Analytical Pyrolysis of the Fungal Melanins from Ochroconis spp. Isolated from Lascaux Cave, France
Abstract
1. Introduction
2. Materials and Methods
3. Results
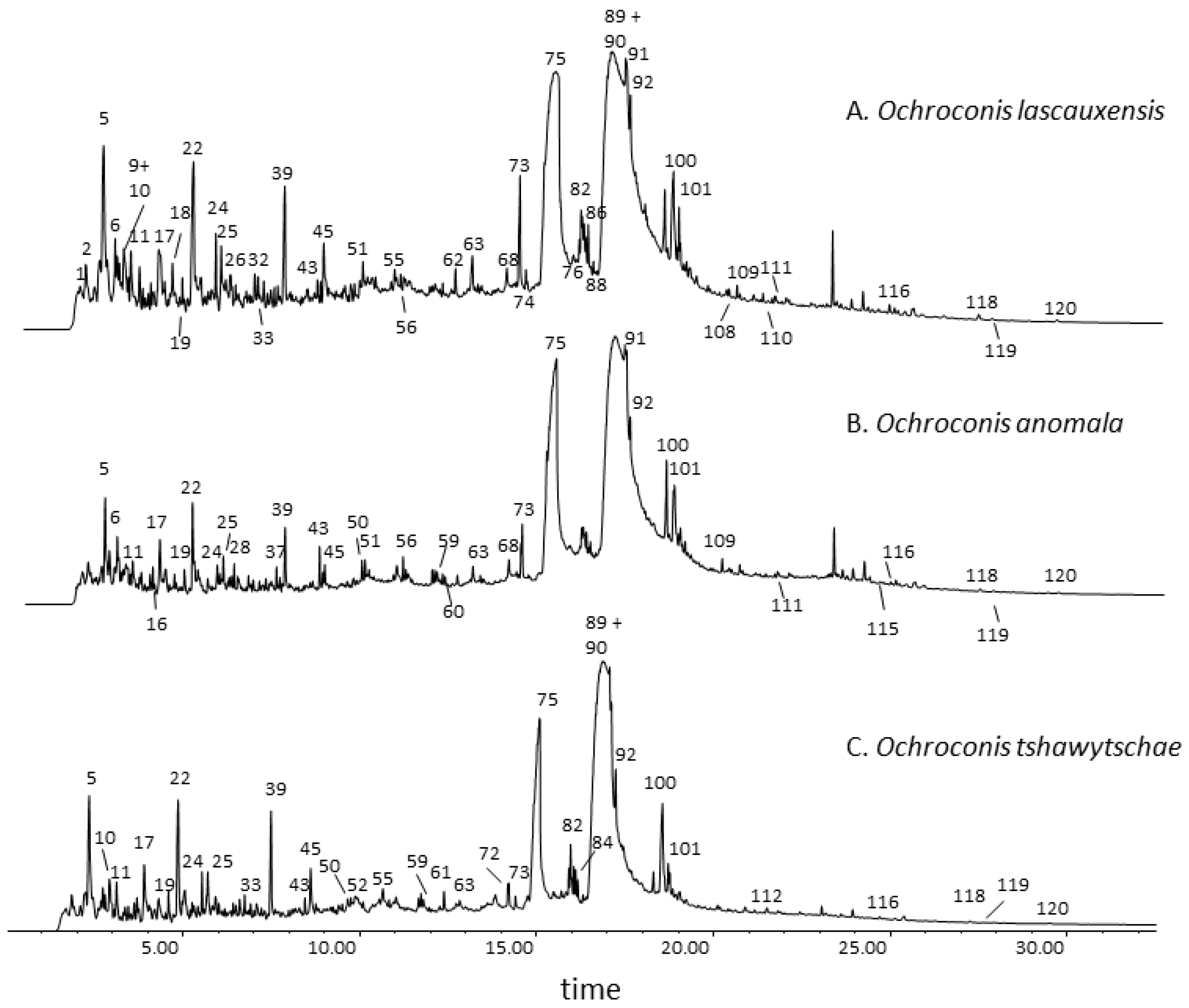
3.1. Pyrolysis-Gas Chromatography–Mass Spectrometry of the Melanins from Three Ochroconis Species
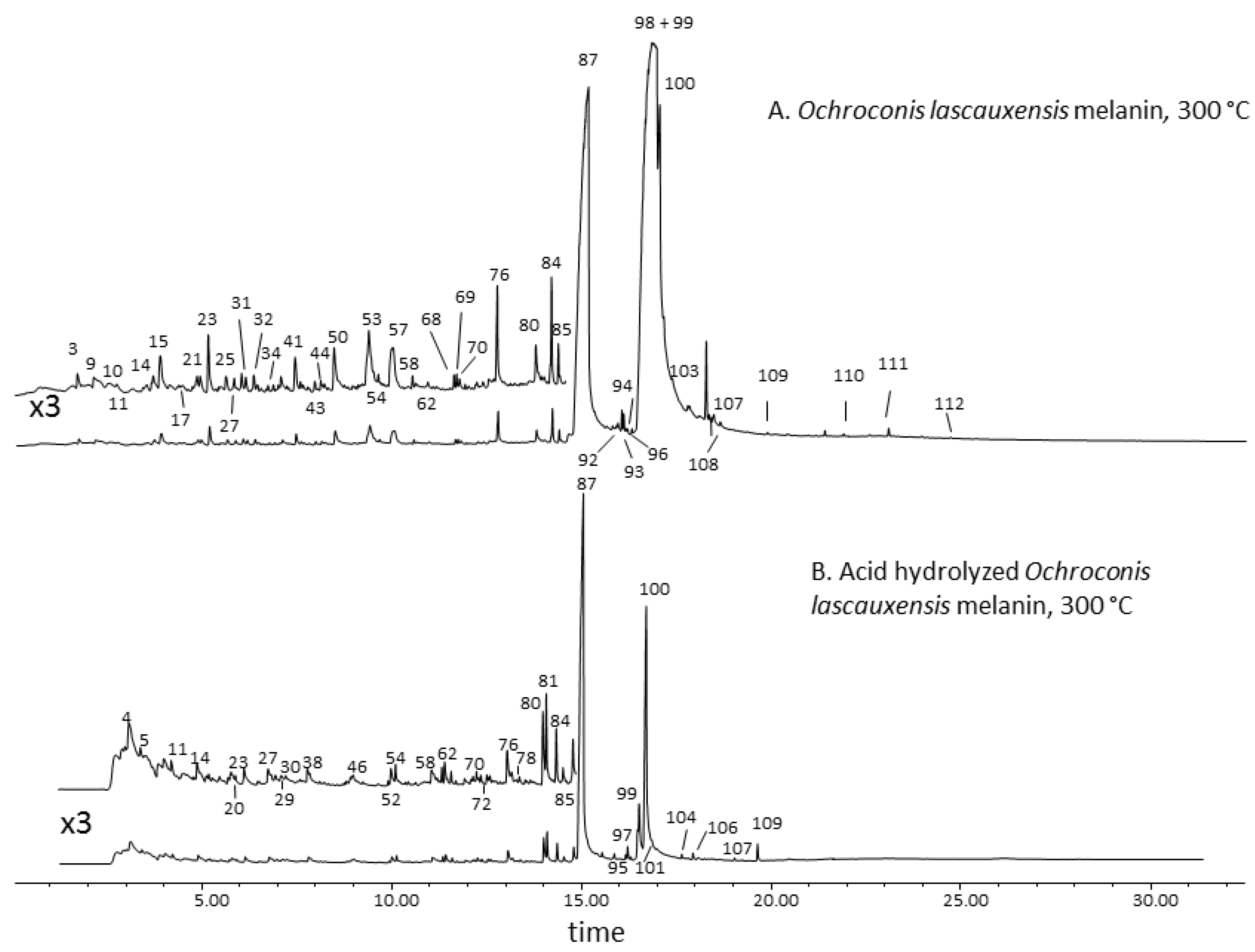
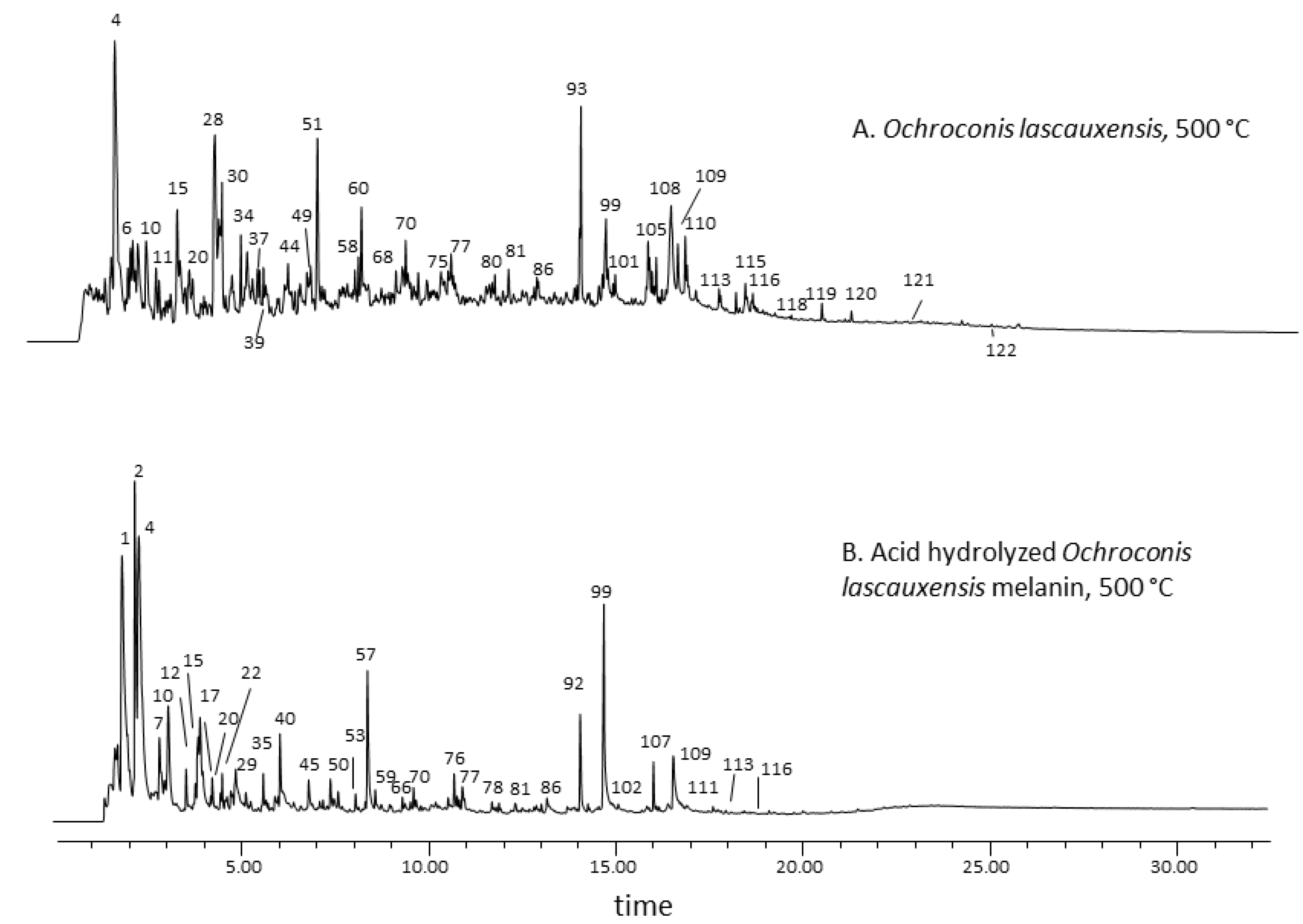
3.2. Thermal Desorption-Gas Chromatography–Mass Spectrometry of the Melanin from Ochroconis lascauxensis
3.3. Second Shot Pyrolysis-Gas Chromatography–Mass Spectrometry of the Melanin from Ochroconis lascauxensis
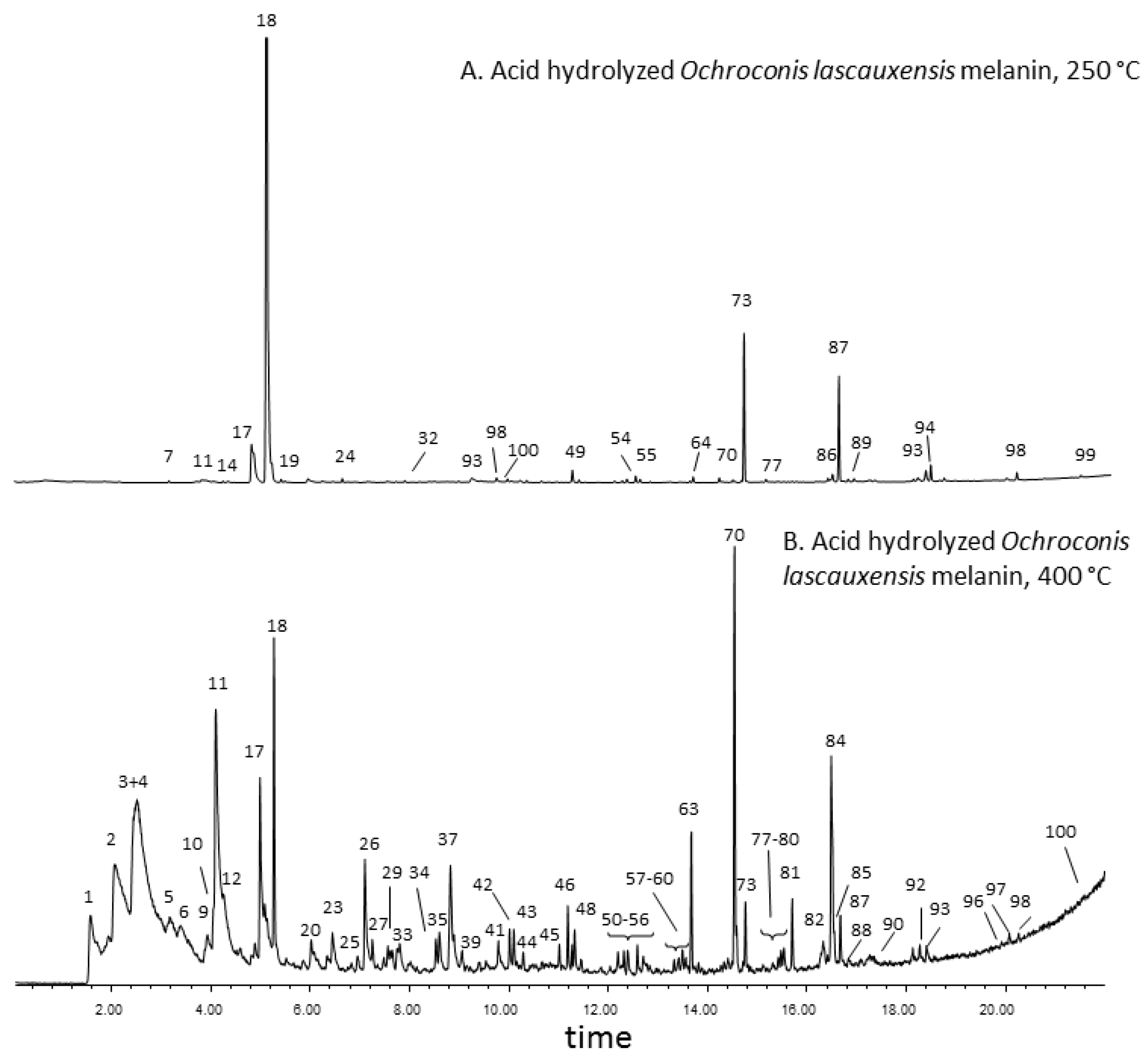
3.4. Thermochemolysis with Tetramethyl Ammonium Hydroxide (TMAH) of the Acid Hydrolyzed Melanin from Ochroconis lascauxensis
4. Discussion
4.1. Aliphatic Hydrocarbons
4.2. Fatty Acids
4.3. Other Aliphatic Compounds
4.4. Proteins
4.5. Carbohydrates and Polysaccharides
4.6. Sterols
4.7. Alkylbenzenes, Alkylthiophenes, and Alkylnaphthalenes
4.8. Oxygen-Containing Compounds
4.9. Acid Hydrolysis
4.10. Conventional Pyrolysis vs. Thermochemolysis
4.11. The Chemical Structure of Ochroconis Melanins
5. Current Challenges and Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allemand, L. Qui sauvera Lascaux? La Rech. 2003, 363, 26–33. [Google Scholar]
- Graff, J. Saving beauty. Time, 15 May 2006. [Google Scholar]
- Roux, E. Les fresques de Lascaux menacées par des moisissures. Le Monde, 26 November 2007. [Google Scholar]
- Bastian, F.; Jurado, V.; Nováková, A.; Alabouvette, C.; Saiz-Jimenez, C. The microbiology of Lascaux Cave. Microbiology 2010, 156, 644–652. [Google Scholar] [CrossRef]
- Martin-Sanchez, P.M.; Nováková, A.; Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Two new species of the genus Ochroconis, O. lascauxensis and O. anomala isolated from black stains in Lascaux Cave, France. Fungal Biol. 2012, 116, 574–589. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanchez, P.M.; Sanchez-Cortes, S.; Lopez-Tobar, E.; Jurado, V.; Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. The nature of black stains in Lascaux Cave, France, as revealed by surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 464–467. [Google Scholar] [CrossRef]
- Martin-Sanchez, P.M.; Nováková, A.; Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Use of Biocides for the Control of Fungal Outbreaks in Subterranean Environments: The Case of the Lascaux Cave in France. Environ. Sci. Technol. 2012, 46, 3762–3770. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, R.A. Melanins; Hermann: Paris, France, 1968. [Google Scholar]
- Bell, A.A.; Wheeler, M.H. Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 1986, 24, 411–451. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014. [Google Scholar] [CrossRef]
- Martin, J.P.; Haider, K. Phenolic polymers of Stachybotrys atra, Stachybotrys chartarum and Epicoccum nigrum in relation to humic acid formation. Soil Sci. 1969, 107, 260–270. [Google Scholar] [CrossRef]
- Schnitzer, M.; De Serra, M.I.O.; Ivarson, K. The Chemistry of Fungal Humic Acid-like Polymers and of Soil Humic Acids. Soil Sci. Soc. Am. J. 1973, 37, 229–236. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C. The chemical nature of the melanins from Coprinus spp. Soil Sci. 1983, 136, 65–74. [Google Scholar] [CrossRef]
- Zhong, J.; Frases, S.; Wang, H.; Casadevall, A.; Stark, R.E. Following Fungal Melanin Biosynthesis with Solid-State NMR: Biopolymer Molecular Structures and Possible Connections to Cell-Wall Polysaccharides. Biochemistry 2008, 47, 4701–4710. [Google Scholar] [CrossRef] [PubMed]
- Prados-Rosales, R.C.; Toriola, S.; Nakouzi, A.; Chatterjee, S.; Stark, R.; Gerfen, G.; Tumpowsky, P.; Dadachova, E.; Casadevall, A. Structural Characterization of Melanin Pigments from Commercial Preparations of the Edible Mushroom Auricularia auricula. J. Agric. Food Chem. 2015, 63, 7326–7332. [Google Scholar] [CrossRef] [PubMed]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef]
- Haider, K.; Martin, J. Humic acid-type phenolic polymers from Aspergillus sydowi culture medium, Stachybotrys spp. cells and autoxidized phenol mixtures. Soil Biol. Biochem. 1970, 2, 145–156. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; Haider, K.; Martin, J.P. Anthraquinones and Phenols as Intermediates in the Formation of Dark-Colored, Humic Acid-Like Pigments by Eurotium echinulatum. Soil Sci. Soc. Am. J. 1975, 39, 649–653. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Martin-Sanchez, P.M.; Sanchez-Cortes, S.; Hermosin, B.; Knicker, H.; Saiz-Jimenez, C. Structure of melanins from the fungi Ochroconis lascauxensis and Ochroconis anomala contaminating rock art in the Lascaux Cave. Sci. Rep. 2017, 7, 13441. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, J.A.; Jiménez-Morillo, N.T.; De la Rosa, J.M.; Almendros, G.; González-Vila, F.J. Pyrolysis-gas chromatography–isotope ratio mass spectrometry of polyethylene. J. Chromatogr. A 2015, 1388, 236–243. [Google Scholar] [CrossRef]
