Physicochemical, Sensory Properties and Lipid Oxidation of Chicken Sausages Supplemented with Three Types of Seaweed
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Processing of Seaweed
2.2. Chicken Sausage Preparation and Processing
2.3. Proximate Composition and Total Dietary Fiber (TDF)
2.4. Water Holding Capacity
2.5. Cooking Loss
2.6. Colour
2.7. Texture Profile Analysis (TPA)
2.8. Sensory Evaluation
2.9. Sample Extraction
2.10. Measurement of Total Phenolic Content (TPC)
2.11. Antioxidant Capacity
2.12. Thiobarbituric Acid Reactive Substance (TBARS)
2.13. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition and Total Dietary Fiber
3.2. Water Holding Capacity, Cooking Loss and Colour
3.3. Texture
3.4. Sensory Characteristics
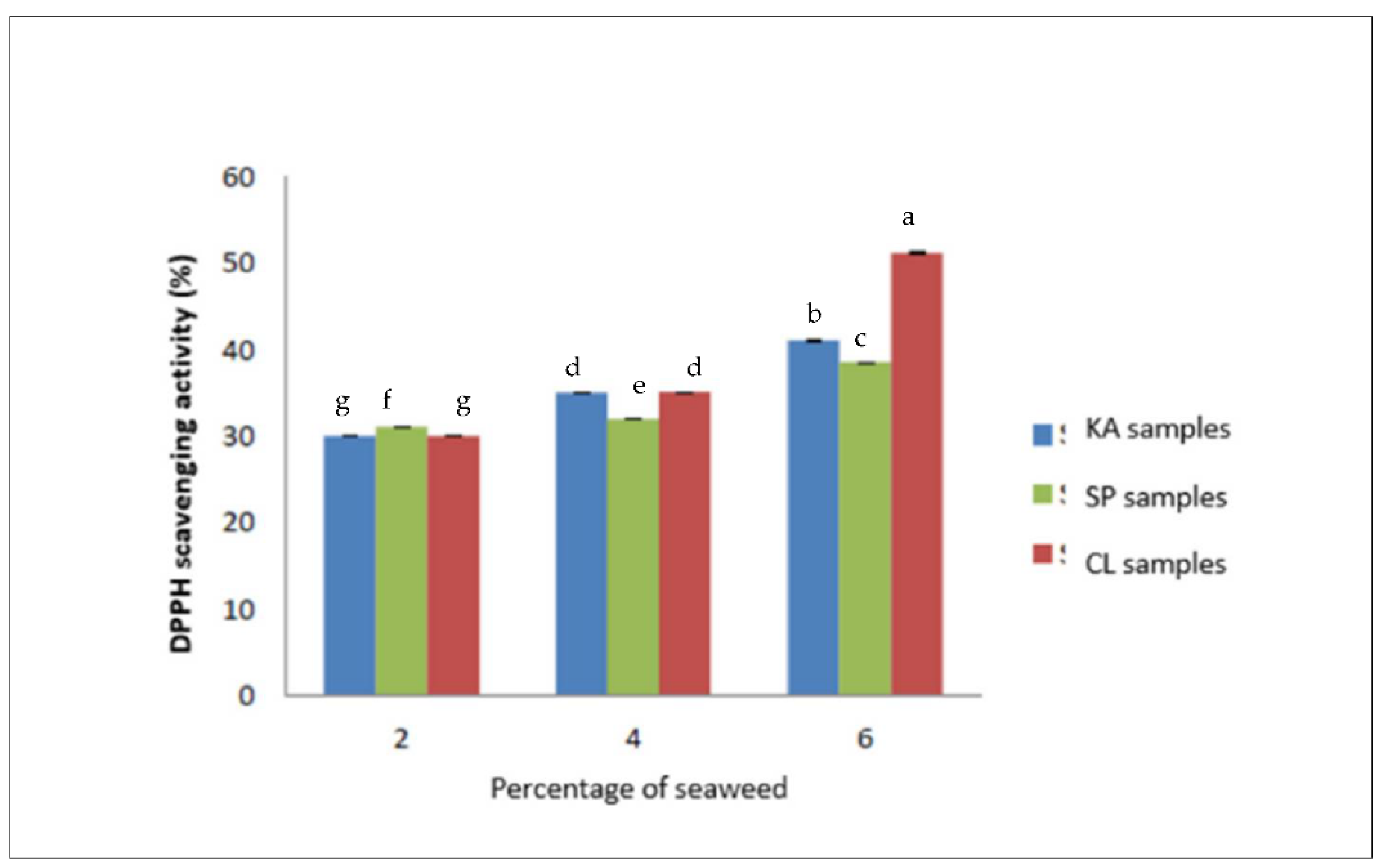
3.5. Total Phenolic Content (TPC) and Antioxidant Activity
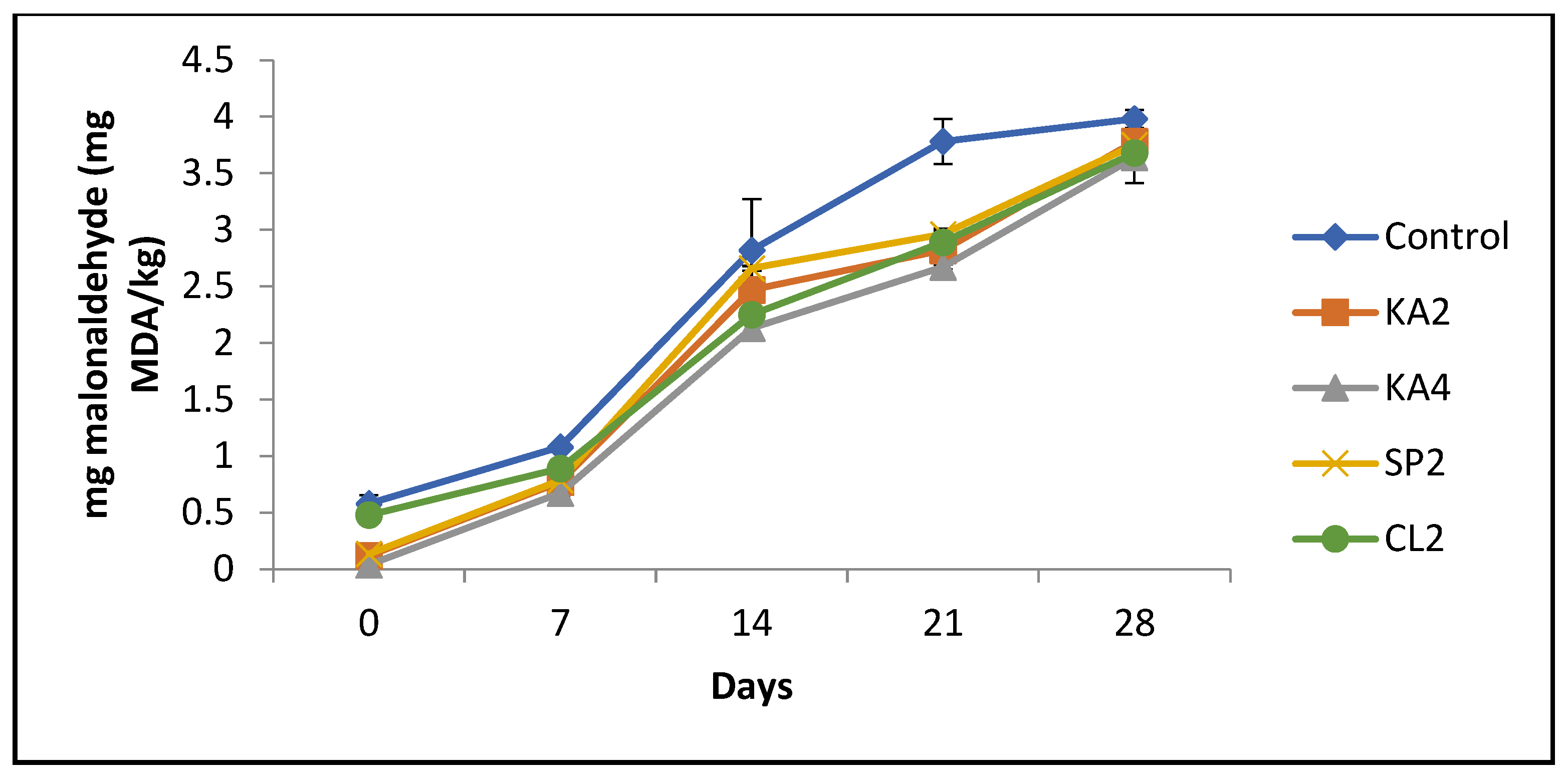
3.6. Lipid Oxidation of Sausage during Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klurfeld, D.M. What is the role of meat in a heathy diet? Anim. Front. 2018, 8, 5–10. [Google Scholar] [CrossRef]
- Cofrades, S.; Benedí, J.; Garcimartin, A.; Sánchez-Muniz, F.; Jimenez-Colmenero, F. A comprehensive approach to formulation of seaweed-enriched meat products: From technological development to assessment of healthy properties. Food Res. Int. 2017, 99, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla-Alonso, B.; Jimenez-Colmenero, F.; Sánchez-Muniz, F.J. Development and assessment of healthy properties of meat and meat products designed as functional foods. Meat Sci. 2013, 95, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Gullón, P.; Astray, G.; Gullón, B.; Franco, D.; Campagnol, P.C.B.; Lorenzo, J.M. Inclusion of seaweeds as healthy approach to formulate new low-salt meat products. Curr. Opin. Food Sci. 2021, 40, 20–25. [Google Scholar] [CrossRef]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Sanchez-Machado, D.I.; Lopez-Cervantes, J.; Lopez-Hernandez, J.; Paseiro-Losada, P.; Simal-Lozano, J. Determination of the uronic acid composition of seaweed dietary fibre by HPLC. Biomed. Chromatogr. 2004, 18, 90–97. [Google Scholar] [CrossRef]
