Current Trends in the Development and Use of Personalized Implants: Engineering Concepts and Regulation Perspectives for the Contemporary Oral and Maxillofacial Surgeon
Abstract
:1. Introduction
2. Engineering Principles for Clinicians
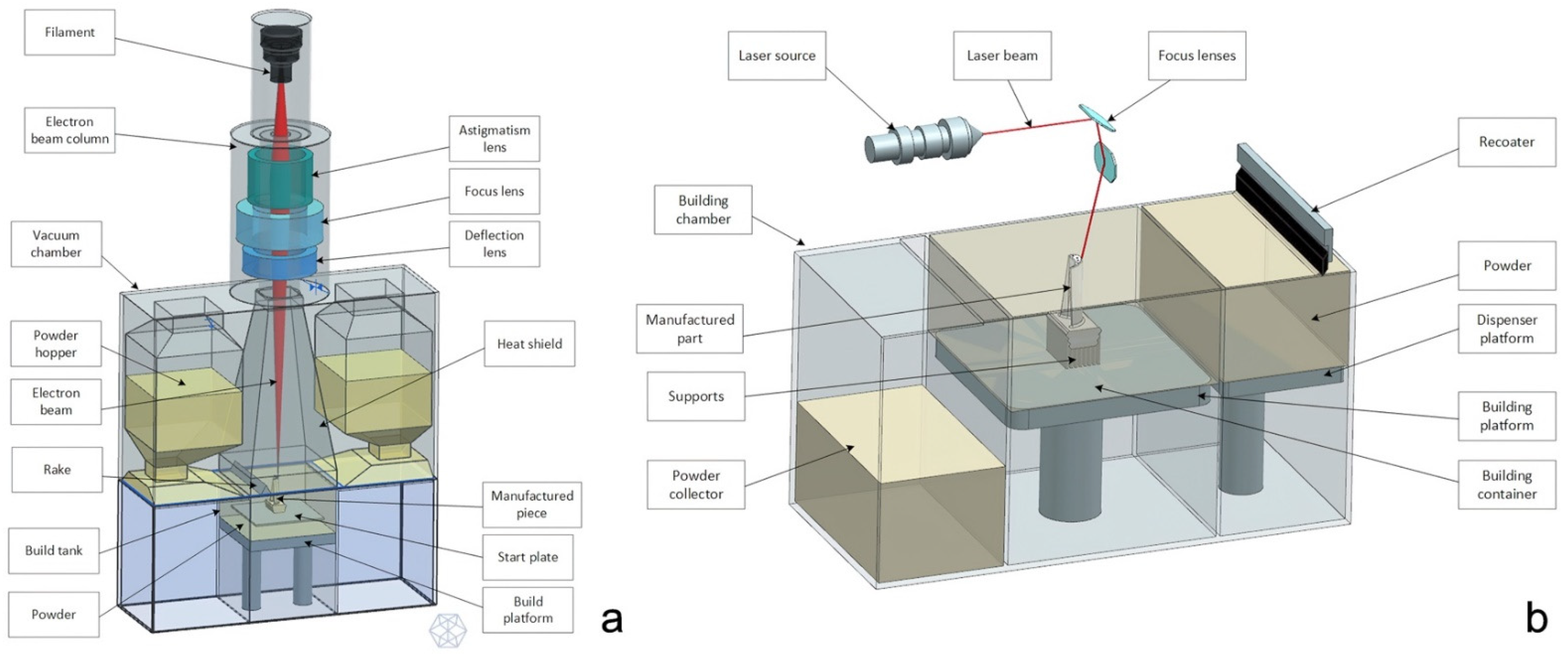
2.1. Additive Manufacturing Concepts for the CMF Surgeon
EBM vs. SLM
- EBM technology has usually a higher building rate in respect to the SLM, therefore it is more suitable for the production of complex shapes, for example lattice structures; EBM technology has usually a higher building rate compared to SLM. This is due to two main factors: first, layer thickness has higher values (50–100 µm in respect to the 20–100 µm of the SLM process); secondly, the immediate electron beam motion from one location to another, thanks to the instantaneous response of magnetic coils, can considerably speed up component fabrication (for example, electron beam speed can reach 8000 m/s in ARCAM machines [11], with the laser reaching 7 m/s in EOS machines [12]). For instance, in the production of truss-like structures (lattice structures), EBM is to be chosen;
- The surface finishing of a component produced with EBM is much lower in respect to an SLM product. Is it sufficient to consider that the roughness parameters of EBM “as built” specimens are about twice those of SLM “as built” ones: in fact the roughness of EBM is 30–40 μm while for SLM specimens is 11–18 um. This is mainly due to the fact that the layer thickness, powder size, and melting pool size in the case of SLM are half the one used in EBM specimens [13];
- The components produced with SLM undergo strong thermic gradients during the 3D printing process, since the preheating temperature of the powders is generally low, since the temperature of the chamber in SLM process is the environment temperature, assumed to be 293 K [14]. That is why they need some thermic post treatments in order to reduce the residual stresses that take origin inside the material because of the rapid and iterative phase change of the metal (solid–liquid–solid). On the contrary, in the EBM technology the temperature of the powder bed is higher and ensures the absence of thermal stresses inside the melted material, around 870 K during the melting process [15];
- On the contrary, maintaining the powder bed at high temperature has a bad influence on the quality of the microstructure of the melted metal that results coarse with large grains. This fact has a direct influence on the mechanical characteristics of the material: an SLM component, in fact, has higher resistance to traction while being less ductile in respect to an EBM product [16,17].
- In the EBM, the pre-heating of the powder allows the unmelted particles to bind together and to act as a support for the overhanging geometrical features. Generally, EBM products need fewer physical supports in respect to the SLM ones.