- Diaz-Herraiz, M.; Laiz, L.; Jurado, V.; Miller, A.Z.; González-Pérez, J.A.; Santos, J.L.; Alonso, E.; Saiz-Jimenez, C. Analytical pyrolysis evidences the presence of granaticins in the violet stains of a Roman tomb. J. Anal. Appl. Pyrolysis 2016, 117, 357–362. [Google Scholar] [CrossRef][Green Version]
- Saiz-Jimenez, C. Pyrolysis/methylation of soil fulvic acids: Benzenecarboxylic acids revisited. Environ. Sci. Technol. 1994, 28, 197–200. [Google Scholar] [CrossRef]
- Schnitzer, M.; Preston, C.M. Effects of acid hydrolysis on the 13C NMR spectra of humic substances. Plant Soil 1983, 75, 201–211. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C. Production of Alkylbenzenes and Alkylnaphthalenes upon Pyrolysis of Unsaturated Fatty Acids A Model Reaction to Understand the Origin of some Pyrolysis Products from Humic Substances? Naturwissenschaften 1994, 81, 451–453. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C. The origin of alkylbenzenes and thiophenes in pyrolysates of geochemical samples. Org. Geochem. 1995, 23, 81–85. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; De Leeuw, J.W. Lignin pyrolysis products: Their structures and their significance as biomarkers. Org. Geochem. 1986, 10, 869–876. [Google Scholar] [CrossRef]
- Tegelaar, E.; De Leeuw, J.; Saiz-Jimenez, C. Possible origin of aliphatic moieties in humic substances. Sci. Total Environ. 1989, 81, 1–17. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; De Leeuw, J. Pyrolysis-gas chromatography-mass spectrometry of soil polysaccharides, soil fulvic acids and polymaleic acid. Org. Geochem. 1984, 6, 287–293. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; De Leeuw, J. Nature of plant components identified in soil humic acids. Sci. Total Environ. 1987, 62, 115–119. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; De Leeuw, J. Chemical structure of a soil humic acid as revealed by analytical pyrolysis. J. Anal. Appl. Pyrolysis 1987, 11, 367–376. [Google Scholar] [CrossRef]
- Chiavari, G.; Torsi, G.; Fabbri, D.; Galleti, G.C. Comparative study of humic substances in soil using pyrolytic techniques and other conventional chromatographic methods. Analyst 1994, 119, 1141–1150. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C. Analytical Pyrolysis of Humic Substances: Pitfalls, Limitations, and Possible Solutions. Environ. Sci. Technol. 1994, 28, 1773–1780. [Google Scholar] [CrossRef]
- Hatcher, P.G.; Minard, R.D. Comment on the origin of benzenecarboxylic acids in pyrolysis methylation studies. Org. Geochem. 1995, 23, 991–994. [Google Scholar] [CrossRef]
- Fabbri, D.; Mongardi, M.; Montanari, L.; Galletti, G.C.; Chiavari, G.; Scotti, R. Comparison between CP/MAS 13C-NMR and pyrolysis-GC/MS in the structural characterization of humins and humic acids of soil and sediments. Anal. Bioanal. Chem. 1998, 362, 299–306. [Google Scholar] [CrossRef]
- Quénéa, K.; Derenne, S.; González-Vila, F.; González-Pérez, J.; Mariotti, A.; Largeau, C. Double-shot pyrolysis of the non-hydrolysable organic fraction isolated from a sandy forest soil (Landes de Gascogne, South-West France). J. Anal. Appl. Pyrolysis 2006, 76, 271–279. [Google Scholar] [CrossRef]
- Martin, J.P.; Haider, K.; Wolf, D. Synthesis of phenols and phenolic polymers by Hendersonula toruloidea in relation to humic acid formation. Soil Sci. Soc. Am. Proc. 1972, 36, 311–315. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; Ortega-Calvo, J.J.; de Leeuw, J.W. The chemical structure of fungal melanins and their possible contribution to black stains in stone monuments. Sci. Total Environ. 1995, 167, 305–314. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; Martinez, F.M.; Cert, A. Low boiling-point compounds produced by pyrolysis of fungal melanins and model phenolic polymers. Soil Biol. Biochem. 1979, 11, 305–309. [Google Scholar] [CrossRef]
- Hatcher, P.G.; Clifford, D.J. Flash pyrolysis and in situ methylation of humic acids from soil. Org. Geochem. 1994, 21, 1081–1092. [Google Scholar] [CrossRef]
- Derenne, S.; Quénéa, K. Analytical pyrolysis as a tool to probe soil organic matter. J. Anal. Appl. Pyrolysis 2015, 111, 108–120. [Google Scholar] [CrossRef]
- Zhang, Z.; Volkman, J.K. Algaenan structure in the microalga Nannochloropsis oculata characterized from stepwise pyrolysis. Org. Geochem. 2017, 104, 1–7. [Google Scholar] [CrossRef]
- Frazier, S.W.; Kaplan, L.; Hatcher, P.G. Molecular characterization of biodegradable dissolved organic matter using bioreactors and [12C/13C] tetramethylammonium hydroxide thermochemolysis GC-MS. Environ. Sci. Technol. 2005, 39, 1479–1491. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C. The chemical structure of humic substances: Recent advances. In Humic Substances in Terrestrial Ecosystems; Piccolo, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 1–44. [Google Scholar]
