Relationship between Anti-Wrinkle Property of Cotton Fabrics and Crosslinking Properties of Glycosyl Polyaldehydes and Polyuronic Acids Finishing Agents: A Molecular Simulation Study
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
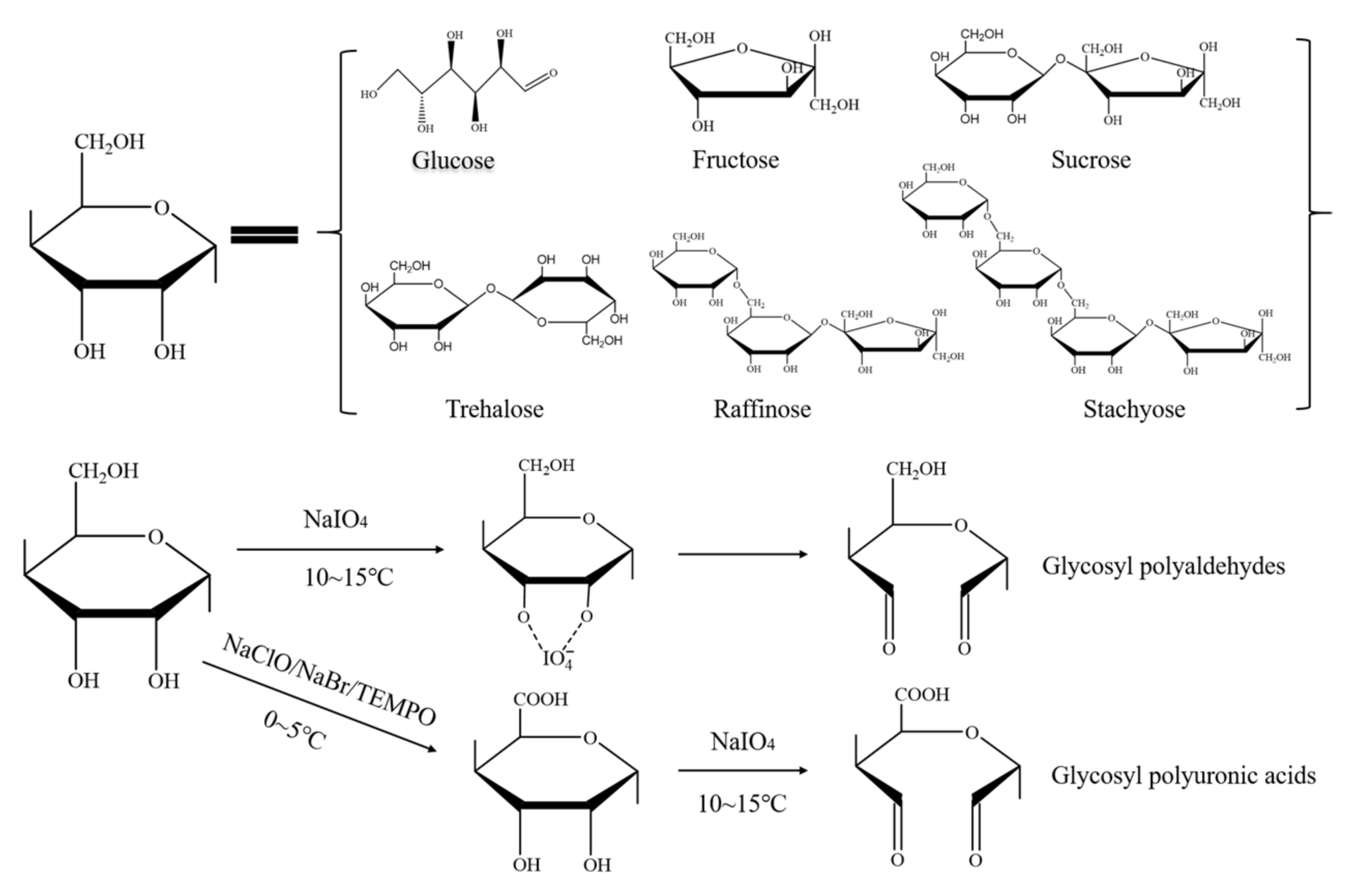
2.2.1. Preparation of the Glycosyl Polyaldehydes
2.2.2. Preparation of the Glycosyl Polyuronic Acid
2.2.3. Anti-Crease Treatment of Cotton Fabric
2.2.4. Properties of Treated Fabrics
2.2.5. Molecular Volume (CSEV)
2.2.6. Number of Rotatable Bonds (RBN)
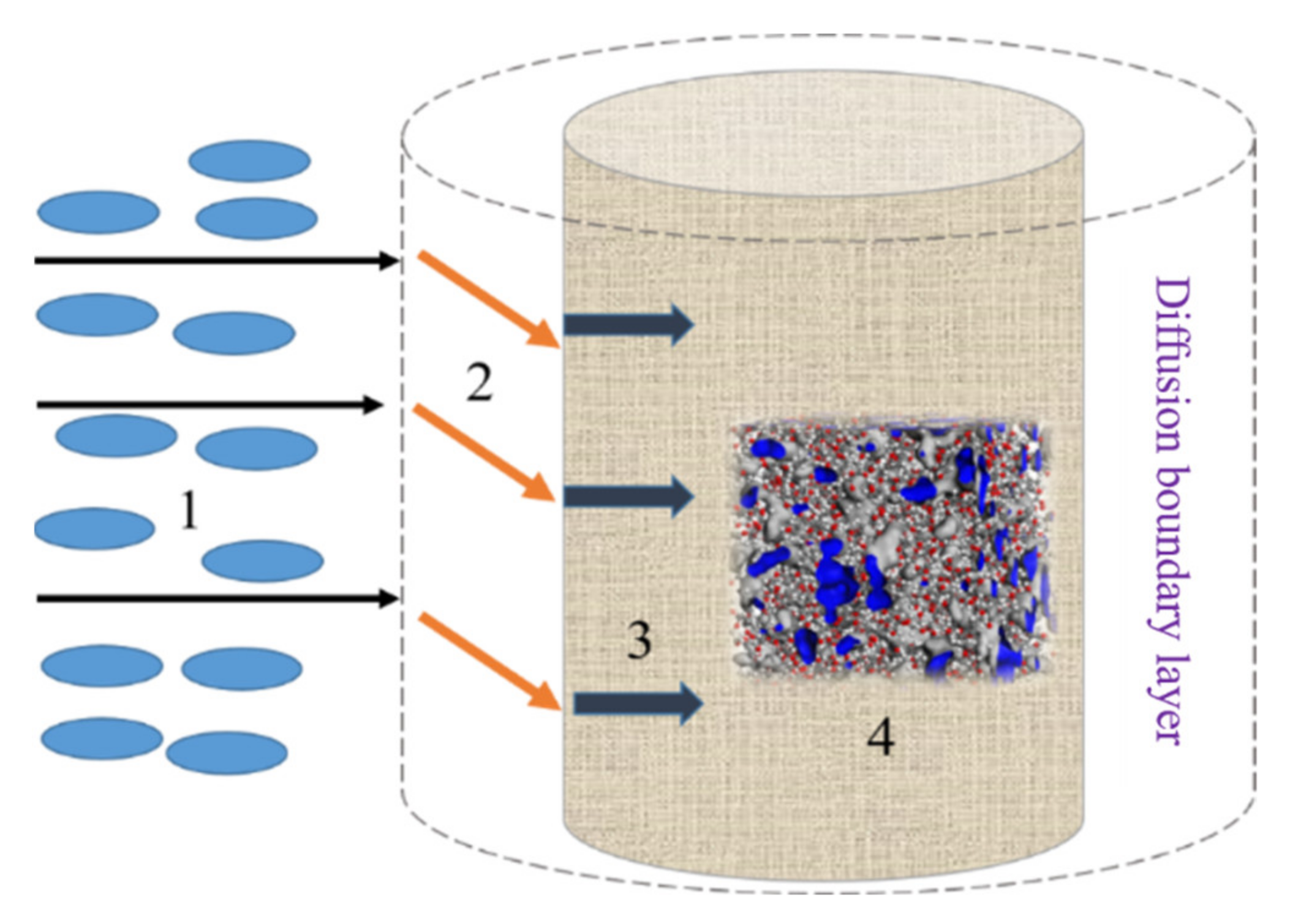
2.2.7. Calculation of Molecular Cross-Linking Distribution Range of Anti-Wrinkle Finishing Agent