- Withstand deformation or even collapse of processed material caused by gravity during the manufacturing process;
- Mitigate the effects of thermal gradients generated during production, since thermal distortions may lead to cracks, curling, sag, delamination, and shrinkage;
- Anchor the part to the build platform;
- In PBF, they stop any layer shifting during the re-coating phase.
2.2. How to Evaluate the Requested CMF Implant? A Guide for the Surgeon
2.2.1. Principles of Mechanical Defects Formation
- Porosity: the presence of pores inside the metallic structure could be critical for the fatigue resistance of the component [19]. Notably, porosities usually represent the trigger points for cracks propagation. These pores usually measure from 1 to 20 µm and can extend up to the surface.
- Balling: melt ball formation occurs when the molten material solidifies into spheres instead of solid layers. The result is a rough and bead-shaped surface that produces an irregular layer deposition with detrimental effects on the density and quality of the part [20]. Balling increases the surface roughness and contributes to the formation of a large number of pores.
- Surface defects: the presence of a rough and non-homogeneous surface represents a critical issue for the final component. In PBF processes, surface roughness has two main contributors: the stair-stepping effect due to the layer-wise production, and the actual roughness of the metal surface. The surface finishing depends on the surface orientation with respect to the growth direction [21]. In particular, downward and upward surfaces are known to have considerably different roughness properties. The former present much lower surface quality. It is important to highlight the dependence of the fatigue resistance on the surface roughness of the stressed surface: the higher the roughness the lower the fatigue performance of the component.
- Geometric defects: the PBF produced parts may exhibit different kinds of dimensional and geometric deviations from the nominal model: shrinkage and oversizing are the most common ones. Other sources of inaccuracy are represented by warping (a curling phenomenon that yields a curved profile of down facing surfaces intended to be flat) and by the formation of super elevated edges. These phenomena deteriorate the surface topology and the dimensional accuracy while interfering with the efficiency of the recoating system as well as damaging the adjacent pieces. Other distortions affect critical features like thin walls, overhanging surfaces, and acute corners [22].
- Residual stresses, cracking, and delamination: the SLM printing is known to create in the molten components great residual stresses that could result in cracking or delamination when the arisen tensile stress exceeds the ultimate tensile strength and overcomes the binding ability between two adjacent layers [23]. As a consequence, a partial disconnection of the part from the base plate could occur.