- Saiz-Jimenez, C.; Ortega-Calvo, J.J.; Hermosin, B. Conventional pyrolysis: A biased technique for providing structural information on humic substances? Naturwissenschaften 1994, 81, 28–29. [Google Scholar] [CrossRef]
- Feofilova, E.P. The fungal cell wall: Modern concepts of its composition and biological function. Microbiology 2010, 79, 711–720. [Google Scholar] [CrossRef]
- Laseter, J.; Weete, J.; Weber, D. Alkanes, fatty acid methyl esters, and free fatty acids in surface wax of Ustilago maydis. Phytochemistry 1968, 7, 1177–1181. [Google Scholar] [CrossRef]
- Weete, J.D. Aliphatic hydrocarbons of the fungi. Phytochemistry 1972, 11, 1201–1205. [Google Scholar] [CrossRef]
- Coelho, R.R.R.; Linhares, L.F.; Martin, J.P. Sugars in hydrolysates of fungal melanins and soil humic acids. Plant Soil 1988, 106, 127–133. [Google Scholar] [CrossRef]
- Růžička, Š.; Edgerton, D.; Norman, M.; Hill, T. The utility of ergosterol as a bioindicator of fungi in temperate soils. Soil Biol. Biochem. 2000, 32, 989–1005. [Google Scholar] [CrossRef]
- Hartgers, W.A.; Damsté, J.S.S.; De Leeuw, J.W. Curie-point pyrolysis of sodium salts of functionalized fatty acids. J. Anal. Appl. Pyrolysis 1995, 34, 191–217. [Google Scholar] [CrossRef][Green Version]
- Sumner, J.L.; Evans, H.C. The fatty acid composition of Dactylaria and Scolecobasidium. Can. J. Microbiol. 1971, 17, 7–11. [Google Scholar] [CrossRef]
- Samerpitak, K.; Van Der Linde, E.; Choi, H.-J.; Ende, A.H.G.G.V.D.; Machouart, M.; Gueidan, C.; De Hoog, G.S. Taxonomy of Ochroconis, genus including opportunistic pathogens on humans and animals. Fungal Divers. 2013, 65, 89–126. [Google Scholar] [CrossRef]
- Uranga, C.C.; Beld, J.; Mrse, A.; Córdova-Guerrero, I.; Burkart, M.D.; Hernández-Martínez, R. Data from mass spectrometry, NMR spectra, GC-MS of fatty acid esters produced by Lasiodiplodia theobromae. Data Brief 2016, 8, 31–39. [Google Scholar] [CrossRef][Green Version]
- Gobé, V.; Lemée, L.; Amblés, A. Structure elucidation of soil macromolecular lipids by preparative pyrolysis and thermochemolysis. Org. Geochem. 2000, 31, 409–419. [Google Scholar] [CrossRef]
- Faure, P.; Schlepp, L.; Mansuy-Huault, L.; Elie, M.; Jardé, E.; Pelletier, M. Aromatization of organic matter induced by the presence of clays during flash pyrolysis-gas chromatography–mass spectrometry (Py–GC–MS). J. Anal. Appl. Pyrolysis 2006, 75, 1–10. [Google Scholar] [CrossRef]
- Fréty, R.; Santos, M.R.; Sales, R.F.; Silva, A.O.; Barbosa, C.B.M.; Pacheco, J.G. Flash Pyrolysis of Oleic Acid as a Model Compound Adsorbed on Supported Nickel Catalysts for Biofuel Production. J. Braz. Chem. Soc. 2014, 25, 2433–2443. [Google Scholar] [CrossRef]
- Cermak, S.C.; Isbell, T.A. Synthesis of δ-stearolactone from oleic acid. J. Am. Oil Chem. Soc. 2000, 77, 243–248. [Google Scholar] [CrossRef]
- Forney, F.; Markovetz, A. The biology of methyl ketones. J. Lipid Res. 1971, 12, 383–395. [Google Scholar] [CrossRef]
- Evershed, R.P.; Stott, A.W.; Raven, A.; Dudd, S.N.; Charters, S.; Leyden, A. Formation of long-chain ketones in ancient pottery vessels by pyrolysis of acyl lipids. Tetrahedron Lett. 1995, 36, 8875–8878. [Google Scholar] [CrossRef]
- Granito, C.; Schultz, H.P. Decarboxylation Studies. II. Preparation of Alkyl Phenyl Ketones. J. Org. Chem. 1963, 28, 879–881. [Google Scholar] [CrossRef]
- Riboulleau, A.; Derenne, S.; Sarret, G.; Largeau, C.; Baudin, F.; Connan, J. Pyrolytic and spectroscopic study of a sulphur-rich kerogen from the “Kashpir oil shales” (Upper Jurassic, Russian platform). Org. Geochem. 2000, 31, 1641–1661. [Google Scholar] [CrossRef]
- Derenne, S.; Largeau, C.; Taulelle, F. Occurrence of non-hydrolysable amides in the macromolecular constituents of Scenedesmus quadricauda cell wall as revealed by 15N NMR: Origin of n-alkylnitriles in pyrolysates of ul-tralaminae-containing kerogens. Geochim. Cosmochim. Acta 1993, 57, 851–857. [Google Scholar] [CrossRef]
- Chen, W.; Yang, H.; Chen, Y.; Xia, M.; Chen, X.; Chen, H. Transformation of Nitrogen and Evolution of N-Containing Species during Algae Pyrolysis. Environ. Sci. Technol. 2017, 51, 6570–6579. [Google Scholar] [CrossRef]
- Vo, T.K.; Ly, H.V.; Lee, O.K.; Lee, E.Y.; Kim, C.H.; Seo, J.-W.; Kim, J.; Kim, S.-S. Pyrolysis characteristics and kinetics of microalgal Aurantiochytrium sp. KRS101. Energy 2017, 118, 369–376. [Google Scholar] [CrossRef]
- Stankiewicz, B.A.; van Bergen, P.F.; Duncan, I.J.; Carter, J.F.; Briggs, D.E.G.; Evershed, R.P. Recognition of chitin and proteins in invertebrate cuticles using analytical pyrolysis/gas chromatography and pyrolysis/gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 1996, 10, 1747–1757. [Google Scholar] [CrossRef]
- Gallois, N.; Templier, J.; Derenne, S. Pyrolysis-gas chromatography–mass spectrometry of the 20 protein amino acids in the presence of TMAH. J. Anal. Appl. Pyrolysis 2007, 80, 216–230. [Google Scholar] [CrossRef]
