The combustion of this kind of residue in a cement kiln is an effective way of using this waste, since the temperature reached in the kiln—approximately 1500 °C—is enough to burn all the organic compounds, while non-organic compounds are trapped in the clinker [
37]. In this case, the waste has a practical use while reducing CO
2 emissions in this industrial sector, as this waste to fuel replaces conventional fossil fuels used in those factories.
The first of the alternatives will be considered in this study as a base case. For this reason, energy and CO2 emissions will be compared to this alternative. This approach will consider the total CO2 emissions avoided, as well as the comparison of the net energy provided by each of the alternatives.
2.4. Fuel Production: Waste to Fuel
The process starts with a waste extraction on trucks. Each sample is characterized, and high calorific value (HCV) and low calorific value (LCV) are measured. This also determines if it necessary to add reagents (soda) to regulate pH and eliminate suspended solids. The mixture must comply with the specifications indicated by the cement industries (
Table 2), for which is necessary to add alkaline mixture. Alkalis are added in order to eliminate cyanide contamination in the aqueous effluent. The final fuel mixture is a mixture in which the viscosity has been reduced. In addition, it also meets specifications for mercury content, viscosity, humidity, and heat of combustion.
Table 3 shows the number of samples analyzed, the tons of waste extracted from the lagoon, and the waste’s calorific value throughout the entire extraction process (
Table 3). The year 2016 presented the largest volume of waste extraction, decreasing from that year onward because of the increase in the viscosity parameter.
The waste from the lagoon is not treated, but processed with the mixed waste batches by carrying out this process, as follows:
Phase separation: the treated waste is a mixture of water with hydrocarbons with a composition of 95% water, 3% solids, and 2% hydrocarbons. This mixture is an emulsion of water/oil or an emulsion of coolant. Through the addition of sulphuric acid, the emulsion is broken, and once the breakage is done, a decantation is carried out. Then 3% of the solids are taken to landfills outside the plant, 95% of the aqueous phase is transferred, if necessary, to a purification phase; if not necessary, it goes directly to the evapo-condensation process and biological treatment, and this is the last treatment process. From there, 2% of the light phase, if possible, proceeds to energy assessment.
Physical–chemical treatment: in the treatment, less waste is contributed to the alternative fuel line. This waste is the emulsion of oil/water in the modelling of metals; the light phase goes to the alternative fuel line to the biological treatment.
Evapo-condensation: this process requires significant amounts of energy, but combined vacuum systems are used to allow low-temperature distillations. It is a triple-effect process composed of three effects or deposits that have a liquid ring heat pump at the end of the third effect. In the first effect, heat is provided, and steam is taken out at 90 ºC; this goes into the second effect, where no additional heat is provided. In the third effect, the boiling temperature drops to 65 ºC, and no heat is provided then either. The compound treated in the evaporator is waste-contaminated with hydrocarbons. The flow can have 80–85% water, and if the hydrocarbon is light, its boiling temperature is 100 ºC or less. It evaporates with water that condenses and leaves distilled water, and if mixed with alcohol is condensed clean. The heavy phases of the hydrocarbons do not evaporate, so they remain in the concentrate that goes to the line of alternative fuels.
Biological treatment: mixing of waste with biodegradable wastewater, water from the agro-food industry, and leachates, with the aim of assimilating the suspended or dissolved substance present in wastewater using microorganisms.
With regards to the electricity consumption of the treatment equipment, the total consumption of the equipment used in the described process, and the proportional part in the case of the last three processes has been taken into account, since the use of these is not explicit and is strictly for the production of fuel from lagoon waste. Another source of electricity consumption is that of laboratory equipment, which is essential for controlling the production process and ensuring compliance with the parameters of admission of the final consumers of the alternative fuel.
Likewise, the heat of combustion has a decreasing value in the extraction series, which leads to a greater mixing with other waste in the process of transforming the waste into fuel (
Table 4).
Table 4 shows the percentage of the lagoon waste that is contained in the fuel transported to the cement factories, as well as the tons of fuel. These tons correspond to the aforementioned mixture of the lagoon waste with other waste. Thus, this fuel is made by mixing waste.
Although it is not related to the increase in waste extraction over the years, and although less was extracted in 2017, it has been observed that the percentage of waste once treated and converted into fuel has decreased over the years. This is because during the extraction process, the lagoon gets deeper, and the extracted material becomes more and more viscous, so in order to reduce its viscosity and meet the specifications set by the cement industries, it has been mixed with other waste. Another reason is that when some cement industries require a lower concentration of Hg in ppm, a higher addition of other waste is required. Thus,
Table 5 shows the distribution of fuel to the nine cement plants during the entire extraction period.