3. Results and Discussion
3.1. Anti-Wrinkle Performance with Glycosyl Formaldehyde-Free Finishing Agents
3.2. Molecular Size Analysis of Glycosyl Anti-Wrinkle Finishing Agent
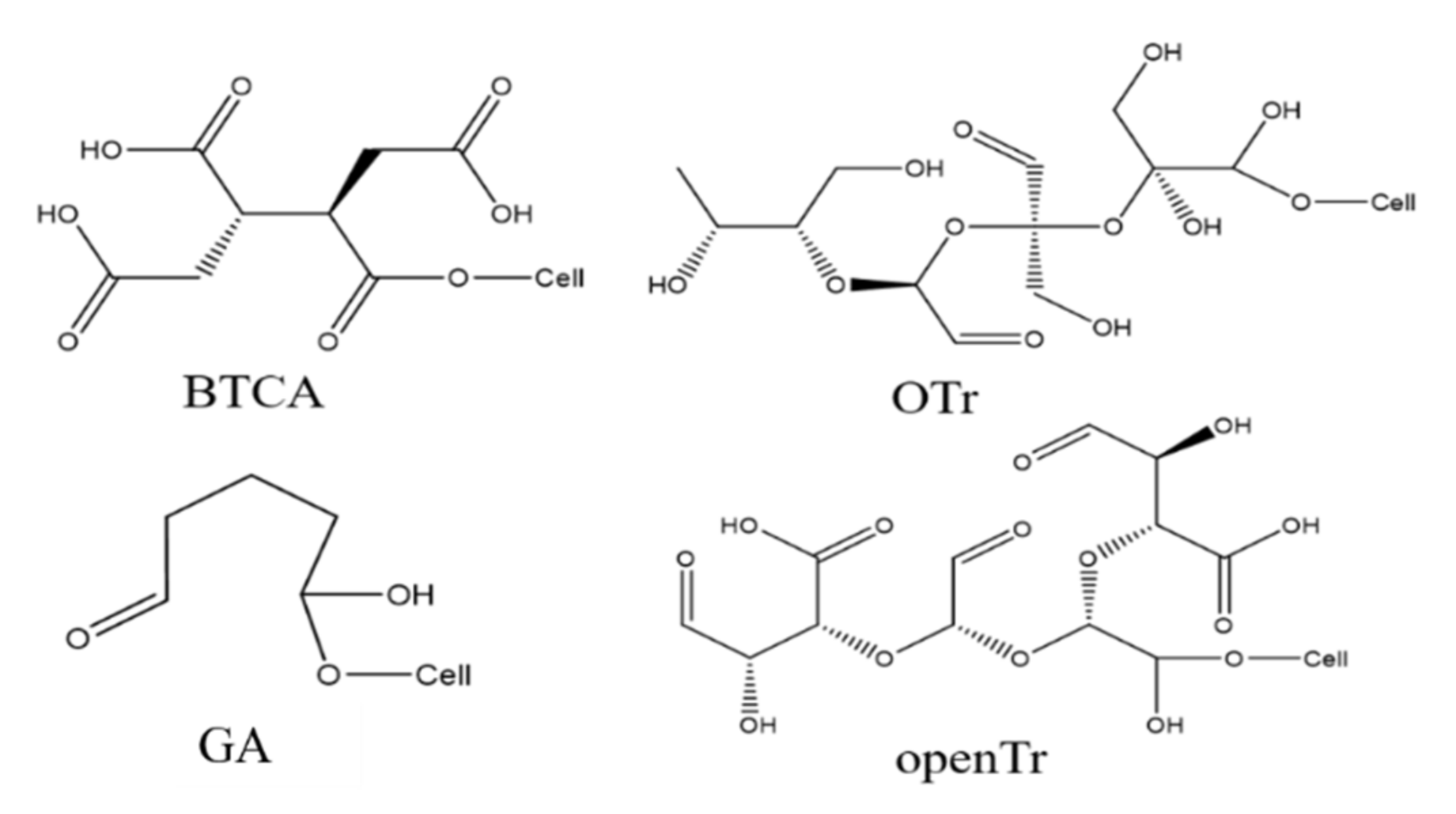
3.3. Cross-Linking Performance of Glycosyl Anti-Wrinkle Finishing Agent
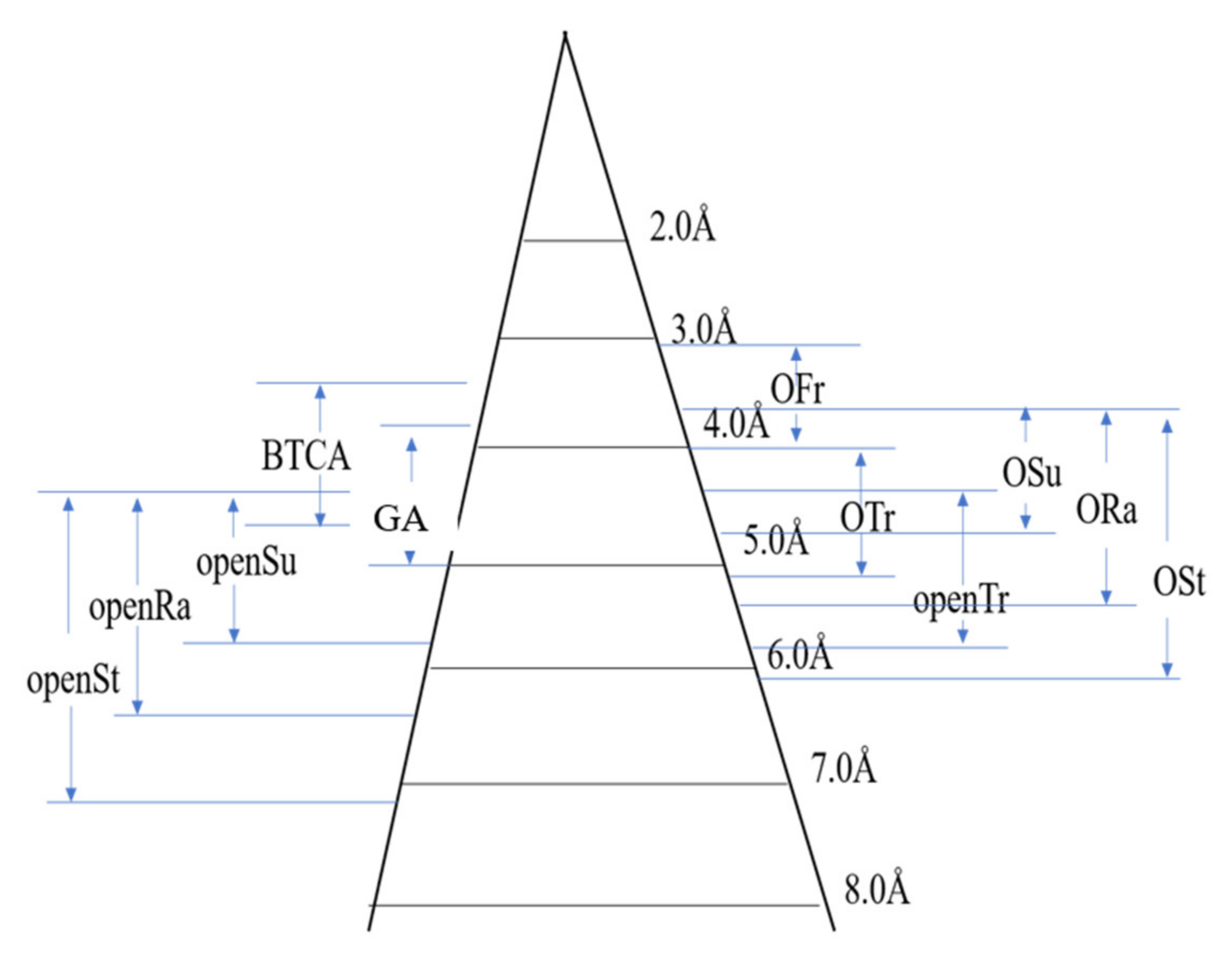
3.4. Effective Crosslinking Radius of Glycosyl Anti-Wrinkle Finishing Agent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dehabadi, V.A.; Buschmann, H.-J.; Gutmann, J.S. Durable press finishing of cotton fabrics: An overview. Text. Res. J. 2013, 83, 1974–1995. [Google Scholar] [CrossRef]