- Gressler, V.; Fujii, M.T.; Martins, A.P.; Colepicolo, P.; Mancini-Filho, J.; Pinto, E. Biochemical composition of two red seaweed species grown on the Brazilian coast. J. Sci. Food Agric. 2011, 91, 1687–1692. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Syad, A.N.; Shunmugiah, K.P.; Kasi, P.D. Seaweeds as nutritional supplements: Analysis of nutritional profile, physicochemical properties and proximate composition of G. acerosa and S. wightii. Biomed. Prev. Nutr. 2013, 3, 139–144. [Google Scholar] [CrossRef]
- Taboada, M.C.; Millán, R.; Miguez, M.I. Nutritional value of the marine algae wakame (Undaria pinnatifida) and nori (Porphyra purpurea) as food supplements. Environ. Boil. Fishes 2013, 25, 1271–1276. [Google Scholar] [CrossRef]
- O’Sullivan, A.; O’Grady, M.; O’Callaghan, Y.; Smyth, T.; O’Brien, N.; Kerry, J. Seaweed extracts as potential functional ingredients in yogurt. Innov. Food Sci. Emerg. Technol. 2016, 37, 293–299. [Google Scholar] [CrossRef]
- Lamont, T.; McSweeney, M. Consumer acceptability and chemical composition of whole-wheat breads incorporated with brown seaweed (Ascophyllum nodosum) or red seaweed (Chondrus crispus). J. Sci. Food Agric. 2021, 101, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Mamat, H.; Akanda, J.; Zainol, K.; Ling, Y.A. The Influence of Seaweed Composite Flour on the Physicochemical Properties of Muffin. J. Aquat. Food Prod. Technol. 2018, 27, 635–642. [Google Scholar] [CrossRef]
- Fradinho, P.; Raymundo, A.; Sousa, I.; Domínguez, H.; Torres, M.D. Edible Brown Seaweed in Gluten-Free Pasta: Technological and Nutritional Evaluation. Foods 2019, 8, 622. [Google Scholar] [CrossRef]
- Budi, P.H.; Thaib, E.A.; Julita, M. Use of Sargassum polycystum ethanol extract as antibacterial for increasing shelf life tilapia fillet (Oreochromis niloticus) stored in chilling temperature. IOP Conf. Series Earth Environ. Sci. 2019, 278, 012012. [Google Scholar] [CrossRef]
