Decision Tree-Based Data Stratification Method for the Minimization of the Masking Effect in Adverse Drug Reaction Signal Detection
Abstract
1. Introduction
2. Methods
2.1. Data Source
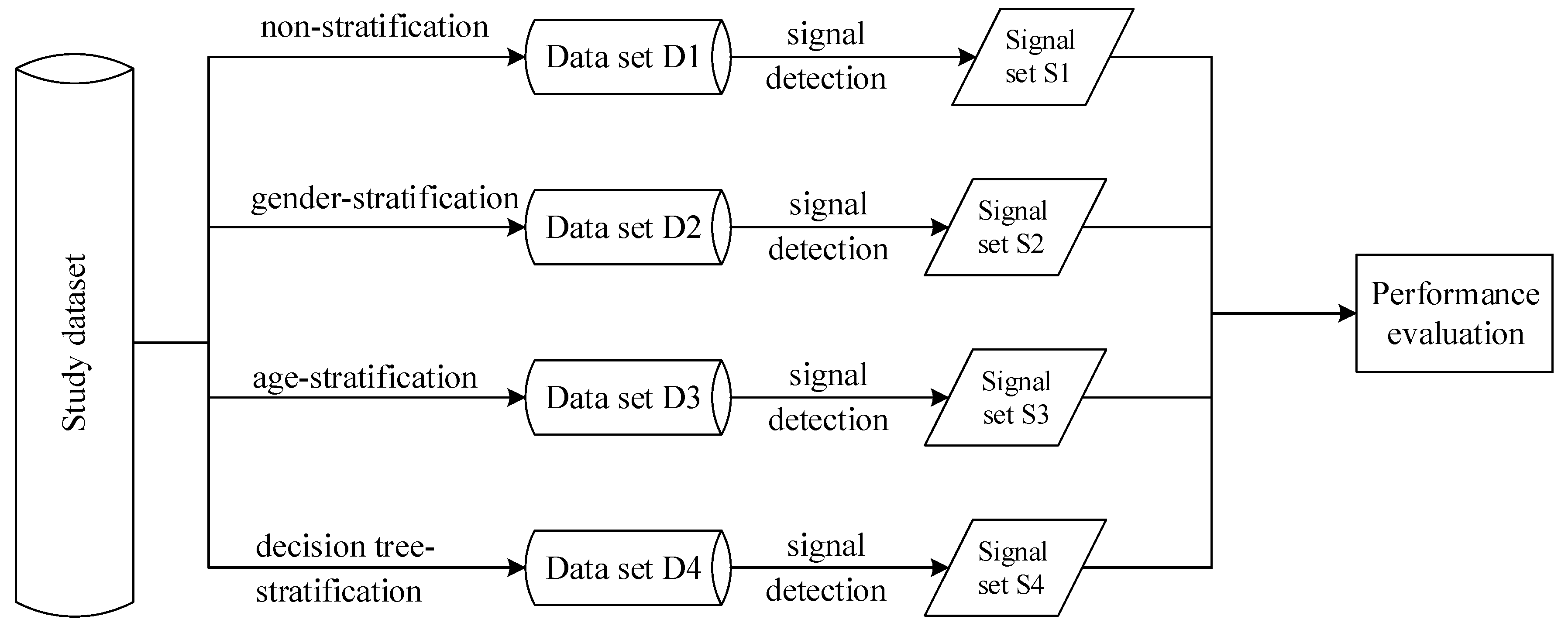
2.2. Stratification Strategy
- (1)
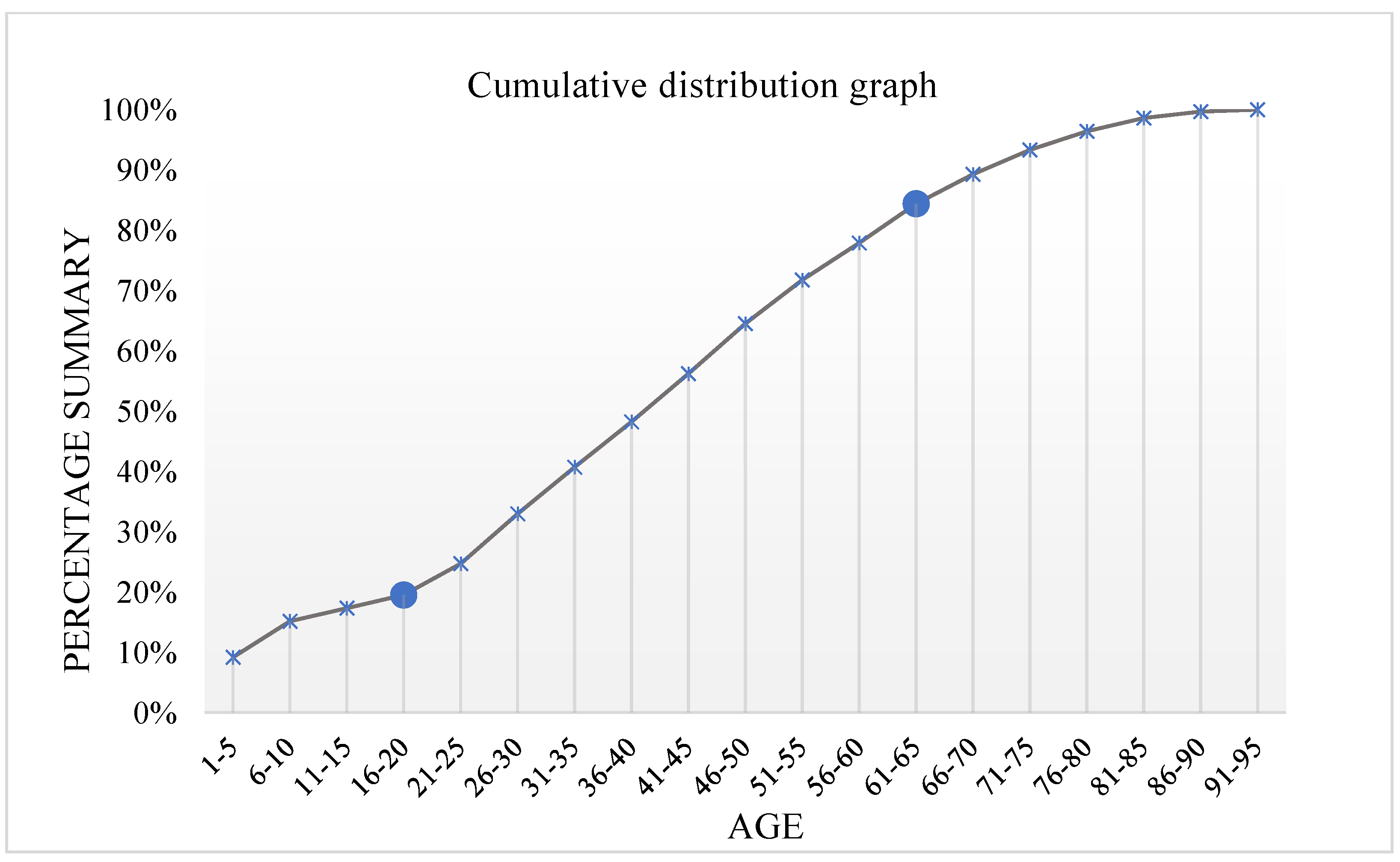
- Determining the age division intervals by using the cumulative distribution of antibiotic-related reports based on the patient’s age;
- (2)
- χ2 was used to analyze the correlation between age and drug category (“Antibiotics” or “Non-antibiotics”), as well as gender and drug category;
- (3)
- Data stratification was conducted by a classification algorithm, based on a decision tree by using drug category as the class label, and the two confounding factors of “gender” and “age” as the stratification conditions;
- (4)
- DPA was performed on the multiple datasets generated by the decision tree;
- (5)
- The performance of this method is compared with that of non-stratification, gender-stratification and age-stratification. Classification performances were assessed by means of precision, recall and F-measure.
2.3. Decision Tree
2.4. Signal Detection Method
2.5. Performance Evaluation
3. Results
3.1. Data Analysis
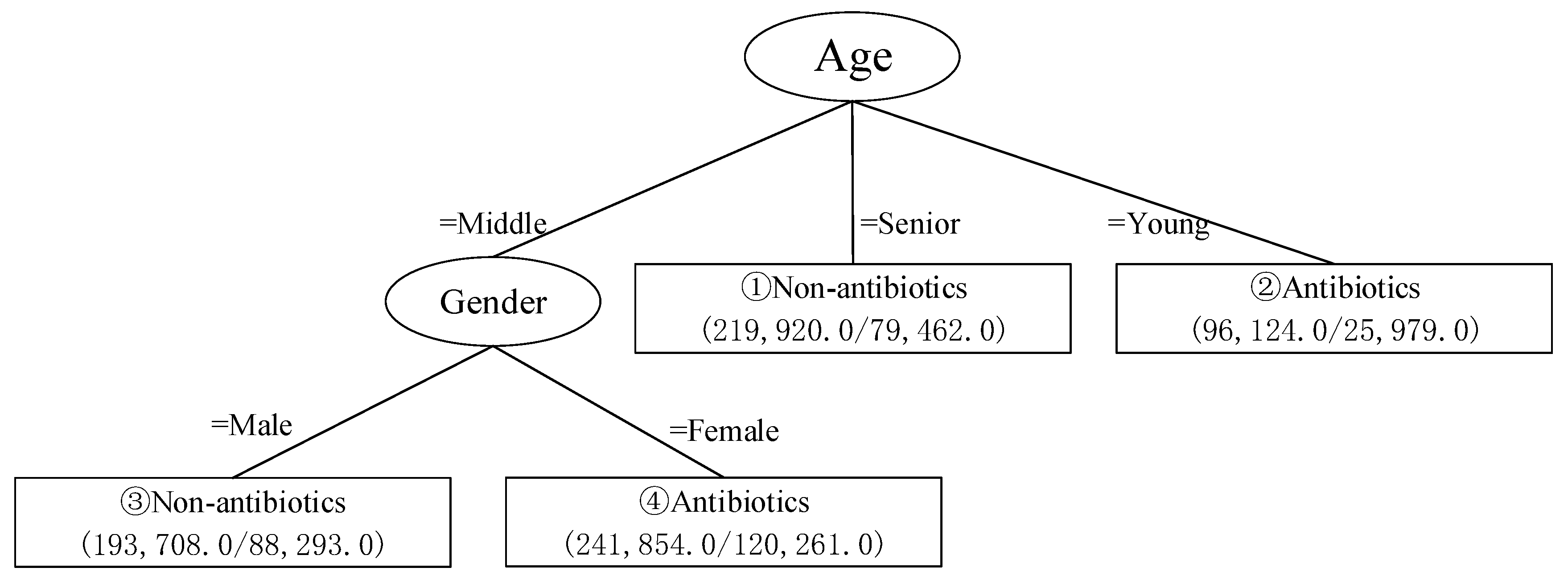
3.2. Decision Tree
- (1)
- The data meeting the condition “Age = Senior” are classified into “Non-antibiotics class”, including 219,920 reports. The accuracy rate is 63.87%.
- (2)
- The data meeting the condition “Age = Young” are classified into “Antibiotics class”, including 96,124 reports. The accuracy rate is 72.97%.
- (3)
- The data meeting the condition “Age = Middle” and “Gender = Male” are classified into “Non-antibiotics class”, including 193,708 reports. The accuracy rate is 54.42%.
- (4)
- The data meeting the condition “Age = Middle” and “Gender = Female” are classified into “Antibiotics class”, including 241,854 reports. The accuracy rate is 50.28%.
3.3. Performance Evaluation
4. Discussion
4.1. Discussion of Methods
4.2. Discussion of Results