- Smith, G.G.; Reddy, G.S.; Boon, J.J. Gas chromatographic–mass spectrometric analysis of the Curie-point pyrolysis products of some dipeptides and their diketopiperazine. J. Chem. Soc. Perkin Trans. II 1988, 2, 203–211. [Google Scholar] [CrossRef]
- Torri, C.; Alba, L.G.; Samorì, C.; Fabbri, D.; Brilman, D.W.F. Hydrothermal Treatment (HTT) of Microalgae: Detailed Molecular Characterization of HTT Oil in View of HTT Mechanism Elucidation. Energy Fuels 2011, 26, 658–671. [Google Scholar] [CrossRef]
- Furtado, N.A.J.C.; Pupo, M.T.; Carvalho, I.; Campo, V.L.; Duarte, M.C.T.; Bastos, J.K. Diketopiperazines produced by an Aspergillus fumigatus Brazilian strain. J. Braz. Chem. Soc. 2005, 16, 1448–1453. [Google Scholar] [CrossRef]
- Kaur, A.; Raja, H.A.; Darveaux, B.A.; Chen, W.-L.; Swanson, S.M.; Pearce, C.J.; Oberlies, N.H. New diketopiperazine dimer from a filamentous fungal isolate of Aspergillus sydowii. Magn. Reson. Chem. 2015, 53, 616–619. [Google Scholar] [CrossRef]
- Teng, W.L.; Khor, E.; Tan, T.K.; Lim, L.Y.; Tan, S.C. Concurrent production of chitin from shrimp shells and fungi. Carbohydr. Res. 2001, 332, 305–316. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Analytical Pyrolysis of Natural Organic Polymers; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Patwardhan, P.R.; Dalluge, D.L.; Shanks, B.H.; Brown, R.C. Distinguishing primary and secondary reactions of cellulose pyrolysis. Bioresour. Technol. 2011, 102, 5265–5269. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef]
- Martín, F.; Del Río, J.; González-Vila, F.; Verdejo, T. Pyrolysis derivatization of humic substances 2. Pyrolysis of soil humic acids in the presence of tetramethylammonium hydroxide. J. Anal. Appl. Pyrolysis 1995, 31, 75–83. [Google Scholar] [CrossRef]
- Del Rio, J.C.; Hatcher, P.G. Structural characterization of humic substances using thermochemolysis with tetramethylammonium hydroxide. ACS Symp. Ser. 1996, 651, 78–95. [Google Scholar]
- Almendros, G.; Dorado, J.; González-Vila, F.J.; Martin, F. Pyrolysis of carbohydrate-derived macromolecules: Its potential in monitoring the carbohydrate signature of geopolymers. J. Anal. Appl. Pyrolysis 1997, 1997, 599–610. [Google Scholar] [CrossRef]
- Wang, T.S.C.; Chen, J.-H.; Hsiang, W.-M. Catalytic synthesis of humic acids containing various amino acids and dipeptides. Soil Sci. 1985, 140, 3–10. [Google Scholar] [CrossRef]
- González-Vila, F.J.; Lankes, U.; Lüdemann, H.-D. Comparison of the information gained by pyrolytic techniques and NMR spectroscopy on the structural features of aquatic humic substances. J. Anal. Appl. Pyrolysis 2001, 1979, 349–359. [Google Scholar] [CrossRef]
- Méjanelle, L.; Lòpez, J.F.; Gunde-Cimerman, N.; Grimalt, J.O. Ergosterol biosynthesis in novel melanized fungi from hypersaline environments. J. Lipid Res. 2001, 42, 352–358. [Google Scholar] [CrossRef]
- Heilbront, I.M.; Kennedy, T.; Spring, F.S.; Swain, G. Studies in the sterol group. Part XXXVII. The structure of lumisterol and its stereoisomers. J. Chem. Soc. 1938, 869–876. [Google Scholar] [CrossRef]
- De Oliveira, B.P.; De La Rosa, J.M.; Miller, A.Z.; Saiz-Jimenez, C.; Gómez-Bolea, A.; Braga, M.A.S.; Dionísio, A. An integrated approach to assess the origins of black films on a granite monument. Environ. Earth Sci. 2010, 63, 1677–1690. [Google Scholar] [CrossRef]
- Faria, S.R.; De La Rosa, J.M.; Knicker, H.; González-Pérez, J.A.; Villaverde, J.; Keizer, J.J. Wildfire-induced alterations of topsoil organic matter and their recovery in Mediterranean eucalypt stands detected with biogeochemical markers. Eur. J. Soil Sci. 2015, 66, 699–713. [Google Scholar] [CrossRef]
- De La Rosa, J.M.; González-Pérez, J.A.; González-Vila, F.J.; Knicker, H.; Araújo, M.D.F. Molecular composition of sedimentary humic acids from South West Iberian Peninsula: A multi-proxy approach. Org. Geochem. 2011, 42, 791–802. [Google Scholar] [CrossRef]
- Du, Z.; Hu, B.; Ma, X.; Cheng, Y.; Liu, Y.; Lin, X.; Wan, Y.; Lei, H.; Chen, P.; Ruan, R. Catalytic pyrolysis of microalgae and their three major components: Carbohydrates, proteins, and lipids. Bioresour. Technol. 2013, 130, 777–782. [Google Scholar] [CrossRef]
- Fuentes, M.; Baigorri, R.; González-Vila, F.J.; González-Gaitano, G.; Mina, J.M.G. Pyrolysis-Gas Chromatography/Mass Spectrometry Identification of Distinctive Structures Providing Humic Character to Organic Materials. J. Environ. Qual. 2010, 39, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- De La Rosa, J.; González-Pérez, J.; González-Vázquez, R.; Knicker, H.; López-Capel, E.; Manning, D.; González-Vila, F. Use of pyrolysis/GC–MS combined with thermal analysis to monitor C and N changes in soil organic matter from a Mediterranean fire affected forest. Catena 2008, 74, 296–303. [Google Scholar] [CrossRef]