2.5. Environmental Specifications in the Cement Factories
In accordance with Directive 2000/76/EC on the incineration of waste [
38], in order to prevent, or if this is not practicable, limit negative effects on the environment through pollution by emissions into the atmosphere [
36] and effects on human health, the use of alternative fuel is chosen. To achieve this purpose, operational conditions and technical requirements are carried out, setting emission limit values for waste incineration and co-incineration plants [
39]. For this reason, the plants are designed, equipped, and constructed so that the exhaust gases do not exceed the established emission limit values in the long term (
Table 2).
With regard to the waste characteristics shown in the
Table 1, they must comply with the emission limit values established in
Table 2. These specifications do not vary from one cement plant to another, but the mercury content does. Therefore, mercury is one of the metals that is controlled in the composition of waste, and materials with low mercury content are selected [
40]. Cement plants usually limit it to 10 ppm, although some limit it to 1.5 ppm.
2.6. Energy Balance Assessment
An energy balance of the waste is carried out throughout the period of extraction from the lagoon, in order to know the consumed energy when the process of using the waste in a cement industry as fuel (
Consumed energyw2f) is carried (Equation (1), in kWh/t). This balance takes into account the gross energy provided by the waste (
Egross), the energy from the extraction of the waste (
Eextr), the energy in the transport—both from the lagoon to the treatment plant (
Et1) and from the treatment plant to each of the cement industries (
Et2)—and the treatment energy to convert the waste into fuel (
Etr). The energy gross is the energy used in the cement manufacturing process.
With regard to the energy balance in cases where the waste is taken directly to the hazardous waste incinerator (
Consumed energyincineration), the energy from the extraction of the waste and the transport energy from the lagoon to the incinerator (
Et) will be taken into account, so that the consumed energy in this case is calculated using Equation (2). Incineration facilities are comprised of a rotary kiln, with an energy production that is used only onsite for internally proposed uses, so there is no external energy use. For this reason, energy production in this facility outside the limit of the system is not considered in the present study. Incineration facilities are the unique in Spain because they can burn hazardous waste with this kind of composition.
In another case, petroleum coke is used as fuel in cement industry kilns, while waste is being eliminated without any use in the hazardous waste incinerator. In this case, waste to fuel the lagoon is a replacement for petroleum coke. The energy of the waste (
Egross) fed into the cement kilns has to be the same energy as the energy of the coke (
Ecoke) it replaces, as reflected in Equation (3):
Equation (4) shows the difference between the two case studies, and
Figure 4 shows it graphically.
Therefore, the case with the best energy efficiency is the waste-to-fuel case if the result of Equation (4) is positive; if negative, it would mean that the best energy efficiency case is the incineration case.
2.7. CO2 Emissions
In order to know the tons of CO
2 avoided, the two alternatives considered in previous sections will be compared. For this purpose, the following emission factors will be used for each energy consumption: an electricity emission factor (
EFelectricity)of 0.181 kg CO
2/kWh [
41] is used for the waste extraction and treatment phases, and a diesel emission factor (
EFdiesel) of 2.79 kg CO
2/l [
41] is used for the transport phase. Finally, a coke emission factor (
EFcoke) of 0.35748 kg CO
2/kWh [
42] is considered for the waste-to-fuel in both case studies. The dangerous waste analyzed would replace coke as fuel in the cement plants, where it would be considered as conventional fuel. For this reason, as a result of a coke reduction consumption, avoided CO
2 is also considered in the waste-to-fuel scenario.
In order to know the tons of CO
2 avoided (
Avoided CO2), the difference of both alternatives is calculated by means of Equation (5).
The tons of CO2 in the extraction phase are not taken into account, because they are the same in both situations.
3. Results
3.1. Consumed Energy: Waste to Fuel
With the data from
Table 3, the gross energy of the waste from
Table 6 is calculated. For this calculation, first the calorific value of the waste is obtained from the number of samples analyzed, changing the calorific value to kWh and dividing it by the quantity of waste extracted; from this, the gross energy I(
Egross) is obtained.
Table 7 shows the energy consumed during the process of waste extraction (
Eextraction) and the transport energy from the lagoon to the treatment center (
Etransport·1). Finally, the energy consumed during the process to treat and blend the extracted residue with other industrial residues (
Etreatment) has been assessed.