- Sihono, S.; Utomo, B.S.B.; Nurhayati, N. Molecular Identification, Nutritional Profile and Heavy Metals Content of Edible Caulerpa from Binuangeun Coast, Banten. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2021, 16, 83–92. [Google Scholar] [CrossRef]
- Tresnati, J.; Yasir, I.; Aprianto, R.; Tuwo, A. Metal bioaccumulation potential of the seaweed Kappaphycus alvarezii. IOP Conf. Series Earth Environ. Sci. 2021, 763, 012059. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, H.W.; Song, D.H.; Choi, J.H.; Park, J.; Kim, M.Y.; Kim, C.J. Quality characteristics and sensory properties of reduced-fat emulsion sausages with brown rice fibre. Korean J. Food Sci. Anim. Resour. 2011, 31, 521–529. [Google Scholar] [CrossRef]
- Pindi, W.; Mah, H.W.; Munsu, E.; Noorakmar, A.W. Effects of addition of Kappaphycus alvareziii on physicochemical properties and lipid oxidation of mechanically deboned chicken meat (MDCM) sausages. Br. Food J. 2017, 119, 2229–2239. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemistry: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.H. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J. Appl. Phycol. 2008, 20, 367–373. [Google Scholar] [CrossRef]
- Hu, H.-Y.; Pereira, J.; Xing, L.-J.; Hu, Y.-Y.; Qiao, C.-L.; Zhou, G.-H.; Zhang, W.-G. Effects of regenerated cellulose emulsion on the quality of emulsified sausage. LWT 2016, 70, 315–321. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Tułodziecka, A.; Szłyk, E. Determination of Antioxidant Capacity of Unprocessed and Processed Food Products by Spectrophotometric Methods. Food Anal. Methods 2011, 5, 807–813. [Google Scholar] [CrossRef][Green Version]
- Yen, G.-C.; Chen, H.-Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Salmiéri, S.; Saucier, L.; Lacroix, M. Antimicrobial and Antioxidant Effects of Milk Protein-Based Film Containing Essential Oils for the Preservation of Whole Beef Muscle. J. Agric. Food Chem. 2004, 52, 5598–5605. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, X.-L.; Zhou, G.