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edwards, I.R.; Aronson, J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000, 356, 1255–1259. [Google Scholar] [CrossRef]
- Slattery, J.; Alvarez, Y.; Hidalgo, A. Choosing thresholds for statistical signal detection with the proportional reporting ratio. Drug Saf. 2013, 36, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Bate, A.; Leufkens, H.G.M.; Lindquist, M.; Orre, R.; Egberts, T. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2002, 11, 3–10. [Google Scholar] [CrossRef]
- Bate, A.; Lindquist, M.; Edwards, I.R.; Orre, R. A data mining approach for signal detection and analysis. Drug Saf. 2002, 25, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Hauben, M.; Bate, A. Decision support methods for the detection of adverse events in post-marketing data. Drug Discov. Today 2009, 14, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Menschik, D.; Bryant-Genevier, M.; Ball, R. Data mining for prospective early detection of safety signals in the vaccine adverse event reporting system (VAERS): A case study of febrile seizures after a 2010–2011 seasonal influenza virus vaccine. Drug Saf. 2013, 36, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Almenoff, J.; Tonning, J.M.; Gould, A.L.; Szarfman, A.; Hauben, M.; Ouellet-Hellstrom, R.; Ball, R.; Hornbuckle, K.; Walsh, L.; Yee, C.; et al. Perspectives on the use of data mining in pharmacovigilance. Drug Saf. 2005, 28, 981–1007. [Google Scholar] [CrossRef] [PubMed]
- Caster, O.; Norén, G.N.; Madigan, D.; Bate, A. Large-scale regression-based pattern discovery: The example of screening the WHO global drug safety database. Stat. Anal. Data Min. ASA Data Sci. J. 2010, 3, 197–208. [Google Scholar] [CrossRef]
- Zeinoun, Z.; Seifert, H.; Verstraeten, T. Quantitative signal detection for vaccines: Effects of stratification, background and masking on GlaxoSmithKline’s spontaneous reports database. Hum. Vaccines 2009, 5, 599–607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hopstadius, J.; Norén, G.N.; Bate, A.; Edwards, I.R. Impact of stratification on adverse drug reaction surveillance. Drug