- Microstructural inhomogeneities and impurities: PBF processes involve highly localized high-heat inputs during very short beam-material interaction times that will therefore significantly affect the microstructure of the part [24], leading to the formation of microstructural inhomogeneities or nonequilibrium microstructures that could have a detrimental influence on the mechanical and functional performances of the part. These kinds of defects includes impurities (inclusions, contaminations from other materials, and formations of surface oxides), grain size characteristics, and crystallographic textures. Furthermore, the presence of unfused powders within pores or in the form of satellite powder clumps could represent a severe problem for the safety of the device [25].
2.2.2. Visual Inspection
2.2.3. Laboratory Inspections
- Metallographic analysis: the component is cut in different positions and the sections are analyzed with a scanning electron microscope, SEM;
- Penetrant liquid testing: the component is immersed in a fluorescent liquid with high capillarity and is then analyzed under a Wood lamp to see where the liquid propagated—this test highlights only surface porosities that could be critical for biological contamination. Penetrant liquid test is a non-destructive control method, nevertheless the analyzed part could undergo contamination by the fluorescent liquid [26];
2.3. Design Tips to Improve the Final Result
- Identify the functional surfaces, where good finishing and precision are essential, in order for the producer to avoid their down facing positioning and to avoid placing supports on it.
- Avoid inserting in the design undercut features, i.e., that have some surfaces overriding a critical angle of inclination in respect to the working plane. The critical angle depends on the process, on the material, and on the parameters of the machine, but generally it is known to be around 45°. Surfaces that override this inclination need to be supported and therefore could undergo a lack of quality [31].
- If not strictly necessary, avoid the positioning of holes with a non-parallel axis in respect to the growth direction of the piece. Do not use too long bridge elements. If it is possible, position holes, pits, and through holes with the axis as closer as possible to the vertical position in order to prevent the formation of unwanted material accumulations.
- Avoid too small holes and too thin features, that would be very affected by the residual stresses and would probably deform. If such details are necessary, they will be performed using a subtractive method once the part is printed.
3. Implications for CMF Implants in Light of the New MDR
- Give evidence of the absence of toxicity in the materials of which the prosthesis is made and in all the residuals of the contaminants as well.
- Ensure the compatibility of the manufacturing materials and substances with the biological tissue, cells, and corporal fluids.
- Ensure the compatibility among the different parts of a device that is intended to have several components to be implanted.
- Verify the impact of the production process on the materials properties.
- Study the mechanical properties of the materials focusing on the strength, ductility, resistance to cracks, resistance to wear and to fatigue.
- Study the surface properties.
- Confirm that the final device respects all the chemical and physical requirements.
4. Possible Corrective Actions
- An evaluation of the biocompatibility not only of the stock material, but especially of the finished product. This kind of analysis involves several tests summed up in the regulation ISO 10993:2021 and should be performed on the finished product. It is fundamental to trace every single phase of the production process in order to understand the various contaminants that enter in contact with the prosthesis. Once the test has given positive results for a specific device, its whole process is intended to be safe from the biocompatibility point of view. The result of the analysis is considered acceptable as long as anything in the process is altered, causing a modification in the contamination chain.
- A microbiological (bioburden) and sterility test according to the ISO 11737 to evaluate the efficiency of the sterilization and of the cleaning phase. Chemical characterization test according to the ISO 17025.
- Resistance tests on melted material samples: tensile test to evaluate the σ-ε curve, rotating fatigue test to understand the performance of the material when stressed with an alternated symmetrical cycle.
- Finite element analysis (FEA) structural characterization of the final geometry of the prosthesis, according to a defined stressing conditions protocol that simulates the actual working conditions of the prosthesis, in order to investigate the static resistance and the efficiency of bone fixation. The magnitude of the loads applied should be then increased by a security factor (Figure 4).
- In case of articulations involving cycled loads of great intensity or of prosthesis in which critical geometrical features are stressed, a fatigue test on a final component should be arranged. This test should be performed with a setup representing the worst-case loading condition. Indeed, a dedicated ISO for CMF implants does not exist yet, but a proper setup should be created taking as guidelines the ISO 14801:2007 or ISO 16428:2005.
- Porosity analysis through metallographic cuts, ultrasound technology or magnetic resonance, to understand the homogeneity of the internal metallographic structure and to investigate the surface porosity as well.