-H. Effects of Meat and Phosphate Level on Water-Holding Capacity and Texture of Emulsion-Type Sausage During Storage. Agric. Sci. China 2009, 8, 1475–1481. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N. Enhancement of the phytochemical and fibre content of beef-patties with Himanthalia Elongata seaweed. Int. J. Food Sci. 2013, 48, 2239–2249. [Google Scholar]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by products of food processing: Characterization, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Sanchez-Zapata, E.; Fernandez-Lopez, J.; Penranda, M.; Fuentes-Zarago, S.E.; Perez-Alverez, A. Technological properties of date paste obtained from date by-products and its effect on quality of cooked meat product. Int. Food Res. J. 2011, 44, 2401–2407. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jimenez-Escrig, A.; Rupérez, P. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Tanase, C.; Coşarcă, S.; Muntean, D.A. Critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef]
- Chinnadurai, S. Estimation of major pigment content in seaweeds collected from Pondicherry coast. J. Food Sci. Technol. 2013, 9, 522–525. [Google Scholar]
- Choi, Y.-S.; Choi, J.-H.; Han, D.-J.; Kim, H.-Y.; Kim, H.-W.; Lee, M.-A.; Chung, H.-J.; Kim, C.-J. Effects of Laminaria japonica on the physico-chemical and sensory characteristics of reduced-fat pork patties. Meat Sci. 2012, 91, 1–7. [Google Scholar] [CrossRef]
- Cofrades, S.; López-López, I.; Solas, M.; Bravo, L.; Jimenez-Colmenero, F. Influence of different types and proportions of added edible seaweeds on characteristics of low-salt gel/emulsion meat systems. Meat Sci. 2008, 79, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hijika fusiformis, a common edible seaweed. Biosci. Biotechnol. 1999, 63, 605–607. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, Y.; Liu, H.; Liu, S.; Qin, Y.; Li, P. Advances in cultivation, wastewater treatment application, bioactive components of Caulerpa lentillifera and their biotechnological applications. PeerJ 2019, 7, e6118. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Ahn, D.U. Mechanism of Lipid Peroxidation in Meat and Meat Products-A Review. Food Sci. Technol. 2005, 14, 152–163. [Google Scholar]