- Zang, X.; Hatcher, P.G. A Py–GC–MS and NMR spectroscopy study of organic nitrogen in Mangrove Lake sediments. Org. Geochem. 2002, 33, 201–211. [Google Scholar] [CrossRef]
- Knicker, H.; Río, J.; Hatcher, P.G.; Minard, R.D. Identification of protein remnants in insoluble geopolymers using TMAH thermochemolysis/GC–MS. Org. Geochem. 2001, 32, 397–409. [Google Scholar] [CrossRef]
- Bracewell, J.; Robertson, G.; Welch, D. Polycarboxylic acids as the origin of some pyrolysis products characteristics of soil organic matter. J. Anal. Appl. Pyrolysis 1980, 2, 239–248. [Google Scholar] [CrossRef]
- Joll, C.A.; Huynh, T.; Heitz, A. Off-line tetramethylammonium hydroxide thermochemolysis of model compound aliphatic and aromatic carboxylic acids: Decarboxylation of some ortho- and/or para-substituted aromatic carboxylic acids. J. Anal. Appl. Pyrolysis 2003, 70, 151–167. [Google Scholar] [CrossRef]
- Van den Berg, J.D.J.; Boon, J.J. Unwanted alkylation during direct methylation of fatty (di)acids using tetramethyl ammonium hydroxide reagent in a Curie-point pyrolysis unit. J. Anal. Appl. Pyrolysis 2001, 61, 45–63. [Google Scholar] [CrossRef]
- Anderson, K.B.; Winans, R.E. Nature and fate of natural resins in the geosphere. 1. Evaluation of pyrolysis-gas chromatography/mass spectrometry for the analysis of natural resins and resinites. Anal. Chem. 1991, 63, 2901–2908. [Google Scholar] [CrossRef]
- Haider, K.; Frederick, L.R.; Flaig, W. Reactions between amino acid compounds and phenols during oxidation. Plant Soil 1965, 22, 49–64. [Google Scholar] [CrossRef]
- Ladd, J.N.; Butler, J.H.A. Humus-enzyme systems and synthesis, organic polymer-enzyme analogs. In Soil Biochemistry; Paul, E.A., McLaren, A.D., Eds.; Mercel Dekker: New York, NY, USA, 1975; Volume 4, pp. 143–194. [Google Scholar]
- Sjoblad, R.D.; Bollag, J.-M. Oxidative coupling of aromatic compounds by enzymes from soil microorganisms. In Soil Biochemistry; Paul, E.A., Ladd, J.N., Eds.; Marcel Dekker: New York, NY, USA, 1981; Volume 5, pp. 113–152. [Google Scholar]
- Haider, K.; Nagar, B.R.; Saiz, C.; Meuzelaar, H.L.C.; Martin, J.P. Studies on soil humic compounds, fungal melanins and model polymers by pyrolysis-mass spectrometry. In Soil Organic Matter Studies; IAEA: Vienna, Austria, 1977; Volume II, pp. 213–222. [Google Scholar]
- Knicker, H.; Almendros, G.; González-Vila, F.J.; Lüdemann, H.-D.; Martin, F. 13C and 15N NMR analysis of some fungal melanins in comparison with soil organic matter. Org. Geochem. 1995, 23, 1023–1028. [Google Scholar] [CrossRef]
- Camacho, E.; Vij, R.; Chrissian, C.; Prados-Rosales, R.; Gil, D.; O’Meally, R.N.; Cordero, R.J.B.; Cole, R.N.; McCaffery, J.M.; Stark, R.E.; et al. The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans. J. Biol. Chem. 2019, 294, 10471–10489. [Google Scholar] [CrossRef] [PubMed]
- Adhyaru, B.B.; Akhmedov, N.G.; Katritzky, A.R.; Bowers, C.R. Solid-state cross-polarization magic angle spinning 13C and 15N NMR characterization of Sepia melanin, Sepia melanin free acid and Human hair melanin in comparison with several model compounds. Magn. Reson. Chem. 2003, 41, 466–474. [Google Scholar] [CrossRef]
- Chatterjee, S.; Prados-Rosales, R.; Tan, S.; Itin, B.; Casadevall, A.; Stark, R.E. Demonstration of a common indole-based aromatic core in natural and synthetic eumelanins by solid-state NMR. Org. Biomol. Chem. 2014, 12, 6730–6736. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-J.; Kim, J.Y.; Kwon, S.L.; Hwang, D.-H.; Choi, Y.-E.; Kim, G.-H. Production and characterization of melanin pigments derived from Amorphotheca resinae. J. Microbiol. 2020, 58, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Pralea, I.-E.; Moldovan, R.-C.; Petrache, A.-M.; Ilies, M.; Heghes, S.-C.; Ielciu, I.; Nicoara, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From extraction to advanced analytical methods: The challenges of melanin analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef] [PubMed]
- Boon, J.J.; De Leeuw, J. Amino acid sequence information in proteins and complex proteinaceous material revealed by pyrolysis-capillary gas chromatography-low and high resolution mass spectrometry. J. Anal. Appl. Pyrolysis 1987, 11, 313–327. [Google Scholar] [CrossRef]
- Schmaler-Ripcke, J.; Sugareva, V.; Gebhardt, P.; Winkler, R.; Kniemeyer, O.; Heinekamp, T.; Brakhage, A.A. Production of Pyomelanin, a Second Type of Melanin, via the Tyrosine Degradation Pathway in Aspergillus fumigatus. Appl. Environ. Microbiol. 2008, 75, 493–503. [Google Scholar] [CrossRef]
- Ao, J.; Bandyopadhyay, S.; Free, S.J. Characterization of the Neurospora crassa DHN melanin biosynthetic pathway in developing ascospores and peridium cells. Fungal Biol. 2019, 123, 1–9. [Google Scholar] [CrossRef]
