Abstract
Resistance to chemotherapy and recurrence are major hurdles to treating hormone receptor-negative breast cancer. The crude extract and natural products obtained from medicinal plants are believed to be multitargeted and possess less toxicity as compared to synthetic compounds. The aerial parts and roots of Moricandia sinaica (Boiss.) Boiss were used to prepare the crude extracts in solvents of different polarities. Human breast cancer cell lines (MCF7 and MDA-MB-231), liver carcinoma (HepG2), and nontumorigenic cells of human origin (human umbilical vein endothelial cells (HUVEC)) were treated with a serial dilution of crude extracts obtained from the aerial and roots of Moricandia sinaica (Boiss.) Boiss. The methanol extract of the shoots exhibited a higher level of cytotoxicity against MDA-MB-231 cells than against any other cancer and nontumorigenic cells lines. Six new compounds were identified by gas chromatography–mass spectrophotometry analysis in the shoots extract of Moricandia sinaica (Boiss.) Boiss, and 2-Tridecen-1-ol was one of the major compounds that represent more than 35% of the extract. M-phase inducer phosphases 1 and 2 (CDC 25A and B) were identified as the specific protein target for 2-Tridecen-1-ol by the Swiss protein target prediction tool. In silico molecular docking showed the binding of 2-Tridecen-1-ol with CDC 25 B with a higher binding energy as compared to CDC 25A. The possible molecular mechanism of anticancer activity of Moricandia sinaica (Boiss.) Boiss in MDA-MB-231 breast cancer is through inhibition of M-phase inducer phosphatases 1 and 2 via 2-Tridecen-1-ol. Further investigations in breast cancer models are needed to explore the therapeutic potential of Moricandia sinaica (Boiss.) Boiss and 2-Tridecen-1-ol as an efficient remedy with a possibly less toxic approach to treat triple-negative breast cancer.
1. Introduction
Breast cancer is a predominant cancer among women [1], and over 2 million cases of breast cancer were reported worldwide in 2018 [2]. The expression of hormone receptors is a predictive feature in breast cancer treatment. Estrogen and progesterone receptor-positive breast cancers have a more favorable prognosis and are more responsive to anti-estrogen therapy than that of estrogen and progesterone receptor-negative breast cancer [3]. Ten to twenty percent of breast cancer cases are triple-negative breast cancers (TNBC). TNBC grows more aggressively, and women diagnosed with TNBC typically have a higher mortality ratio [4]. Moreover, there are very limited treatment options for TNBC, these mainly being chemotherapy and surgery, but the recurrence rate is higher compared to hormone receptor-positive breast cancers. Limited treatment options and resistance to cytotoxic drugs are major contributing factors to the poor prognosis of TNBC [5].
Cancer cells gradually develop resistance to chemotherapy, which poses a major problem in cancer treatment. Natural products from medicinal plants and dietary sources have shown promising results in cancer treatment when used either alone or in combination with conventional chemotherapy [6]. Herbal drugs are believed to possess less side effects that arise from conventional chemotherapy [7]. It has been shown that natural compounds often exert effects by targeting multiple pathways in cancer biology, and thus are more effective than single-target chemotherapy agents [8]. The natural products either alone or in combination with conventional anticancer drugs would be an effective remedy to treat breast cancer with minimum side effects. Combining natural compounds with conventional chemotherapy can produce positive outcomes; for example, the anticancer activity of doxorubicin was improved upon by co-treatment with genistein in drug-resistant human breast cancer MCF-7/Adr cells [9]. The supplementation of rosemary extracts with tamoxifen, trastuzumab, and paclitaxel increased the cytotoxicity and ER-α and HER2 receptors downregulation in multiple drug-resistant breast cancer cells [10].
Moricandia is a genus of plants of the family Brassicaceae, and many plants from this genus have been reported for their anticancer and antioxidant activities. The Moricandia genus has most likely originated in the Mediterranean Basin and then spread to North Africa, the Middle East, Central Asia, and Southern Europe in arid and semi-arid environments. They usually have upright and branched stems, and the leaves are without stipules, having a featherlike shape [11]. Eight species are currently recognized in this genus, some of which have been reported to have medicinal value, or are used as traditional folk medicines. Among all, Moricandia arvensis (L.) DC has been extensively studied. It is an ornamental plant that is also used in traditional cooking. The Decoctions of leaves and stem of Moricandia arvensis (L.) DC are employed to treat sexually transmitted bacterial infection (syphilis) [12,13]. The antigenotoxic and antimutagenic activities of extracts of M. arvensis and its sub-species “eu-arvensis”, which is found in the southern region of Tunisia, have been reported [14,15]. The in vivo analgesic, anti-inflammatory, and antipyretic properties M. sinaica have been reported quite recently [16]. Keeping in view the medicinal application of the plants from this genus, extensive phytochemical screening has been done and a large number of bioactive compounds have been identified from medicinally important plants of this genus. The methanol extract of M. arvensis contains a high amount of Quercetin and kaempferol [17]. The positive impact of quercetin and kaempferol on type 2 diabetes mellitus has been shown in experimental animal models [18,19]. Various sulfur compounds, glucosinolates and isothiocyanates, and indole glucosinolates polyphenols have been reported from Moricandia arvensis [20,21,22,23], Quercetin, and kaempferol from M. sinaica [16], four different types of triacytylated peonidin from the flowers of Moricandia ramburii Webb [24].