The pump that extracts the waste to the road tanker mainly explains the energy consumed in the extraction process (Eextraction). This pump, with a power of 45 kW, operates between 1.5 and 2.0 h to fill a road tanker (24 t). Then the residue is transferred to the treatment and transformation center—the distance between the lagoon and this center is 30 km. In this case, the fuel (diesel) used for this process is calculated (Etransport 1).
The energy treatment process (Etreatment) is assessed for all processes carried out in the treatment center. As a result, the energy per ton is obtained in the treatment process of waste-to-fuel mixture.
Table 8 shows the energy consumption in the transport from the treatment center to each one of the cement plants (
Etransport·2). For these calculations, it was taken into account that the road tankers were the same model used for each of the transports to the cement plants, so the difference in consumption is the distance from the treatment center to each one of the cement plants.
To calculate the transport from the treatment plant to each cement plant, the distance to be travelled, the liters of fuel consumed by the road tankers, the number of road tankers required for transport, the energy consumption of the road tankers, and the tons of waste to be transported were determined. As a result, the transport energy (Etransport·2), which is related to distance, was obtained for each cement plant.
Finally, using all the energies obtained and applying Equation (1), the consumed energies provided by the waste in each one of the plants were obtained, as was the global consumed energy of the whole process (
Table 9).
Therefore, it was possible to assess an average consumed energy value of 6156.55 kWh/twaste; the standard deviation was 50 kWh/twaste, and it is explained by the different distance between the treatment center and the nine cement plants. It was concluded that the entire process is viable in terms of energy input.
3.2. Consumed Energy: Waste Incineration
Table 10 shows the three energies needed to calculate the consumed energy of Equation (2). The calculations of this energies are carried out in the same way as those explained for
Table 6 and
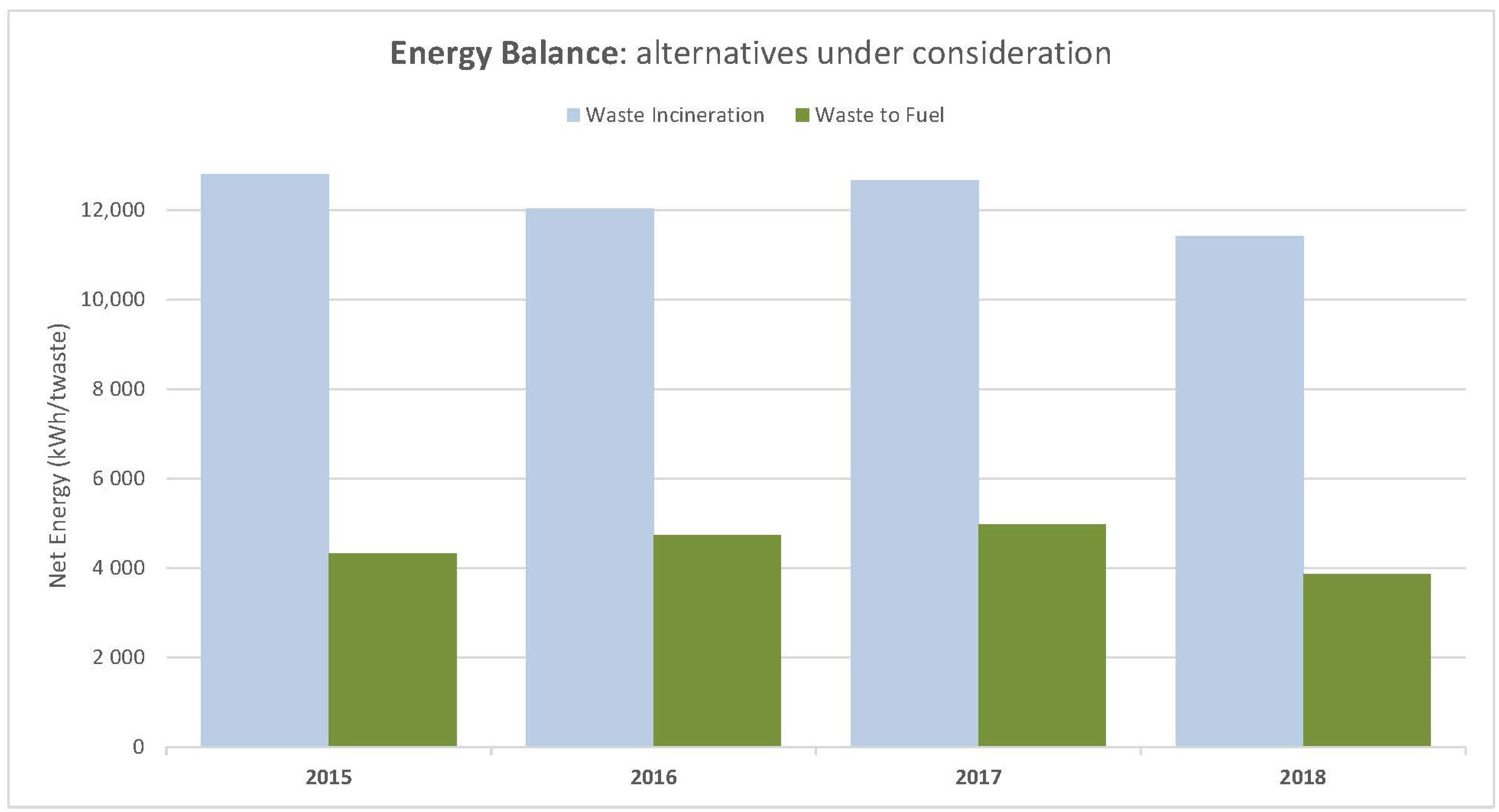
Table 7. Therefore, with these calculations, it is possible to know the average energy consumed from Equation (2), which in this case was 12,189.19 kWh/t. This result reflects that the waste-to-fuel case is more efficient, because the value is higher in the waste incineration case than in the waste-to-fuel case.