- KARPIŃSKA-TYMOSZCZYK, M. The effect of oil-soluble rosemary extract, sodium erythorbate, their mixture, and packaging method on the quality of Turkey meatballs. J. Food Sci. Technol. 2011, 50, 443–454. [Google Scholar] [CrossRef]
| Characteristics | Kappaphycus alvarezii (KA) | Sargassum polycystum (SP) | Caulerpa lentilifira (CL) |
|---|---|---|---|
| Moisture content, % | 16.67 ± 0.20 A | 15.28 ± 0.01 B | 11.91 ± 0.01 C |
| Ash content, % | 33.99 ± 0.39 A | 34.12 ± 0.21 A | 32.70 ± 1.65 A |
| Protein content, % | 5.08 ± 0.02 B | 5.20 ± 0.02 B | 11.68 ± 0.15 A |
| Fat content, % | 1.26 ± 0.04 B | 0.24 ± 0.10 B | 2.88 ± 0.05 A |
| Total dietaryfiber, % | 24.79 ± 0.58 B | 38.05 ± 0.21 A | 34.42 ± 0.41 B |
| Total phenolic content (mg GAE/g) | 1.65 ± 0.06 B | 2.25 ± 0.06 B | 7.18 ± 0.66 A |
| DPPH (SA%) | 52.40 ± 0.30 C | 54.29 ± 0.06 B | 57.22 ± 0.91 A |
| Ingredients | Treatments (g/100 g) | |||
|---|---|---|---|---|
| Control | F2 | F4 | F6 | |
| Chicken meat | 65 | 65 | 65 | 65 |
| Fat | 10 | 10 | 10 | 10 |
| Ice water | 14.9 | 14.9 | 14.9 | 14.9 |
| Isolate soy protein | 5 | 5 | 5 | 5 |
| Potato starch | 3 | 3 | 3 | 3 |
| Salt | 1.5 | 1.5 | 1.5 | 1.5 |
| Other seasoning | 0.6 | 06 | 0.6 | 0.6 |
| Total | 100 | 100 | 100 | 100 |
| KA | 0 | 2 | 4 | 6 |
| SP | 0 | 2 | 4 | 6 |
| CL | 0 | 2 | 4 | 6 |
| Parameter | Seaweed Powder Percentage | KA | SP | CL |
|---|---|---|---|---|
| Moisture | 0% | 68.87 ± 0.09 a | 68.87 ± 0.09 a | 68.87 ± 0.09 a |
| 2% | 67.94 ± 0.58 bB | 68.75 ± 0.03 aA | 68.88 ± 0.28 aA | |
| 4% | 67.29 ± 0.22 bA | 66.65 ± 0.53 bB | 68.30 ± 0.26 aA | |
| 6% | 65.43 ± 0.85 cB | 66.56 ± 0.44 bA | 67.73 ± 0.05 bA | |
| Ash | 0% | 0.64 ± 0.68 b | 0.64 ± 0.68 b | 0.64 ± 0.69 a |
| 2% | 0.91 ± 0.09 bA | 1.60 ± 0.49 bA | 1.15 ± 0.05 aA | |
| 4% | 1.33 ± 0.02 abB | 3.57 ± 0.54 aA | 1.14 ± 0.02 aB | |
| 6% | 2.17 ± 0.08 aB | 3.93 ± 0.20 aA | 1.28 ± 0.10 aC | |
| Protein | 0% | 19.23 ± 0.66 a | 19.23 ± 0.66 a | 19.23 ± 0.66 a |