One of the major hurdles to treat TNBC is that they do not express hormone receptors that are normally used as targets to design anticancer therapies. Cell division cycle 25 (CDC25) phosphatases have been proposed as an efficient therapeutic target to treat TNBC quite recently [25]. CDC25 are a family of dual-specific protein phosphatases that activate cyclin-dependent kinases (CDKs) and regulate the eukaryotic cell cycle progression in a dose-dependent manner and are important targets for cancer therapy [26].
This study focuses on natural and dietary compounds, in order to identify new and more effective treatment strategies for TNBC. Two types of human breast cancer cell lines (MCF7 and MDA-MB-231) were used in this study. The breast cancer cell lines MCF7 and MDA-MB-231 provide a comparative insight into the action of a drug in hormone receptor-positive and hormone receptor-negative breast cancer, respectively. MCF-7 was isolated from a 69 year-old Caucasian woman in 1970 [27] having all three receptors, for estrogen, progesterone, and HER2 [28], while MDA-MB-231 does not express any of these receptors [29].
We have previously reported the anti-angiogenic activity of methanol extract of the stem and leaves of M. sinaica in zebrafish embryos and human umbilical vein endothelial cells and phytochemical constituents present in the stem and leaves extracts [30]. Here, we report the cytotoxicity of the extracts against human breast cancer cell lines (MDA-MB-231 and MCF7), human liver carcinoma (HepG2), and a normal cell line; human umbilical vein endothelial cells (HUVEC). The antioxidant potential of various extracts and phytochemicals is present in the shoots of M. sinaica. The Swiss protein target prediction tool (http://www.swisstargetprediction.ch/) was used to identify which protein(s) could be a possible target of the major compound present in the shoots of M. sinaica. The molecular docking approach was adopted to verify the in silico binding efficiency of major compounds to the target proteins.
2. Materials and Methods
2.1. Plant
The aerial parts of Moricandia sinaica (Boiss.) Boiss. were collected in March 2019 from the district of Riyadh, Saudi Arabia. After taxonomical identification, the specimen was deposited at the Herbarium of the College of Science, King Saud University, Riyadh, Saudi Arabia. The crude extraction of dried parts of M. sinaica was done by the Soxhlet extraction method as described previously [30].
2.2. Screening Crude Extracts for Anticancer Activity
The anticancer activity was measured by evaluation of the cell proliferation using the Vybrant-MTT Cell Proliferation Assay Kit (Cat # V13154 Invitrogen). Human cancer cell lines HepG2, MCF-7, MDA = MB-231, and HUVEC cells were seeded at 5 × 104 cells/well (in 100 μL of DMEM) in 96-well microplates. After 24 h incubation at 37 °C, different concentrations of each crude extract were added. Methanol (0.1%)-treated cells were considered as a mock control and untreated cells as a negative control. Doxorubicin was used as a positive control. Cells were incubated for 24 h, and 10 μL of the MTT solution (5 mg/mL) was added to each well and incubated for another 4 h. After the completion of incubation time, 1 mL of acidified isopropanol was added and incubated on a shaker for a further 10 min at room temperature to solubilize formazan product, and the color development was assayed at 570 nm using a microplate reader (BioTek, Elx-800, Winooski, VT, USA).
2.3. GC/MS Analysis
Bioactive compounds from different extracts of the M. sinaica plant were analyzed using GC/MS analysis (Perkin Elmer Clarus 600 gas chromatograph/mass spectrometer) (Turbomass, PerkinElmer, Inc., Waltham, MA, USA). Then, 1 μL of each extract was injected into the Elite-5MS column (30 m, 0.25 µm thickness, 0.25 µm internal diameter). Pure helium was used as the mobile phase (flow rate: 1 mL/min). The temperature program was as follows: Hold at 40 °C for 2 min, then heat to 200 °C at a rate of 5 °C/min and hold for 2 min, and then heat to 300 °C at a rate of 5 °C/min and hold for 2 min.
2.4. Estimation of Total Phenol and Flavonoid Contents
The total phenolic content of the extracts was determined using the Folin–Ciocalteu spectrophotometric reagent. The total flavonoid content of the three different extracts was determined by the aluminum chloride spectrophotometric method as previously described in [31].
2.5. Quantification of Apoptosis by Annexin V-FITC/(PI) Staining
MDA-MB231 cells (1 × 106) were grown in a 6-well culture plate for 24 h. The cells were treated with vehicle (methanol) as control or extracts in duplicate for 48 h. The cells were harvested by trypsin and washed twice with ice-cold PBS. The FITC Annexin V Apoptosis Detection Kit (Thermo Fisher Scientific, Waltham, CA, USA) was used by following the manufacturer’s instructions.
2.6. Cell Cycle Analysis
MDA-MB231 cells were treated as described above in Section 2.5, and both treated and control cells were washed with PBS and harvested by scraping. The cells were pelleted by centrifugation at 2000 rpm for 5 min and washed twice with PB and fixed in 70% cold ethanol. The cells were stained with PI solution (50 µg/mL) for 30 min at 37 °C in the dark. The stained cells were analyzed using a Beckman coulter LS600 flow cytometer.