Saf. 2008, 31, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Mickael, A.; Francesco, S.; Ismaïl, A.; Philip, R.; Nicholas, M.; Bernard, M.; Tubert-Bitter, P.; Pariente, A. A method for the minimization of competition bias in signal detection from spontaneous reporting databases. Drug Saf. 2016, 39, 251–260. [Google Scholar]
- Barbieri, D.; Chawla, N.; Zaccagni, L.; Grgurinović, T.; Šarac, J.; Čoklo, M.; Missoni, S. Predicting cardiovascular risk in Athletes: Resampling improves classification performance. Int. J. Environ. Res. Public Health 2020, 17, 7923. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, J.; Wang, F.; Wang, B.; Liu, L.; Hou, W.; Fan, H.; Tong, Y.; Zhang, J.; Lu, Z. Overprescribing in China, driven by financial incentives, results in very high use of antibiotics, injections, and corticosteroids. Health Aff. 2012, 31, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, J.R. C4.5: Programs for Machine Learning; Morgan Kaufmann Publishers: San Francisco, CA, USA, 1993. [Google Scholar]
- Garner, S.R. WEKA: The Waikato Environment for Knowledge Analysis; Department of Computer Science, University of Waikato: Hamilton, OH, USA, 1995. [Google Scholar]
- Li, C.B.; Li, S.J. Data Warehouse and Data Mining Practice and Application; Publishing House of Electronics Industry: Beijing, China, 2014; pp. 328–330. [Google Scholar]
- Zhao, L.; Ye, X.; Wang, C.; Qian, W.; Du, W.; He, J. Application of stratification analysis in adverse drug reaction signal detection. Chin. J. Pharmacovigil. 2011, 8, 158–160. [Google Scholar]
- Walley, J.D.; Zhang, Z.; Wei, X. Antibiotic overuse in China: Call for consolidated efforts to develop antibiotic stewardship programmes. Lancet Infect. Dis. 2021, 21, 597. [Google Scholar] [CrossRef]
- Wang, H.-W.; Hochberg, A.M.; Pearson, R.K.; Hauben, M. An experimental investigation of masking in the US FDA adverse event reporting system database. Drug Saf. 2010, 33, 1117–1133. [Google Scholar] [CrossRef] [PubMed]
- Hopstadius, J.; Norén, G.N.; Bate, A.; Edwards, I.R. Stratification for spontaneous report databases. Drug Saf. 2008, 31, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