- Beltrán-García, M.J.; Prado, F.M.; Oliveira, M.S.; Ortiz-Mendoza, D.; Scalfo, A.C.; Pessoa, A., Jr.; Medeiros, M.H.G.; White, J.F.; Bechara, E.J. Singlet Molecular Oxygen Generation by Light-Activated DHN-Melanin of the Fungal Pathogen Mycosphaerella fijiensis in Black Sigatoka Disease of Bananas. PLoS ONE 2014, 9, e91616. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; DeAraujo, A.; Mazurek, J.; Schilling, M.; Mitchell, R. Pyomelanin production in Penicillium chrysogenum is stimulated by L-tyrosine. Microbiology 2015, 161, 1211–1218. [Google Scholar] [CrossRef]
- Frases, S.; Salazar, A.; Dadachova, E.; Casadevall, A. Cryptococcus neoformans Can Utilize the Bacterial Melanin Precursor Homogentisic Acid for Fungal Melanogenesis. Appl. Environ. Microbiol. 2006, 73, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Suwannarach, N.; Kumla, J.; Watanabe, B.; Matsui, K.; Lumyong, S. Characterization of melanin and optimal conditions for pigment production by an endophytic fungus, Spissiomyces endophytica SDBR-CMU319. PLoS ONE 2019, 14, e0222187. [Google Scholar] [CrossRef] [PubMed]
- Yew, S.M.; Chan, C.L.; Kuan, C.S.; Toh, Y.F.; Ngeow, Y.F.; Na, S.L.; Lee, K.W.; Hoh, C.-C.; Yee, W.-Y.; Ng, K.P. The genome of newly classified Ochroconis mirabilis: Insights into fungal adaptation to different living conditions. BMC Genom. 2016, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.B. Stachybotrys chartarum: A fungus for our time. Phytochemistry 2003, 64, 53–60. [Google Scholar] [CrossRef]
- Gonzalez-Vila, F.J.; Saiz-Jimenez, C.; Lentz, H.; Lüdemann, H.-D. 13C nuclear magnetic resonance spectra of fungal melanins. Z. Naturforsch. 1978, 33c, 291–293. [Google Scholar] [CrossRef]
- Pukalski, J.; Marcol, N.; Wolan, N.; Płonka, P.M.; Ryszka, P.; Kowalski, T.; Latowski, D. Detection of a pheomelanin-like pigment by EPR spectroscopy in the mycelium of Plenodomus biglobosus. Acta Biochim. Pol. 2020, 67, 295–301. [Google Scholar] [CrossRef]
| Peak | Compound | Peak | Compound |
|---|---|---|---|
| 1 | Methylfuran | 61 | Methylfluorene |
| 2 | Benzene | 62 | Diketodipyrrole |
| 3 | Pyridine | 63 | Tetradecanoic acid |
| 4 | Pyrrole | 64 | n-Undecylbenzene |
| 5 | Toluene | 65 | n-Octadecene |
| 6 | Furfural | 66 | n-Octadecane |
| 7 | Methylpyrrole | 67 | Hexadecanone |
| 8 | Methylpyrrole | 68 | Pentadecanoic acid |
| 9 | Methylpyridine | 69 | n-Dodecylbenzene |
| 10 | n-Ethylbenzene | 70 | n-Nonadecene |
| 11 | Styrene | 71 | n-Nonadecane |
| 12 | Dimethylpyridine | 72 | Hexadecanenitrile |
| 13 | Dimethylpyrrole | 73 | Tetradecylfuran |
| 14 | n-Propylbenzene | 74 | Hexadecanoic acid methyl ester |
| 15 | Benzaldehyde | 75 | Hexadecanoic acid |
| 16 | Methylfurfural | 76 | Hexahydro-(methylpropyl)-pyrrolo-pyrazinedione |
| 17 | Phenol | 77 | Diketopyrazine (from Pro-Val dipeptide) |
| 18 | Trimethylbenzene | 78 | Diethoxy-tetrahydro-dipyrrolopyrazine |
| 19 | n-Butylbenzene | 79 | n-Eicosene |
| 20 | Methylpropylbenzene | 80 | n-Eicosane |
| 21 | Methylphenol | 81 | Diketopyrazine (from Pro-Val dipeptide) |
| 22 | Methylphenol | 82 | Octadecenenitrile |
| 23 | n-Undecane | 83 | n-Heneicosene |
| 24 | Benzyl nitrile | 84 | Octadecadienoic acid methyl ester |
| 25 | n-Pentylbenzene | 85 | Octadecenoic acid methyl ester |
| 26 | n-Ethylphenol | 86 | Octadecanenitrile |
| 27 | Methylbutylbenzene | 87 | n-Heneicosane |
| 28 | n-Dodecene | 88 | Octadecanoic acid methyl ester |
| 29 | n-Dodecane | 89 | Octadecadienoic acid |
| 30 | Naphthalene | 90 | Octadecenoic acid |
| 31 | Dianhydroglucopyranose | 91 | Octadecanoic acid |
| 32 | Vinylphenol | 92 | Hexadecanamide |
| 33 | Benzenepropanenitrile | 93 | n-Docosene |
| 34 | n-Hexylbenzene | 94 | n-Docosane |
| 35 | Methylpentylbenzene | 95 | n-Pentadecylbenzene |
| 36 | Picolinamide | 96 | n-Tricosene |
| 37 | n-Tridecene | 97 | n-Tricosane |
| 38 | n-Tridecane | 98 | n-Hexadecylbenzene |
| 39 | Indole | 99 | Hydroxyoctadecanoic acid γ-lactone |
| 40 | Methylnaphthalene | 100 | Octadecenamide |
| 41 | n-Heptylbenzene | 101 | Octadecanamide |
| 42 | Methylhexylbenzene | 102 | n-Heptadecylbenzene |
| 43 | n-Tetradecene | 103 | Hexahydro-(phenylmethyl)-pyrrolo-pyrazinedione |
| 44 | n-Tetradecane | 104 | N,N-Dimethyl-octadecenamide |
| 45 | Methylindole | 105 | N,N-Dimethyl-octadecanamide |
| 46 | Dimethylnaphthalene | 106 | Docosanenitrile |
| 47 | Phenylhexanone | 107 | Bis-(ethylhexyl)-phthalate |
| 48 | n-Octylbenzene | 108 | Oxoheptadecylpyrrolidine |
| 49 | Phenylpyridine | 109 | Tricosanenitrile |
| 50 | n-Pentadecene | 110 | Tetracosanenitrile |
| 51 | n-Pentadecane | 111 | Oxooctadecadienylpyrrolidine |
| 52 | Dimethylindole | 112 | Oxooctadecenylpyrrolidine |
| 53 | Phenylfuran | 113 | Oxooctadecylpyrrolidine |
| 54 | Naphthalenamine | 114 | Squalene |
| 55 | n-Nonylbenzene | 115 | Anthiaergosta-1,5,7,9,22-pentaene |
| 56 | n-Hexadecene | 116 | Anthiaergostan-5,7,9,16,22-pentene |
| 57 | n-Hexadecane | 117 | Hexadecanoic acid hexadecyl ester |
| 58 | Fluorene | 118 | Ergosta-4,6,8(14),22-tetraen-3-one |