2.7. Molecular Docking
AutoDock Vina 1.1.2 [32] was used to perform molecular docking. The three-dimensional structure of the catalytic domain of CDC25B was downloaded from the Protein Data Bank [1C25 and 1CWR]. The protein structure was cleaned by removing the water molecules and other ions/atoms using MGLTools 1.5.6. Nonpolar hydrogens were merged followed by addition of Kollman charges. The structure of the ligand (trans-2-tridecen-1-ol) was obtained from PubChem (CID: 5364949). The ligand was made flexible by “detect root” using MGLTools 1.5.6. Coordinates of the receptors and ligand were saved in pdbqt format. The analysis of the lowest-energy conformation was performed using Maestro 12.3, Discovery Studio 2020 Client, and PyMOL 2.23.
2.8. Statistics
Differences among groups were evaluated by one-way analysis of variance (ANOVA) followed by Dunnett’s t-test. Results were considered significant at p < 0.05. Origin (version 6.1052; Origin Lab Corp Northampton, MA, USA) was used for statistical analysis. IC50 values were calculated by Probit Analysis as indicated in [33]. For total phenolic and flavonoid content results, the data were expressed as mean ± standard error of the mean of triplicate experiments.
3. Results
3.1. Total Phenolic and Flavonoid Analysis
The MeOH extract of the shoots of M. sinaica exhibited the highest total phenolic content (259.7 ± 0.57 mg GAE/g powder), followed by the MeOH extract of leaves (171.2 ± 1.68 mg GAE/g powder). On the other hand, crude extract from the stem exhibited the lowest total phenolic content (96.5 ± 2.98 mg GAE/g powder) (Figure 1A). Similarly, crude extracts of the shoots and leaves exhibited the highest total flavonoid content (69.5 ± 1.07 mg and 57.7 ± 0.57 mg QE/g powder, respectively), while crude extract from the stem exhibited the lowest total flavonoid content (Figure 1B).
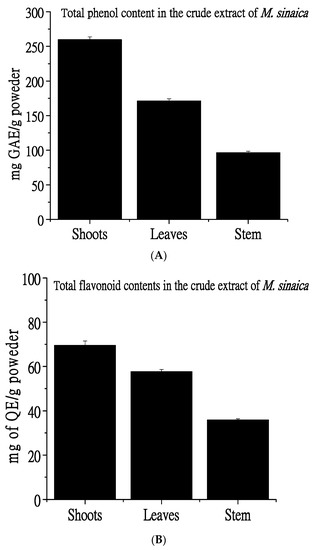
Figure 1.
Total phenol and flavonoid content. (A). The shoots exhibited the highest phenol and flavonoid contents, and the total phenolic contents of shoots, leaves, and stem extracts were 259.7 ± 0.57, 171.2 ± 1.68, and 96.5 ± 2.98 mg gallic acid equivalents (GAE)/gm. (B) Total flavonoid contents of shoots, leaves, and stem extract were 69.5 ± 1.07, 57.7 ± 0.57, and 35.9 ± 2.57 mg quercetin equivalent /g of the extract.
3.2. Methanol Extract of the Shoots of M. sinaica Selectively Perturbed Cell Survival of Breast Cancer MDA-MB-231 Cells
The cytotoxicity of methanol, ethyl acetate, and chloroform crude extracts prepared from the stem, leaves, and shoots of M. sinaica in human liver and breast cancer cell lines is shown in Table 1. The data indicate that crude extract prepared from the stem did not affect cell proliferation of HepG2 cells, and induced weak cytotoxicity in MDA and MCF7 cells. Crude extract from the leaves showed weak cytotoxicity (IC50 values ≥ 300 µg/mL) in HepG2 and MCF7 cells, but no cytotoxicity was observed in MDA-MB-231 cells. Similarly, the methanol and ethyl acetate extract of the shoots of M. sinaica did not exhibit cytotoxicity in HepG2 cells, and exhibited very weak cytotoxicity in MCF7 breast cancer cells. However, methanol, ethyl acetate, and chloroform extracts of the shoots of M. sinaica demonstrated strong cytotoxicity in MDA-MB-231 cells with IC50 values of 114.32, 148.93 and 143.98 µg/mL, respectively. M. sinaica was not toxic to normal cells, except the methanol extract of the leaves, which affected the cell survival of HUVEC with an IC50 value of 245.34 µg/mL. Doxorubicin is a known anti-cancer agent that was used as positive control in this evaluation. Doxorubicin affected the cell survival of all cancer cell lines with IC50 values less than 10 µM, and also showed strong toxicity in normal HUVEC cells with IC50 values of 3.14 µM (Table 1).

Table 1.
The effect of crude extract of plant parts of M. sinaica on cell survival of human cancer and normal cell lines.
3.3. MeOH Extract of the Shoots of M. sinaica-Induced Apoptosis in MDA-MB-231 Cells
MDA-MB-231 cells were treated with sub-IC50 concentrations (14 μg/mL) of MeOH extracts shoots. These cells demonstrated increased Annexin-V FITC/PI-staining as compared to mock (methanol, 0.55% v/v)-treated control cells (Figure 2). The percentage of early and late apoptotic cells upon treatment were increased to 13 ± 0.5% and 5.6 ± 0.3%, respectively, upon treatment, as compared to controls (1.1 ± 0.2% and 1.7% ± 0.3%, respectively), in mock-treated control cells (Figure 2).