- Wear test in aggressive environment replicating the actual working conditions of the prosthesis, together with a count of the residual particulate released by the contact surfaces after the test in order to give evidence of the absence or presence of fretting phenomena. In this case, ISO 17853:2011 should be taken as reference.
5. Suggestions for Your Point-of-Care 3D Printing Lab
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC (Text with EEA Relevance). 2017. Available online: http://data.europa.eu/eli/reg/2017/745/oj/eng (accessed on 24 November 2021).
- Council Directive 93/42/EEC of 14 June 1993 Concerning Medical Devices. 1993, Volume OJ L. Available online: http://data.europa.eu/eli/dir/1993/42/oj/eng (accessed on 24 November 2021).
- Regulation (EU) 2020/561 of the European Parliament and of the Council of 23 April 2020 Amending Regulation (EU) 2017/745 on Medical Devices, as Regards the Dates of Application of Certain of Its Provisions (Text with EEA Relevance). 2020. Available online: http://data.europa.eu/eli/reg/2020/561/oj/eng (accessed on 24 November 2021).
- ISO. ISO 13485—Medical Devices. Available online: https://www.iso.org/iso-13485-medical-devices.html (accessed on 24 November 2021).
- Elledge, R.; Mercuri, L.G.; Attard, A.; Green, J.; Speculand, B. Review of emerging temporomandibular joint total joint replacement systems. Br. J. Oral Maxillofac. Surg. 2019, 57, 722–728. [Google Scholar] [CrossRef]
- Lee, D.H.; Reasoner, K.; Stewart, A. From Concept to Counter: A Review of Bringing an Orthopaedic Implant to Market. J. Am. Acad. Orthop. Surg. 2020, 28, e604–e611. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Health Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [Green Version]
- Williams, F.C.; Hammer, D.A.; Wentland, T.R.; Kim, R.Y. Immediate Teeth in Fibulas: Planning and Digital Workflow with Point-of-Care 3D Printing. J. Oral Maxillofac. Surg. 2020, 78, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Gibson, I.; Rosen, D.W.; Stucker, B. Additive Manufacturing Technologies. In 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing, 2nd ed.; Springer: New York, NY, USA, 2015; ISBN 978-1-4939-2113-3. [Google Scholar]
- Grasso, M.; Colosimo, B.M. Process defects andin situmonitoring methods in metal powder bed fusion: A review. Meas. Sci. Technol. 2017, 28, 044005. [Google Scholar] [CrossRef] [Green Version]
- Arcam, A.B. Arcam Q20 Technical Data. 2015. Available online: www.arcam.com/wp-content/uploads/Arcam-Q20-final.pdf (accessed on 24 November 2021).
- EOS. Laser sintering system EOSINT M 280 for the production of tooling inserts, prototype parts and end products directly in metal. In The Technology: Laser Sintering—The Key to E-Manufacturing; EOS GmbH Electro Optical Systems Corporate Headquarters: Krailling/Munich, Germany, 2017. [Google Scholar]
- Vayssette, B.; Saintier, N.; Brugger, C.; El May, M. Surface roughness effect of SLM and EBM Ti-6Al-4V on multiaxial high cycle fatigue. Theor. Appl. Fract. Mech. 2020, 108, 102581. [Google Scholar] [CrossRef]
- Ansari, M.J.; Nguyen, D.-S.; Park, H.S. Investigation of SLM Process in Terms of Temperature Distribution and Melting Pool Size: Modeling and Experimental Approaches. Materials 2019, 12, 1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gokuldoss, P.K.; Kolla, S.; Eckert, J. Additive Manufacturing Processes: Selective Laser Melting, Electron Beam Melting and Binder Jetting—Selection Guidelines. Materials 2017, 10, 672. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Li, S.; Zhang, M.; Liu, Y.; Sercombe, T.B.; Wang, S.; Hao, Y.; Yang, R.; Murr, L.E. Comparison of the microstructures and mechanical properties of Ti–6Al–4V fabricated by selective laser melting and electron beam melting. Mater. Des. 2016, 95, 21–31. [Google Scholar] [CrossRef]