| The Target ADR | The Other ADRs | |
|---|---|---|
| the target drug | A | B |
| the other drugs | C | D |
| Drug Category | Number of Reports (Proportion) |
|---|---|
| Non-antibiotics | 392,113 (52.17%) |
| Antibiotics | 359,493 (47.83%) |
| Total | 751,606 |
| Male | Female | Young (≤20) | Middle (21~60) | Senior (≥61) | |
|---|---|---|---|---|---|
| Antibiotics | 170,739 | 188,754 | 25,979 | 225,676 | 140,458 |
| Non-antibiotics | 194,618 | 197,495 | 70,145 | 209,886 | 79,462 |
| χ2 | 343.42 | 36,435.81 | |||
| Method | TP | FP | FN | R | P | F |
|---|---|---|---|---|---|---|
| non-stratification | 7293 | 28,781 | 6661 | 52.26% | 20.22% | 29.16% |
| gender-stratification | 8369 | 34,236 | 5585 | 59.98% | 19.64% | 29.59% |
| age-stratification | 8977 | 35,591 | 4977 | 64.33% | 20.14% | 30.68% |
| decision tree-stratification | 9605 | 38,225 | 4349 | 68.83% | 20.08% | 31.09% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Cheng, L.; Han, P.; Zhu, Y.; Huang, W. Decision Tree-Based Data Stratification Method for the Minimization of the Masking Effect in Adverse Drug Reaction Signal Detection. Appl. Sci. 2021, 11, 11380. https://doi.org/10.3390/app112311380
Wei J, Cheng L, Han P, Zhu Y, Huang W. Decision Tree-Based Data Stratification Method for the Minimization of the Masking Effect in Adverse Drug Reaction Signal Detection. Applied Sciences. 2021; 11(23):11380. https://doi.org/10.3390/app112311380
Chicago/Turabian StyleWei, Jianxiang, Lu Cheng, Pu Han, Yunxia Zhu, and Weidong Huang. 2021. "Decision Tree-Based Data Stratification Method for the Minimization of the Masking Effect in Adverse Drug Reaction Signal Detection" Applied Sciences 11, no. 23: 11380. https://doi.org/10.3390/app112311380
APA StyleWei, J., Cheng, L., Han, P., Zhu, Y., & Huang, W. (2021). Decision Tree-Based Data Stratification Method for the Minimization of the Masking Effect in Adverse Drug Reaction Signal Detection. Applied Sciences, 11(23), 11380. https://doi.org/10.3390/app112311380