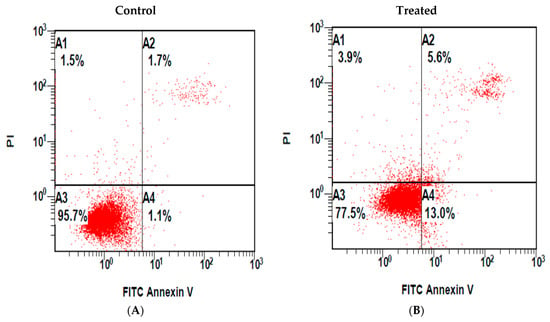
Figure 2.
Detection of apoptosis by Annexin V-FITC/PI staining in methanol extract-treated cells. MDA-MB-231 cells were incubated for 24 h (A) without (control) and (B) with the methanol extract of shoots of M. sinaica and stained with both FITC and PI. Quadrant (A1) represents the necrotic dead cells, (A2) shows the late apoptosis cells, (A3) viable cells, and (A4) indicates early apoptosis.
3.4. Methanol Extract of the Shoots of M. sinaica-Induced G1 Cell Cycle Arrest in MDA-MB-231 Cells
Cell cycle deregulation is a hallmark of cancer; therefore, we subsequently explored whether crude methanol extracts of M. sinaica were able to disrupt MDA-MB231 cell cycle progression by flow cytometry and PI staining. We showed that methanol extract of the shoots of M. sinaica induced G1 cell cycle arrest in MDA-MB-231 cells, as indicated by the increased proportion of cells at the G1 phase (66.1 ± 1.5%) compared with the control group (60.29 ± 1.2%). The results also showed that the percentage of cells at the G2/M phase were significantly decreased to 17.10 ± 1.2% compared with untreated cells (23.40 ± 1%) (Figure 3).
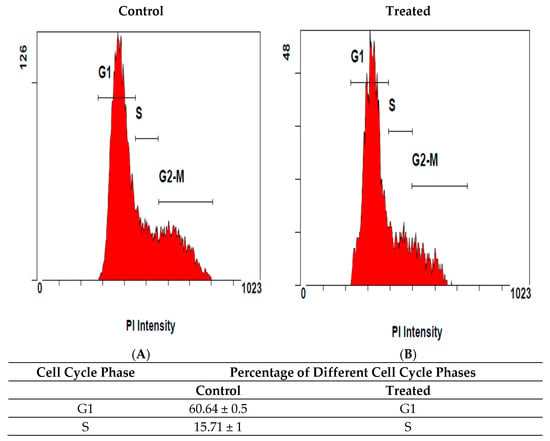
Figure 3.
Cell cycle distribution profiles for the effects of the methanol extract of shoots of M. sinaica on MDA-MB-231. Cells were incubated for 24 h (A) without (control) and (B) with the methanol extract of shoots of M. sinaica and stained with PI. Following Fluorescence Activated Cell Sorting (FACS) analysis, DNA histograms were analyzed using CXP software V. 3.0. Data represent mean ± S.D. and the difference was statistically significant at (p < 0.05) compared to control.
3.5. Identification of Phytochemicals and In Silico Molecular Docking Studies
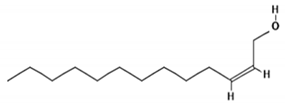
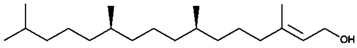
Gas chromatography coupled with mass spectrophotometry was used to identify phytochemicals present in crude extract of the shoots of M. sinaica, and the data are presented in Table 2. The major identified compound was 2-Tridecen-1-ol, which represents 35% of the crude extract, followed by 1,2-benzenedicarboxylic acid (19%); and hexadecanoic acid (17.4%).

Table 2.
Photochemical present in MeOH extract of shoots of M. sinaica.
In order to investigate the possible mechanism through which the crude extract of shoots of M. sinaica exert the anticancer activity, it is very important to determine the protein targets for each of these major compounds. The chemical structure for each compound was deduced from the PubChem database, and potential protein targets were obtained using an online protein target prediction tool [17]. The target prediction report for 2-Tridecen-1-ol is presented in Table S1. M-phase inducer phosphatases 1 (CDC25A; uniport ID P30304) and M-phase inducer phosphatases 2 (CDC25B; uniport ID P30305) were identified as the proteins target for molecule “2-Tridecen-1-ol” with the highest probability. Table S2 represents the target prediction report for 1,2-benzenedicarboxylic acid. Tyrosyl-DNA phosphodiesterase 1 (TDP1; uniport port ID Q9NUW8) was identified as a possible protein target for molecule 1,2-benzenedicarboxylic acid with the highest probability among other predicted protein targets. The protein target report for hexadecanoic acid is shown in Table S3. Various tissue-specific fatty acid-binding proteins have been predicted with the highest % probability to be the protein target for hexadecanoic acid.
In silico molecular docking was used to find out that the binding of 2-Tridecen-1-ol might bind to the active domain of CDC25A and CDC25B. The three-dimensional structure of the catalytic domain of the human CDC25A (IC25) and CDC25 B (ICWR) was downloaded from RCSB protein data bank (https://www.rcsb.org/). Molecular docking yielded nine different conformations in order of increasing binding energy. The docking result of the ligand with the lowest binding energy is analyzed here. The lowest binding energy for 1C25 and 1CWR was found to be −4.2 and −4.4 kcal/mol, respectively. The docked structure of trans-2-tridecen-1-ol and 1C25 is shown in Figure 4A. Glu378 and Phe379 formed hydrogen bonds with 1C25 at a distance of 2.26 and 2.63 Å, respectively. Other amino acids such as Val367, Ile376, and Ile381 are also involved in the binding through hydrophobic interactions. The docked trans-2-tridecen-1-ol in structure 1CWR is shown in Figure 4B. The ligand interacted at the hydrophobic region of the protein, and Leu398, Cys484, Arg485, Arg488, and Met405 were involved in this protein–ligand complex formation.