| 2% | 19.49 ± 0.38 aA | 19.88 ± 0.01 aA | 19.71 ± 0.19 aA | |
| 4% | 19.18 ± 0.17 aA | 19.84 ± 0.02 aA | 19.64 ± 0.03 aA | |
| 6% | 19.03 ± 0.02 aA | 19.29 ± 0.42 aA | 19.56 ± 0.03 aA | |
| Fat | 0% | 10.11 ± 0.37 a | 10.11 ± 0.37 a | 10.11 ± 0.37 a |
| 2% | 9.58 ± 0.11 bA | 9.76 ± 0.93 bA | 9.37 ± 0.46 bA | |
| 4% | 8.25 ± 0.74 cB | 9.46 ± 0.33 bA | 8.97 ± 0.11 cAB | |
| 6% | 7.29 ± 0.42 cB | 8.50 ± 0.13 bA | 8.85 ± 0.39 cA | |
| Dietary fiber | 0% | 0.07 ± 0.05 c | 0.07 ± 0.05 c | 0.07 ± 0.05 c |
| 2% | 0.93 ± 0.05 bC | 1.22 ± 0.01 bA | 1.02 ± 0.01 cB | |
| 4% | 1.02 ± 0.03 bC | 1.29 ± 0.01 bB | 1.47 ± 0.03 bA | |
| 6% | 1.38 ± 0.20 aB | 2.67 ± 0.11 aA | 1.63 ± 0.06 aB |
| Treatment | Water Holding Capacity (%) | Cooking Loss (%) | Colour | ||
|---|---|---|---|---|---|
| Lightness (L*) | Redness (a*) | Yellowness (b*) | |||
| Control | 8.86 ± 0.67 ab | 12.22 ± 0.65 a | 63.50 ± 1.53 a | 0.84 ± 0.10 e | 13.46 ± 0.20 ab |
| KA2 | 2.87 ± 0.81 fg | 11.23 ± 0.96 ab | 60.27 ± 2.05 b | 0.99 ± 0.11 d | 13.24 ± 0.25 ab |
| KA4 | 1.80 ± 0.20 gh | 10.69 ± 0.09 b | 57.62 ± 1.31 c | 1.08 ± 1.32 cd | 13.13 ± 0.41 ab |
| KA6 | 1.60 ± 0.22 h | 9.79 ± 0.17 c | 56.11 ± 1.30 c | 1.21 ± 0.10 bce | 12.93 ± 0.24 b |
| SP2 | 7.31 ± 1.37 bc | 11.50 ± 0.06 a | 42.86 ± 1.88 e | 1.03 ± 0.12 c | 11.95 ± 0.14 d |
| SP4 | 5.22 ± 0.61 de | 9.54 ± 0.18 c | 37.54 ± 1.44 f | 1.34 ± 0.12 be | 10.87 ± 0.32 d |
| SP6 | 4.69 ± 1.13 def | 7.84 ± 0.41 d | 33.53 ± 0.60 g | 1.61 ± 0.07 a | 10.82 ± 0.44 c |
| CL2 | 5.55 ± 0.51 cd | 11.27 ± 0.08 a | 45.93 ± 0.60 d | −4.17 ± 0.06 g | 13.66 ± 0.36 b |
| CL4 | 3.75 ± 0.99 ef | 6.51 ± 0.33 ef | 39.00 ± 1.98 f | −4.15 ± 0.22 g | 12.74 ± 0.20 cd |
| CL6 | 2.56 ± 0.39 g | 6.15 ± 0.77 f | 36.72 ± 0.94 f | −2.40 ± 0.11 f | 11.48 ± 0.79 ab |
| Parameter | Seaweed Powder | KA | SP | CL |
|---|---|---|---|---|
| Hardness (N) | 0% | 23.24 ± 1.49 d | 23.24 ± 1.49 d | 23.24 ± 1.49 d |
| 2% | 39.96 ± 1.77 cC | 57.41 ± 3.95 bA | 42.15 ± 1.96 aB | |
| 4% | 56.55 ± 1.89 bB | 62.37 ± 2.68 bA | 43.82 ± 6.49 aC | |