- Koike, M.; Greer, P.; Owen, K.; Lilly, G.; Murr, L.E.; Gaytan, S.M.; Martinez, E.; Okabe, T.H. Evaluation of Titanium Alloys Fabricated Using Rapid Prototyping Technologies—Electron Beam Melting and Laser Beam Melting. Materials 2011, 4, 1776–1792. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, X.; Stringer, J. Support Structures for Additive Manufacturing: A Review. J. Manuf. Mater. Process. 2018, 2, 64. [Google Scholar] [CrossRef] [Green Version]
- Edwards, P.D.; O’Conner, A.; Ramulu, M. Electron Beam Additive Manufacturing of Titanium Components: Properties and Performance. J. Manuf. Sci. Eng. 2013, 135, 061016. [Google Scholar] [CrossRef]
- Li, R.; Liu, J.; Shi, Y.; Wang, L.; Jiang, W. Balling behavior of stainless steel and nickel powder during selective laser melting process. Int. J. Adv. Manuf. Technol. 2012, 59, 1025–1035. [Google Scholar] [CrossRef]
- Fox, J.C.; Moylan, S.P.; Lane, B.M. Effect of Process Parameters on the Surface Roughness of Overhanging Structures in Laser Powder Bed Fusion Additive Manufacturing. Procedia CIRP 2016, 45, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Grasso, M.; Laguzza, V.; Semeraro, Q.; Colosimo, B.M. In-Process Monitoring of Selective Laser Melting: Spatial Detection of Defects Via Image Data Analysis. J. Manuf. Sci. Eng. 2017, 139, 051001. [Google Scholar] [CrossRef]
- Harrison, N.J.; Todd, I.; Mumtaz, K. Reduction of micro-cracking in nickel superalloys processed by Selective Laser Melting: A fundamental alloy design approach. Acta Mater. 2015, 94, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Van Humbeeck, J.; Kruth, J.-P. A study of the microstructural evolution during selective laser melting of Ti–6Al–4V. Acta Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Smith, R.J.; Hirsch, M.; Patel, R.; Li, W.; Clare, A.T.; Sharples, S.D. Spatially resolved acoustic spectroscopy for selective laser melting. J. Mater. Proc. Technol. 2016, 236, 93–102. [Google Scholar] [CrossRef]
- Rucka, M. Special Issue: “Non-Destructive Testing of Structures”. Materials 2020, 13, 4996. [Google Scholar] [CrossRef]
- Turó, A.; Chávez, J.A.; García-Hernández, M.J.; Bulkai, A.; Tomek, P.; Tóth, G.; Gironés, A.; Salazar, J. Ultrasonic inspection system for powder metallurgy parts. Measurement 2013, 46, 1101–1108. [Google Scholar] [CrossRef]
- Millon, C.; Vanhoye, A.; Obaton, A.-F.; Penot, J.-D. Development of laser ultrasonics inspection for online monitoring of additive manufacturing. Weld. World 2018, 62, 653–661. [Google Scholar] [CrossRef]
- Taud, H.; Martinez-Angeles, R.; Parrot, J.F.; Hernandez-Escobedo, L. Porosity estimation method by X-ray computed tomography. J. Pet. Sci. Eng. 2005, 47, 209–217. [Google Scholar] [CrossRef]
- Farber, L.; Tardos, G.; Michaels, J.N. Use of X-ray tomography to study the porosity and morphology of granules. Powder Technol. 2003, 132, 57–63. [Google Scholar] [CrossRef]
- Badiru, A.B.; Valencia, V.V.; Liu, D. (Eds.) Additive Manufacturing Handbook: Product Development for the Defense Industry, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-11910-6. [Google Scholar]
- Pinto-Borges, H.; Carvalho, O.; Henriques, B.; Silva, F.; Ramos, A.; Souza, J.C.M. A Preliminary Analysis of the Wear Pathways of Sliding Contacts on Temporomandibular Joint Total Joint Replacement Prostheses. Metals 2021, 11, 685. [Google Scholar] [CrossRef]
- Beckers, R.; Kwade, Z.; Zanca, F. The EU medical device regulation: Implications for artificial intelligence-based medical device software in medical physics. Phys. Med. 2021, 83, 1–8. [Google Scholar] [CrossRef]