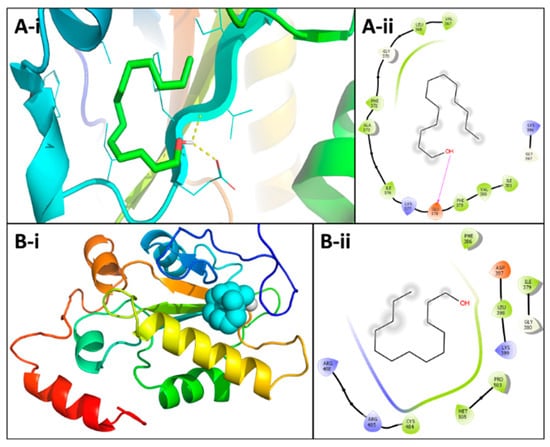
Figure 4.
Molecular docking (A-i). The mode of interaction of compound 2-tridecen-1-ol in the ac-tive region of CDC25B (A-ii). The ligand 2-tridecen-1-ol (green colored) (B-i) and the important residues in the active site of the enzyme are presented by a tube model (B-ii).
4. Discussion
CDC25 phosphatases are rate-limiting in tumorigenesis, and are overexpressed in a variety of human cancers, including breast cancer [34]. CDC25A and CDC25B have also been used as molecular targets in breast cancer treatments [25,35]. Primary breast tumors overexpressed Cdc25A, which correlated with increased levels of cyclin-dependent kinase 2 (Cdk2) enzymatic activity, suggesting that both Cdc25A and Cdk2 are suitable therapeutic targets in early-stage breast cancer [36]. The inhibition of CDC25 alone or in combination with other drugs was also proposed as a therapeutic treatment for triple-negative breast cancer by one recent study [37]. The in-silico protein target prediction report suggested Tyrosyl-DNA phosphodiesterase 1 (TDP1; uniport ID: Q9NUW8) as a putative target for the second major molecule (1,2-benzenedicarboxylic acid). TDP1 has been reported to be up-regulated in more than 90% of breast tumors tissues by screening tumor tissue arrays; moreover, inhibition of TDP1 has been suggested as an effective combination therapy for breast cancer with camptothecin [38]. 2-Tridecen-1-ol was identified as one of the major constituents present in the MeOH extracts of shoots of M. sinaica. 2-Tridecen-1-ol has been reported to be present in the crude extract of other medicinal plants such as Cadaba fruticosa (L.) Druce [39] and Rumex hastatus D. [40]. In order to search for the mechanism of action, we ask the question of how the crude extract of the shoots of M. sinaica exerted its anticancer activity in breast cancer cells. The putative protein targets for the active molecules present in the extract were investigated by in silico studies. The online protein target prediction tool [41] was used to identify which proteins might be the target binder for the three major compounds present in the crude extracts of the shoots of M. sinaica. The Swiss protein prediction investigation has revealed that M-phase inducer phosphatases 1 and 2 (CDC25A and CDC25B) are specific protein targets of 2-tridecen-1-ol. Moreover, the molecular docking simulations also showed that molecule 2-tridecen-1-ol could fit within the active site of both phosphatases with the lowest binding energy of −4.2 and −4.4 kcal/mol for CDC25A and CDC25B, respectively. The ligand (2-tridecen-1-ol) interacted at the hydrophobic region of the protein residues: Leu398, Cys484, Arg485, Arg488, and Met405, which were involved in the protein–ligand complex formation. The same region was reported as an active binding site for the inhibitory activity of these enzymes by other molecular docking studies [26,42].
The anticancer activity of crude extracts from various plants of genus Moricandia have been reported previously. The aerial parts and roots of Moricandia nitens exhibited significant cytotoxicity against human colon (HCT) and liver (HepG2) cancer cell lines, with IC50 values in the micro-molar range [43]. The anticancer activity of Moricandia nitens against human chronic myelogenous leukemia cell line K562 and chloroform extract of Moricandia arvensis in B16-F0 melanoma cells has been reported [23,44].
M. sinaica is a herbaceous plant that is native to Saudi Arabia and Pakistan [45]. However, its biological properties and anticancer potential are largely unexplored. The suppression of tumor cell proliferation by certain medicinal and dietary plants has previously been attributed to the presence of phenols and flavonoids [46,47]. The cytotoxic effects of methanol leaf extract of Moricandia arvensis in colorectal cancer cells has also been demonstrated to be related to the antioxidant activity of phenol and flavonoids [12,13]. The incidence of colon and breast cancer in certain populations has been recorded as very low; upon further analysis, it was found that those people consume certain diets, which were high in flavonoids and lignans [48]. Higher amounts of phenol and flavonoids contents were found in the shoots of M. sinaica as compared leaves and stems. The crude extracts prepared from the shoots of M. sinaica showed a significant level of cytotoxicity in human breast cancer cell lines in this study, which could be correlated to the higher amounts of phenolic and flavonoid contents in this extract. The antioxidant activities of M. Sinaica were also reported by another study quite recently, which show the EC50 values of 12.31 ± 0.15 µg/mL in the DPPH assay. Moreover, the aerial part also contained a high amount of total phenolic and flavonoid contents [16]. Hence, the high amount of phenol and flavonoid contents that are reported in this study agree with already published data.