| 59 | n-Heptadecene | 119 | Stigmasta-3,5-dien-7-one |
| 60 | n-Heptadecane | 120 | Hexadecanoic acid octadecyl ester |
| Peak | Compound | Peak | Compound |
|---|---|---|---|
| 1 | Chloromethane | 58 | Dodecanoic acid |
| 2 | Sulfur dioxide | 59 | n-Nonylbenzene |
| 3 | Hexanal | 60 | n-Hexadecene |
| 4 | Benzene | 61 | n-Hexadecane |
| 5 | Dimethyldisulfide | 62 | Diethylphthalate |
| 6 | Toluene | 63 | Methylthiobenzothiazole |
| 7 | Butanoic acid | 64 | Benzophenone |
| 8 | Pyridine | 65 | Phenylhexanoic acid |
| 9 | Furfural | 66 | Tridecanoic acid |
| 10 | Maleic anhydride | 67 | n-Decylbenzene |
| 11 | Pentanoic acid | 68 | Heptadecatriene |
| 12 | n-Propylbenzene | 69 | Heptadecadiene |
| 13 | Benzaldehyde | 70 | n-Heptadecene |
| 14 | Hexanoic acid | 71 | Phenyloctanone |
| 15 | Anhydrodeoxypentenulose | 72 | 2-Pentadecanone |
| 16 | Phenol | 73 | Methyldecylbenzene |
| 17 | n-Butylbenzene | 74 | Diketodipyrrole |
| 18 | n-Butylthiophene | 75 | Phenylheptanoic acid |
| 19 | Acetophenone | 76 | Tetradecanoic acid |
| 20 | Heptanoic acid | 77 | n-Undecylbenzene |
| 21 | Methylphenol | 78 | 2-Hexadecanone |
| 22 | Nonanal | 79 | Phenanthrene |
| 23 | Levoglucosenone | 80 | Pentadecanoic acid |
| 24 | Benzeneacetonitrile | 81 | Dibutylphthalate |
| 25 | n-Pentylbenzene | 82 | n-Dodecylbenzene |
| 26 | n-Pentylthiophene | 83 | Hexadecanenitrile |
| 27 | Octanoic acid | 84 | Tetradecylfuran |
| 28 | 2-Decanone | 85 | Hexadecanoic acid methyl ester |
| 29 | Naphthalene | 86 | Hexadecenoic acid |
| 30 | Benzoic acid | 87 | Hexadecanoic acid |
| 31 | Dianhydromannopyranose | 88 | Hexahydro-(methylpropyl)-pyrrolo-pyrazinedione |
| 32 | Dianhydroglucopyranose | 89 | Diketopyrazine (from Pro-Val dipeptide) |
| 33 | Anhydrogalactosan | 90 | Diethoxy-tetrahydro-dipyrrolopyrazine |
| 34 | Anhydromannosan | 91 | Heptadecanoic acid |
| 35 | n-Hexylbenzene | 92 | Octadecenenitrile |
| 36 | n-Hexylthiophene | 93 | Octadecedienoic acid methyl ester |
| 37 | Phenylbutanone | 94 | Octadecenoic acid methyl ester |
| 38 | Nonanoic acid | 95 | Octadecanoic acid methyl ester |
| 39 | n-Tridecene | 96 | Octadecanenitrile |
| 40 | 2-Undecanone | 97 | Hydroxyhexadecanoic acid γ-lactone |
| 41 | Indole | 98 | Octadecadienoic acid |
| 42 | Methylnaphthalene | 99 | Octadecenoic acid |
| 43 | Pentylfuranone | 100 | Octadecanoic acid |
| 44 | Phenylpentanone | 101 | Octadecanoic acid ethyl ester |
| 45 | n-Heptylbenzene | 102 | n-Pentadecylbenzene |
| 46 | Decanoic acid | 103 | Hexadecanamide |
| 47 | n-Tetradecene | 104 | n-Tricosene |
| 48 | Biphenyl | 105 | n-Hexadecylbenzene |
| 49 | n-Tetradecane | 106 | Hydroxyoctadecanoic acid γ-lactone |
| 50 | Galactosan | 107 | Octadecenamide |
| 51 | Phenylhexanone | 108 | Octadecanamide |
| 52 | Octylbenzene | 109 | Bis-(2-ethylhexyl)-phthalate |
| 53 | Mannosan | 110 | Squalene |
| 54 | n-Pentadecene | 111 | Hexadecanoic acid dodedecyl ester |
| 55 | n-Pentadecane | 112 | Anthiaergosta-1,5,7,9,22-pentaene |
| 56 | Dihydroindolone | 113 | Anthiaergostan-5,7,9,16,22-pentene |
| 57 | Levoglucosan |
| Peak | Compound | Peak | Compound |
|---|---|---|---|
| 1 | Benzene | 62 | Acenaphthene |
| 2 | Pyridine | 63 | Dimethylnaphthalene |
| 3 | Pyrrole | 64 | Diphenylmethane |
| 4 | Toluene | 65 | Dimethylnaphthalene |
| 5 | Methylpyrrole | 66 | n-Octylbenzene |
| 6 | Methylpyrrole | 67 | Methylheptylbenzene |
| 7 | n-Ethylbenzene | 68 | Phenylpyridine |
| 8 | n-Ethylthiophene | 69 | n-Pentadecene |
| 9 | Methylpyridine | 70 | n-Pentadecane |
| 10 | Styrene | 71 | Methylbiphenyl |
| 11 | Dimethylpyridine | 72 | Isoindoledione |
| 12 | n-Propylbenzene | 73 | Dihydroindolone |
| 13 | n-Propylthiophene | 74 | Dibenzofuran |
| 14 | Methylethylbenzene | 75 | n-Nonylbenzene |
| 15 | Phenol | 76 | n-Hexadecene |
| 16 | Trimethylbenzene | 77 | n-Hexadecane |
| 17 | Benzonitrile | 78 | Fluorene |
| 18 | Benzofuran | 79 | n-Decylbenzene |
| 19 | Ethymethylbenzene | 80 | n-Heptadecane |
| 20 | Indane | 81 | Diketodipyrrole |
| 21 | Indene | 82 | Tetradecanoic acid |
| 22 | n-Butylbenzene | 83 | n-Undecylbenzene |
| 23 | n-Butylthiophene | 84 | n-Octadecene |
| 24 | Methylphenol | 85 | n-Octadecane |
| 25 | Methylpropylbenzene | 86 | Phenanthrene |
| 26 | Acetophenone | 87 | Phenylbenzofuran |
| 27 | n-Undecene | 88 | Pentadecanoic acid |
| 28 | Methylphenol | 89 | n-Dodecylbenzene |
| 29 | n-Undecane | 90 | n-Nonadecene |
| 30 | Methoxyphenol | 91 | n-Nonadecane |
| 31 | Benzeneacetonitrile | 92 | Hexadecanenitrile |
| 32 | Methylbenzofuran | 93 | Tetradecylfuran |
| 33 | Methylpropenylbenzene | 94 | Hexadecanoic acid methyl ester |
| 34 | Methylbenzonitrile | 95 | Methylphenanthrene |
| 35 | n-Pentylbenzene | 96 | Methylphenanthrene |
| 36 | n-Pentylthiophene | 97 | Hexahydro-(methylpropyl)-pyrrolopyrazinedione |
| 37 | Ethylphenol | 98 | Diethoxy-tetrahydro-dipyrrolopyrazine |
| 38 | n-Dodecene | 99 | Hexadecanoic acid |