- Martelli, N.; Eskenazy, D.; Déan, C.; Pineau, J.; Prognon, P.; Chatellier, G.; Sapoval, M.; Pellerin, O. New European Regulation for Medical Devices: What Is Changing? Cardiovasc. Interv. Radiol. 2019, 42, 1272–1278. [Google Scholar] [CrossRef]
- Vignesh, U.; Mehrotra, D.; Bhave, S.M.; Katrolia, R.; Sharma, S. Finite element analysis of patient-specific TMJ implants to replace bilateral joints with simultaneous correction of facial deformity. J. Oral Biol. Craniofacial Res. 2020, 10, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Keutzer, L.; Simonsson, U.S. Medical Device Apps: An Introduction to Regulatory Affairs for Developers. JMIR mHealth uHealth 2020, 8, e17567. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, B.; Van Boxtel, R. The Medical Device Regulation of the European Union Intensifies Focus on Clinical Benefits of Devices. Ther. Innov. Regul. Sci. 2020, 54, 613–617. [Google Scholar] [CrossRef]
- Melvin, T.; Torre, M. New medical device regulations: The regulator’s view. EFORT Open Rev. 2019, 4, 351–356. [Google Scholar] [CrossRef]



| Critical Feature Description | Recommendation for Prosthesis Design |
|---|---|
| Vertical thin walled structures with thickness from 1 to 0.1 mm | Avoid thin walled parts having thickness smaller than 0.2 mm |
| Pipe-like, hollow cylinders with different external diameters and thicknesses | |
| Small quarters of spherical shells presenting undercut surfaces | |
| Abrupt transition from semi-void section to full section | Avoid abrupt section variations whenever possible |
| Small full cross section parallelepipeds and cylinders having variable fillets/radii at their bases | It is possible to print small features with small or no fillets/radii at their conjunctions with other surfaces |
| Horizontal cylindrical holes with diameters in the range 2–8 mm, without internal supports | Horizontal holes are feasible having maximum diameter of about 8 mm, but they can be inaccurate; better results can be achieved by assuming a drop-like cross section shape |
| (Undercut) surfaces with different slope | Surface quality is affected by the staircase effect, but it can be partially improved by varying the orientation of the part with respect to the platform. Small undercut surfaces are feasible without supports |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tel, A.; Bordon, A.; Sortino, M.; Totis, G.; Fedrizzi, L.; Ocello, E.; Sembronio, S.; Robiony, M. Current Trends in the Development and Use of Personalized Implants: Engineering Concepts and Regulation Perspectives for the Contemporary Oral and Maxillofacial Surgeon. Appl. Sci. 2021, 11, 11694. https://doi.org/10.3390/app112411694
Tel A, Bordon A, Sortino M, Totis G, Fedrizzi L, Ocello E, Sembronio S, Robiony M. Current Trends in the Development and Use of Personalized Implants: Engineering Concepts and Regulation Perspectives for the Contemporary Oral and Maxillofacial Surgeon. Applied Sciences. 2021; 11(24):11694. https://doi.org/10.3390/app112411694
Chicago/Turabian StyleTel, Alessandro, Alessandra Bordon, Marco Sortino, Giovanni Totis, Lorenzo Fedrizzi, Elisabetta Ocello, Salvatore Sembronio, and Massimo Robiony. 2021. "Current Trends in the Development and Use of Personalized Implants: Engineering Concepts and Regulation Perspectives for the Contemporary Oral and Maxillofacial Surgeon" Applied Sciences 11, no. 24: 11694. https://doi.org/10.3390/app112411694
APA StyleTel, A., Bordon, A., Sortino, M., Totis, G., Fedrizzi, L., Ocello, E., Sembronio, S., & Robiony, M. (2021). Current Trends in the Development and Use of Personalized Implants: Engineering Concepts and Regulation Perspectives for the Contemporary Oral and Maxillofacial Surgeon. Applied Sciences, 11(24), 11694. https://doi.org/10.3390/app112411694