The cell cycle is the result of the tightly regulated event that controls the proper division of a parent cell into two daughter cells. Understanding the molecular events responsible for cell cycle checkpoints remains a target in anticancer drug design [49]. Methanol extract of the shoots of M. sinaica induced G1 cell cycle arrest in MDA-MB-231 cells. Many medicinal plant-based anticancer products or extracts exercise their anticancer effect by altering the cell cycle and subsequent induction of apoptosis in cancer cells [50].
Breast tumors expressed a higher concentration of hypoxia-inducible factor (HIF-1) α than normal breast tissue [51]. Furthermore, breast tumors harbor increased tumor angiogenesis, providing a solid justification for antiangiogenic therapies in early, locally advanced, and metastatic breast cancer [31]. Suppression of the proliferation of breast cancer cell lines by the crude extract of M. sinaica, shown in this study, may be due to its antiangiogenic activity, which has been previously reported in transgenic zebrafish embryos.
5. Conclusions
Triple-negative breast cancers (TNBC) are more aggressive in nature and are difficult to treat. In order to treat TNBC more effectively, a multiple-anticancer-targets approach is required. The results obtained from this study suggest that the MeOH extract of the shoots of M. sinaica efficiently and selectively inhibited the proliferation of MDA-MB-231 breast cancer cells by inducing apoptosis, and cell cycle arrest. The major biological constituent, 2-tridecen-1-ol, might be an inhibitor of the enzymatic activity of CDC25 phosphatases, which resulted in the G1/M cell cycle. Further investigations in established breast cancer models are ongoing in our lab to explore the therapeutic potential of Moricandia sinaica (Boiss.) Boiss as an efficient and safe therapy to treat triple-negative breast cancer.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/11/3/1244/s1. Table S1: Swiss protein target prediction report for compound “2-Tridecen-1-ol”. Table S2: Swiss protein target prediction report for compound 1,2-benzenedicarboxylic. Table S3: Swiss protein target prediction report for compound “hexadecanoic acid”.
Author Contributions
Conceptualization, M.F.K. and M.A.M.W.; methodology, M.F.K., F.A.N. and A.A.B.; validation, M.F.K. and F.A.N.; investigation, M.F.K. and M.A.M.W.; resources, M.F.K. and M.A.M.W.; data curation, A.A.B.; writing—original draft preparation, M.F.K. and F.A.N.; writing—review and editing, M.F.K., F.A.N., A.S.A. and M.A.M.W.; supervision, M.F.K.; project administration, M.F.K.; funding acquisition, M.F.K., A.S.A. and M.A.M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Deanship of Scientific Research, King Saud University through the Vice Deanship of Scientific Research Chairs. The funding body had NO role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data used and/or analyzed during the current study will be available from the corresponding author on reasonable request.
Acknowledgments
The authors thank the Research support unit (RSSU) at the Deanship of Scientific Research, King Saud University for their technical support.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Yang, V.; Gouveia, M.J.; Santos, J.; Koksch, B.; Amorim, I.; Gartner, F.; Vale, N. Breast cancer: Insights in disease and influence of drug methotrexate. RSC Med. Chem. 2020, 11, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Mosly, D.; Turnbull, A.; Sims, A.; Ward, C.; Langdon, S. Predictive markers of endocrine response in breast cancer. World J. Exp. Med. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.R.; Brown, M.; Cress, R.D.; Parise, C.A.; Caggiano, V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer Registry. Cancer 2007, 109, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Nedeljkovic, M.; Damjanovic, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Li, A.; Inagaki, Y.; Gao, J.; Li, J.; Kokudo, N.; Li, X.K.; Tang, W. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci. Trends 2010, 4, 297–307. [Google Scholar]
- Liao, G.S.; Apaya, M.K.; Shyur, L.F. Herbal medicine and acupuncture for breast cancer palliative care and adjuvant therapy. Evid. Based Complement. Altern. Med. 2013, 2013, 437948. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, Y.; Li, Y.; Xu, D.P.; Li, S.; Li, H.B. Spices for Prevention and Treatment of Cancers. Nutrients 2016, 8, 495. [Google Scholar] [CrossRef]
- Xue, J.P.; Wang, G.; Zhao, Z.B.; Wang, Q.; Shi, Y. Synergistic cytotoxic effect of genistein and doxorubicin on drug-resistant human breast cancer MCF-7/Adr cells. Oncol. Rep. 2014, 32, 1647–1653. [Google Scholar] [CrossRef]