3.3. Comparison of the Two Cases: Waste-to-Fuel and Waste Incineration
Comparing the consumed energies of both alternatives (Equation (4) and
Table 11), it can be concluded that consumed energy in the proposed fuel mixture alternative is better than the incineration alternative, reflecting an average energy savings of 6032.65 kWh/t.
Figure 4 shows the results for each year for the two alternatives explained above, where the difference in energy is reflected when applying one of the other alternatives.
3.4. CO2 Emissions
The calculation of CO
2 emissions avoided was made by comparing the novel process of transforming waste into fuel, which is used in cement factories, and waste incineration as a conventional process (
Table 12).
Equation (5) resulted in 89,277.90 tCO2, which are the tons avoided by using the waste in the cement plants instead of burning the waste in the incinerator. The most significant CO2 emissions reduction can be found due to the replacement of coke in cement kilns, which means getting a second use of this hazardous waste. This is the basis of circular economy.
4. Conclusions
Dangerous waste is difficult to manage, and the process usually followed is destroying it in authorized incinerators. However, this residue may be an opportunity, if it would be possible to process it with other residues and transform it into fuel. In addition, the case study presented fulfills the criteria of the European Union, in which the hierarchized waste management strategies are reduction, reuse, and recycling. Waste-to-energy is considered a reuse strategy, and one of the most interesting options once the waste is generated.
Pumpable residue can be extracted from any settling pond, and after physical and energy content characterization, the waste might be mixed with other liquid residues to fulfill fuel conditions in a specific industry. Hydrocarbon residues have shown a high energy content; thus, they were given the opportunity in this study to mix with other, less energetic residues.
The old hydrocarbon settling pond in the municipality of Arganda has demonstrated the ability to transform a problem into an opportunity to reduce greenhouse gas (GHG) emissions in industry, by Directive 2003/87/EC. Consequently, the waste-to-fuel process implemented in the Arganda project is already decreasing CO2 emissions. This would mean economic implications in this regulated sector if CO2 EU rights continue to increase in cost. The European Union aims to be neutral in terms of CO2 emissions, so it would support this conclusion.
Even if the energy balance for each cement plan were positive, the highest energy consumption determined in this study was transport to the cement plants (99.8%). This would suggest a more favorable energy balance when a consumption factory is closer to the studied setting pond and transformation plant. This would be highlighted as the most remarkable limitation. Thus, some other close industries included in Directive 2003/87/EC could consider this fuel as an alternative to reduce GHG emissions.
This study states that waste-to-fuel processing is a feasible method, which could be implemented in other regions and in contaminated soils. Compared to the conventional alternative (incineration) and the new alternative (waste-to-fuel), it can be concluded that the most recent process avoided a net emission of 89,277.90 t CO2, with an average value of 22,319.40 t CO2 each year.
From the environmental point of view, the decontamination of the old hydrocarbon setting pond, considered potentially dangerous, is a net benefit for the environment, which contributes positively to the flora and fauna of the area.
These results could serve as a stimulus for the use of the non-pumpable waste that still exists in the lagoon (phase D, described in the section on Materials and Methods). The energy characterization phase, together with the estimation of the capacity and processing required for the transformation of waste into energy, will be the subject of further research.