| 6% | 67.10 ± 1.72 aB | 81.84 ± 9.12 aA | 46.04 ± 6.90 aC | |
| Cohesiveness | 0% | 0.66 ± 0.02 a | 0.66 ± 0.02 a | 0.66 ± 0.02 a |
| 2% | 0.64 ± 0.01 aA | 0.65 ± 0.00 aA | 0.71 ± 0.01 aA | |
| 4% | 0.67 ± 0.02 aA | 0.65 ± 0.00 aA | 0.64 ± 0.04 aA | |
| 6% | 0.67 ± 0.01 aA | 0.65 ± 0.00 aA | 0.67 ± 0.11 aA | |
| Springiness | 0% | 0.86 ± 0.09 a | 0.86 ± 0.09 a | 0.86 ± 0.09 a |
| 2% | 0.91 ± 0.06 aA | 0.86 ± 0.02 aA | 0.88 ± 0.03 aA | |
| 4% | 0.91 ± 0.01 aA | 0.82 ± 0.01 aA | 0.87 ± 0.04 aA | |
| 6% | 0.89 ± 0.09 aA | 0.86 ± 0.09 aA | 0.87 ± 0.08 aA | |
| Chewiness | 0% | 14.78 ± 3.14 d | 14.78 ± 3.14 d | 14.78 ± 3.14 d |
| 2% | 21.23 ± 1.58 cC | 34.99 ± 1.27 bA | 26.94 ± 2.96 aB | |
| 4% | 34.33 ± 1.53 bA | 32.84 ± 2.17 bA | 25.07 ± 2.80 aB | |
| 6% | 39.67 ± 0.97 aB | 42.72 ± 6.52 aA | 26.10 ± 2.11 aC |
| Sensory Assessement | Seaweed Powder Percentage | KA | SP | CL |
|---|---|---|---|---|
| Shape | 0% | 6.41 ± 0.71 ab | 6.41 ± 0.71 a | 6.41 ± 0.71 a |
| 2% | 6.69 ± 0.89 aA | 5.60 ± 0.64 bA | 4.86 ± 0.55 bB | |
| 4% | 6.21 ± 1.00 abA | 4.44 ± 1.23 cB | 4.68 ± 0.67 bB | |
| 6% | 6.14 ± 1.03 bA | 3.85 ± 1.22 dB | 3.88 ± 0.98 cB | |
| Colour | 0% | 6.24 ± 0.74 ab | 6.24 ± 0.74 a | 6.24 ± 0.74 a |
| 2% | 6.58 ± 0.86 aA | 4.94 ± 0.90 bB | 4.23 ± 0.54 bB | |
| 4% | 6.08 ± 1.04 abA | 3.74 ± 1.19 cB | 4.21 ± 0.58 bB | |
| 6% | 5.89 ± 1.09 Ba | 3.60 ± 1.37 cB | 3.38 ± 0.74 cB | |
| Aroma | 0% | 6.26 ± 0.72 ab | 6.26 ± 0.72 a | 6.26 ± 0.72 a |
| 2% | 6.42 ± 0.92 aA | 6.57 ± 0.89 aA | 4.42 ± 0.48 bB | |
| 4% | 6.10 ± 1.15 abA | 4.43 ± 1.43 bB | 4.25 ± 0.56 bB | |
| 6% | 5.71 ± 0.99 bA | 4.07 ± 1.56 bA | 3.61 ± 0.93 cA | |
| Taste | 0% | 6.00 ± 0.71 ab | 6.00 ± 0.71 a | 6.00 ± 0.71 a |
| 2% | 6.37 ± 0.87 aA | 6.05 ± 0.77 aA | 4.62 ± 0.70 bB | |
| 4% | 5.58 ± 1.01 bA | 4.68 ± 0.98 bB | 4.42 ± 0.83 bB | |
| 6% | 5.88 ± 0.94 abA | 3.04 ± 1.07 cB | 3.68 ± 0.79 cB | |
| Hardness | 0% | 6.05 ± 0.63 ab | 6.05 ± 0.63 a | 6.05 ± 0.63 a |
| 2% | 6.22 ± 0.75 aA | 6.40 ± 0.55 aA | 4.56 ± 0.65 bB | |
| 4% | 5.61 ± 0.98 bcA | 4.90 ± 0.96 bA | 4.42 ± 0.72 bB | |