- Gonzalez-Vallinas, M.; Molina, S.; Vicente, G.; Sanchez-Martinez, R.; Vargas, T.; Garcia-Risco, M.R.; Fornari, T.; Reglero, G.; Ramirez de Molina, A. Modulation of estrogen and epidermal growth factor receptors by rosemary extract in breast cancer cells. Electrophoresis 2014, 35, 1719–1727. [Google Scholar] [CrossRef]
- Pratap, A.; Gupta, S.K. Biology and Ecology of Wild Crucifers; Gupta, S.K., Ed.; CRC Press: Boca Raton, FL, USA; Tayler and Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Skandrani, I.; Leloup, L.; Kovacic, H.; Dijoux-Franca, M.G.; Ghedira, K.; Chekir Ghedira, L. Antioxidant, Antimutagenic, Tanning and Calpain Induction Activities of Methanolic Extract of Tunisian Plant (Moricandia Arvensis). Iran. J. Pharm. Res. 2017, 16, 119–134. [Google Scholar] [PubMed]
- Skandrani, I.; Sghaier, M.B.; Neffati, A.; Boubaker, J.; Bouhlel, I.; Kilani, S.; Mahmoud, A.; Ghedira, K.; Chekir-Ghedira, L. Antigenotoxic and free radical scavenging activities of extracts from Moricandia arvensis. Drug Chem. Toxicol. 2007, 30, 361–382. [Google Scholar] [CrossRef]
- Skandrani, I.; Limem, I.; Neffati, A.; Boubaker, J.; Ben Sghaier, M.; Bhouri, W.; Bouhlel, I.; Kilani, S.; Ghedira, K.; Chekir-Ghedira, L. Assessment of phenolic content, free-radical-scavenging capacity genotoxic and anti-genotoxic effect of aqueous extract prepared from Moricandia arvensis leaves. Food Chem. Toxicol. 2010, 48, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Skandrani, I.; Bouhlel, I.; Limem, I.; Boubaker, J.; Bhouri, W.; Neffati, A.; Ben Sghaier, M.; Kilani, S.; Ghedira, K.; Ghedira-Chekir, L. Moricandia arvensis extracts protect against DNA damage, mutagenesis in bacteria system and scavenge the superoxide anion. Toxicol. Vitro 2009, 23, 166–175. [Google Scholar] [CrossRef] [PubMed]
- El-Mekkawy, S.; Shahat, A.A.; Alqahtani, A.S.; Alsaid, M.S.; Abdelfattah, M.A.O.; Ullah, R.; Emam, M.; Yasri, A.; Sobeh, M. A Polyphenols-Rich Extract from Moricandia sinaica Boiss. Exhibits Analgesic, Anti-Inflammatory and Antipyretic Activities In Vivo. Molecules 2020, 25, 5049. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Morrone, F.; Argentieri, M.P.; Gambacorta, L.; Conforti, F.; Avato, P. Phytochemical and Biological Profile of Moricandia arvensis (L.) DC.: An Inhibitor of Pancreatic Lipase. Molecules 2018, 23, 2829. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol—A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. Foods 2017, 30, 203–219. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Belkhiri, A.; Lockwood, G.B. An indole derivative and glucosinolates from Moricandia arvensis. Phytochemistry 1990, 29, 1315–1316. [Google Scholar] [CrossRef]
- Braham, H.; Mighri, Z.; Jannet, H.B.; Matthew, S.; Abreu, P.M. Antioxidant phenolic glycosides from Moricandia arvensis. J. Nat. Prod. 2005, 68, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Skandrani, I.; Boubaker, J.; Bhouri, W.; Limem, I.; Kilani, S.; Ben Sghaier, M.; Neffati, A.; Bouhlel, I.; Ghedira, K.; Chekir-Ghedira, L. Leaf extracts from Moricandia arvensis promote antiproliferation of human cancer cells, induce apoptosis, and enhance antioxidant activity. Drug Chem. Toxicol. 2010, 33, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Tatsuzawa, F.; Ito, K.; Muraoka, H.; Namauo, T.; Kato, K.; Takahata, Y.; Ogawa, S. Triacylated peonidin 3-sophoroside-5-glucosides from the purple flowers of Moricandia ramburii Webb. Phytochemistry 2012, 76, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Granieri, L.; Shrestha, M.; Wang, D.Y.; Vorobieva, I.; Rubie, E.A.; Jones, R.; Ju, Y.; Pellecchia, G.; Jiang, Z.; et al. Identification of CDC25 as a Common Therapeutic Target for Triple-Negative Breast Cancer. Cell Rep. 2018, 23, 112–126. [Google Scholar] [CrossRef]
- Kabakci, Z.; Kappeli, S.; Cantu, C.; Jensen, L.D.; Konig, C.; Toggweiler, J.; Gentili, C.; Ribaudo, G.; Zagotto, G.; Basler, K.; et al. Pharmacophore-guided discovery of CDC25 inhibitors causing cell cycle arrest and tumor regression. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Lee, A.V.; Oesterreich, S.; Davidson, N.E. MCF-7 cells--changing the course of breast cancer research and care for 45 years. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef]
- Farooq, M.; Nasr, F.A.; Almoutiri, N.D.; Al-yahya, N.; Wadaan, M.A.; Abutaha, N. The phytochemical screening and antiangiogenic activity of audthan al himar (Moricandia sinaica Boiss.) extracts in zebrafish embryos and human umbilical vein endothelial cells. J. King Saud Univ. Sci. 2020, 32, 2370–2376. [Google Scholar] [CrossRef]