| 39 | n-Dodecane | 100 | n-Eicosene |
| 40 | Naphthalene | 101 | n-Eicosane |
| 41 | Benzoic acid | 102 | Phenylnaphthalene |
| 42 | Vinylphenol | 103 | Octadecadienoic acid |
| 43 | Phenylfuran | 104 | Dimethylphenanthrene |
| 44 | Benzenepropanenitrile | 105 | Octadecenenitrile |
| 45 | n-Hexylbenzene | 106 | n-Tetradecylbenzene |
| 46 | n-Hexylthiophene | 107 | Octadecanenitrile |
| 47 | Methylpentylbenzene | 108 | Octadecenoic acid |
| 48 | n-Tridecene | 109 | Octadecanoic acid |
| 49 | n-Tridecane | 110 | Hexadecanamide |
| 50 | Methylnaphthalene | 111 | n-Tricosene |
| 51 | Indole | 112 | n-Tricosane |
| 52 | Methylnaphthalene | 113 | n-Hexadecylbenzene |
| 53 | n-Heptylbenzene | 114 | Hydroxyoctadecanoic acid γ-lactone |
| 54 | Methylhexylbenzene | 115 | Octadecenamide |
| 55 | n-Tetradecene | 116 | Octadecanamide |
| 56 | Methylindole | 117 | n-Heptadecylbenzene |
| 57 | Biphenyl | 118 | n-Octadecylbenzene |
| 58 | n-Tetradecane | 119 | Tricosanenitrile |
| 59 | Methylbiphenyl | 120 | Tetracosanenitrile |
| 60 | Methylindole | 121 | Anthiaergosta-1,5,7,9,22-pentaene |
| 61 | Phenylthiophene | 122 | Anthiaergostan-5,7,9,16,22-pentene |
| Peak | Compound | Peak | Compound |
|---|---|---|---|
| 1 | Chloromethane + Sulfur dioxide | 51 | n-Decylthiophene |
| 2 | Benzene | 52 | n-Heptadecene |
| 3 | Pyridine | 53 | n-Heptadecane |
| 4 | Toluene | 54 | 2-Pentadecanone |
| 5 | n-Ethylbenzene | 55 | Pentadecanal |
| 6 | Styrene | 56 | Tetradecanoic acid methyl ester |
| 7 | Hexanoic acid methyl ester | 57 | n-Undecylbenzene |
| 8 | n-Propylbenzene | 58 | n-Undecylthiophene |
| 9 | Isocyanobenzene | 59 | n-Octadecene |
| 10 | Phenol | 60 | n-Octadecane |
| 11 | Benzenamine | 61 | 2-Hexadecanone |
| 12 | Trimethylbenzene | 62 | Phenanthrene |
| 13 | Benzofuran | 63 | Hexadecanal |
| 14 | Butanedioic acid dimethyl ester | 64 | Pentadecanoic acid methyl ester |
| 15 | Methylphenol | 65 | Pentyloctylbenzene |
| 16 | n-Butylbenzene | 66 | Butylnonylbenzene |
| 17 | N-Methylbenzenamine | 67 | Propyldecylbenzene |
| 18 | N,N-Dimethylbenzenamine | 68 | n-Dodecylbenzene |
| 19 | Octanoic acid methyl ester | 69 | n-Nonadecane |
| 20 | n-Pentylbenzene | 70 | 2-Heptadecanone |
| 21 | n-Dodecene | 71 | Tetramethylfuran |
| 22 | 2-Decanone | 72 | Heptadecanal |
| 23 | Naphthalene | 73 | Hexadecanoic acid methyl ester |
| 24 | Nonanoic acid methyl ester | 74 | Methylphenanthrene |
| 25 | Benzothiazole | 75 | Decylphenol |
| 26 | Quinoline | 76 | Hexadecanoic acid |
| 27 | n-Hexylbenzene | 77 | Dibutyl phthalate |
| 28 | n-Tridecene | 78 | n-Tridecylbenzene |
| 29 | n-Tridecane | 79 | 2-Octadecanone |
| 30 | Indole | 80 | n-Tridecylthiophene |
| 31 | Methylnaphthalene | 81 | Octadecanal |
| 32 | Decanoic acid methyl ester | 82 | n-Tetradecylbenzene |
| 33 | Methylnaphthalene | 83 | n-Heneicosane |
| 34 | Heptanedioic acid dimethyl ester | 84 | 2-Nonadecanone |
| 35 | n-Heptylbenzene | 85 | Octadecanenitrile |
| 36 | Methylquinoline | 86 | Hydroxyhexadecanoic acid γ-lactone |
| 37 | Biphenyl | 87 | Octadecanoic acid methyl ester |
| 38 | n-Tetradecane | 88 | Dodecylphenol |
| 39 | Dodecanal | 89 | Octadecanoic acid |
| 40 | Octanedioic acid dimethyl ester | 90 | n-Pentadecylbenzene |
| 41 | n-Octylbenzene | 91 | n-Hexadecylbenzene |
| 42 | n-Pentadecene | 92 | 2-Heneicosenone |
| 43 | n-Pentadecane | 93 | Hydroxyoctadecanoic acid γ-lactone |
| 44 | Dodecanoic acid methyl ester | 94 | Eicosanoic acid methyl ester |
| 45 | n-Nonylbenzene | 95 | n-Heptadecylbenzene |
| 46 | n-Hexadecene | 96 | n-Octadecylbenzene |
| 47 | n-Hexadecane | 97 | Hexadecanephenone |
| 48 | Fluorene | 98 | Bis-(2-ethylhexyl)-phthalate |
| 49 | Diethyl phthalate | 99 | Tetracosanoic acid methyl ester |
| 50 | n-Decylbenzene | 100 | Octadecanephenone |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiz-Jimenez, C.; Martin-Sanchez, P.M.; Gonzalez-Perez, J.A.; Hermosin, B. Analytical Pyrolysis of the Fungal Melanins from Ochroconis spp. Isolated from Lascaux Cave, France. Appl. Sci. 2021, 11, 1198. https://doi.org/10.3390/app11031198
Saiz-Jimenez C, Martin-Sanchez PM, Gonzalez-Perez JA, Hermosin B. Analytical Pyrolysis of the Fungal Melanins from Ochroconis spp. Isolated from Lascaux Cave, France. Applied Sciences. 2021; 11(3):1198. https://doi.org/10.3390/app11031198
Chicago/Turabian StyleSaiz-Jimenez, Cesareo, Pedro M. Martin-Sanchez, Jose A. Gonzalez-Perez, and Bernardo Hermosin. 2021. "Analytical Pyrolysis of the Fungal Melanins from Ochroconis spp. Isolated from Lascaux Cave, France" Applied Sciences 11, no. 3: 1198. https://doi.org/10.3390/app11031198
APA StyleSaiz-Jimenez, C., Martin-Sanchez, P. M., Gonzalez-Perez, J. A., & Hermosin, B. (2021). Analytical Pyrolysis of the Fungal Melanins from Ochroconis spp. Isolated from Lascaux Cave, France. Applied Sciences, 11(3), 1198. https://doi.org/10.3390/app11031198