| 6% | 5.39 ± 0.99 cA | 3.50 ± 0.88 cB | 3.72 ± 0.78 cB | |
| Juiciness | 0% | 5.90 ± 0.64 ab | 5.90 ± 0.64 a | 5.90 ± 0.64 a |
| 2% | 6.20 ± 0.75 aA | 4.60 ± 0.47 bB | 4.58 ± 0.64 bB | |
| 4% | 5.44 ± 0.99 bA | 4.63 ± 0.92 bA | 4.46 ± 0.68 bA | |
| 6% | 5.57 ± 0.93 bA | 3.01 ± 0.92 cB | 3.79 ± 0.89 cB | |
| Overall | 0% | 6.26 ± 0.67 ab | 6.26 ± 0.67 a | 6.26 ± 0.67 a |
| 2% | 6.45 ± 0.88 aA | 5.92 ± 0.60 aAB | 4.94 ± 0.64 bB | |
| 4% | 5.81 ± 1.00 bcA | 5.32 ± 0.50 bB | 4.82 ± 0.68 bB | |
| 6% | 5.65 ± 0.95 cA | 3.46 ± 1.10 cB | 4.00 ± 0.86 cB |
| Seaweed Powder | KA | SP | CL |
|---|---|---|---|
| 0% | 0.15 ± 0.26 c | 0.15 ± 0.26 c | 0.15 ± 0.26 d |
| 2% | 1.92 ± 0.04 bA | 1.57 ± 0.00 bA | 1.98 ± 0.13 cA |
| 4% | 3.59 ± 0.65 aA | 3.44 ± 0.15 aA | 4.27 ± 0.00 bA |
| 6% | 4.13 ± 0.79 aB | 3.59 ± 0.07 aC | 6.65 ± 0.09 aA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munsu, E.; Mohd Zaini, H.; Matanjun, P.; Ab Wahab, N.; Sulaiman, N.S.; Pindi, W. Physicochemical, Sensory Properties and Lipid Oxidation of Chicken Sausages Supplemented with Three Types of Seaweed. Appl. Sci. 2021, 11, 11347. https://doi.org/10.3390/app112311347
Munsu E, Mohd Zaini H, Matanjun P, Ab Wahab N, Sulaiman NS, Pindi W. Physicochemical, Sensory Properties and Lipid Oxidation of Chicken Sausages Supplemented with Three Types of Seaweed. Applied Sciences. 2021; 11(23):11347. https://doi.org/10.3390/app112311347
Chicago/Turabian StyleMunsu, Elisha, Hana Mohd Zaini, Patricia Matanjun, Noorakmar Ab Wahab, Nurul Shaeera Sulaiman, and Wolyna Pindi. 2021. "Physicochemical, Sensory Properties and Lipid Oxidation of Chicken Sausages Supplemented with Three Types of Seaweed" Applied Sciences 11, no. 23: 11347. https://doi.org/10.3390/app112311347
APA StyleMunsu, E., Mohd Zaini, H., Matanjun, P., Ab Wahab, N., Sulaiman, N. S., & Pindi, W. (2021). Physicochemical, Sensory Properties and Lipid Oxidation of Chicken Sausages Supplemented with Three Types of Seaweed. Applied Sciences, 11(23), 11347. https://doi.org/10.3390/app112311347