- Aref, H.L.; Karima, B.H.S.; Fekih, A.; Chemli, R.; Mars, M.; Aouni, M.; Chaumon, J.P.; Said, K. Variability in antimicrobial activity of latex from two varieties of Ficus carica. Afr. J. Microbiol. Res. 2011, 5, 1361–1367. [Google Scholar]
- Trott, O.; Olson, A.J. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Akçay, A. The Calculation of LD50 Using Probit Analysis. FASEB J. 2013, 27. [Google Scholar] [CrossRef]
- Beillerot, A.; Battaglia, E.; Bennasroune, A.; Bagrel, D. Protection of CDC25 phosphatases against oxidative stress in breast cancer cells: Evaluation of the implication of the thioredoxin system. Free Radic. Res. 2012, 46, 674–689. [Google Scholar] [CrossRef] [PubMed]
- Cangi, M.G.; Cukor, B.; Soung, P.; Signoretti, S.; Moreira, G.; Ranashinge, M.; Cady, B.; Pagano, M.; Loda, M. Role of the Cdc25A phosphatase in human breast cancer. J. Clin. Investig. 2000, 106, 753–761. [Google Scholar] [CrossRef]
- Zacksenhaus, E.; Liu, J.C.; Granieri, L.; Vorobieva, I.; Wang, D.Y.; Ghanbari-Azarnier, R.; Li, H.Q.; Ali, A.; Chung, P.E.D.; Ju, Y.J.; et al. CDC25 as a common therapeutic target for triple-negative breast cancer—The challenges ahead. Mol. Cell Oncol. 2018, 5. [Google Scholar] [CrossRef]
- Dean, R.A.; Fam, H.K.; An, J.H.; Choi, K.H.; Shimizu, Y.; Jones, S.J.M.; Boerkoel, C.F.; Interthal, H.; Pfeifer, T.A. Identification of a Putative Tdp1 Inhibitor (CD00509) by in Vitro and Cell-Based Assays. J. Biomol. Screen. 2014, 19, 1372–1382. [Google Scholar] [CrossRef]
- Chen, C.L.; Chu, J.S.; Su, W.C.; Huang, S.C.; Lee, W.Y. Hypoxia and metabolic phenotypes during breast carcinogenesis: Expression of HIF-1 alpha, GLUT1, and CAIX. Virchows Arch. 2010, 457, 53–61. [Google Scholar] [CrossRef]
- Murugesan Amudha, S.R. Assessing the Bioactive Constituents of Cadaba Fruticosa (L.) Druce Through Gc-Ms. Int. J. Pharm. Pharm. Sci. 2014, 6, 3. [Google Scholar]
- Ahmad, S.; Ullah, F.; Sadiq, A.; Ayaz, M.; Imran, M.; Ali, I.; Zeb, A.; Ullah, F.; Shah, M.R. Chemical composition, antioxidant and anticholinesterase potentials of essential oil of Rumex hastatus D. Don collected from the North West of Pakistan. BMC Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Evain-Bana, E.; Schiavo, L.; Bour, C.; Lanfranchi, D.A.; Berardozzi, S.; Ghirga, F.; Bagrel, D.; Botta, B.; Hanquet, G.; Mori, M. Synthesis, biological evaluation and molecular modeling studies on novel quinonoid inhibitors of CDC25 phosphatases. J. Enzym. Inhib. Med. Chem. 2017, 32, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Moaty, H.I. Bioactive Compounds of Moricandia Nitens and Its Anticancer Effect. Indo Am. J. Pharm Sci. 2016, 3, 1283–1290. [Google Scholar] [CrossRef]
- Aidi Wannes, W.; Saidani Tounsi, M.; Marzouk, B. A review of Tunisian medicinal plants with anticancer activity. J. Complement. Integr. Med. 2017, 15. [Google Scholar] [CrossRef]
- Perfectti, F.; Gomez, J.M.; Gonzalez-Megias, A.; Abdelaziz, M.; Lorite, J. Molecular phylogeny and evolutionary history of Moricandia DC (Brassicaceae). PeerJ 2017, 5, e3964. [Google Scholar] [CrossRef] [PubMed]
- Bhagya, N.; Chandrashekar, K.R. Identification and quantification of cytotoxic phenolic acids and flavonoids in Ixora brachiata by UHPLC-DAD and UHPLC-ESI-MS/MS. Int. J. Mass Spectrom. 2020, 450. [Google Scholar] [CrossRef]
- Paudel, M.R.; Chand, M.B.; Pant, B.; Pant, B. Assessment of Antioxidant and Cytotoxic Activities of Extracts of Dendrobium crepidatum. Biomolecules 2019, 9, 478. [Google Scholar] [CrossRef]
- Taraphdar, A.; Roy, M.; Bhattacharya, R. Natural products as inducers of apoptosis: Implication for cancer therapy and prevention. Curr. Sci. 2001, 80, 1387–1396. [Google Scholar]
- Jackson, R.C.; Di Veroli, G.Y.; Koh, S.B.; Goldlust, I.; Richards, F.M.; Jodrell, D.I. Modelling of the cancer cell cycle as a tool for rational drug development: A systems pharmacology approach to cyclotherapy. PLoS Comput. Biol. 2017, 13. [Google Scholar] [CrossRef]
- Saleh, K.A.; Albinhassan, T.H.; Elbehairi, S.E.I.; Alshehry, M.A.; Alfaifi, M.Y.; Al-Ghazzawi, A.M.; Al-Kahtani, M.A.; Alasmari, A.D.A. Cell Cycle Arrest in Different Cancer Cell Lines (Liver, Breast, and Colon) Induces Apoptosis under the Influence of the Chemical Content of Aeluropus lagopoides Leaf Extracts. Molecules 2019, 24, 507. [Google Scholar] [CrossRef]
- Uzzan, B.; Nicolas, P.; Cucherat, M.; Perret, G.Y. Microvessel density as a prognostic factor in women with breast cancer: A systematic review of the literature and meta-analysis. Cancer Res. 2004, 64, 2941–2955. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).