- Harifi, T.; Montazer, M. Past, present and future prospects of cotton cross-linking: New insight into nano particles. Carbohydr. Polym. 2012, 88, 1125–1140. [Google Scholar] [CrossRef]
- Chang, H.-L.; Chen, C.-C. Crosslinking of Cotton with DMDMDHEU in the Presence of Sodium Chloride. Text. Res. J. 2016, 66, 803–809. [Google Scholar] [CrossRef]
- Chen, J.C.; Yao, W.H.; Chen, C.H.; Chen, C.C. Dyeing kinetics from a finite bath of direct dyes on DMDHEU-AA crosslinked cotton. Text. Res. J. 2002, 72, 55–60. [Google Scholar]
- Lund, K.; Brelid, H. Kinetics of Cross-Linking Softwood Kraft Pulp with 1,2,3,4-Butanetetracarboxylic Acid. Ind. Eng. Chem. Res. 2013, 52, 11502–11509. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Chu, X.; Zhu, P. Mechanism of Antiwrinkle Finishing of Cotton Fabrics Using Mixed Polycarboxylic Acids. Int. J. Polym. Sci. 2020, 2020, 3876595. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, B.; Yan, K.; Hu, T. Non-phosphorus catalysts for the ester cross-linking of cellulose with 1,2,3,4-butanetetracarboxylic acid. Fibers Polym. 2017, 18, 682–688. [Google Scholar] [CrossRef]
- Ji, B.; Zhao, C.; Yan, K.; Sun, G. Effects of acid diffusibility and affinity to cellulose on strength loss of polycarboxylic acid crosslinked fabrics. Carbohydr. Polym. 2016, 144, 282–288. [Google Scholar] [CrossRef]
- Munster, L.; Vicha, J.; Klofac, J.; Masar, M.; Hurajova, A.; Kuritka, I. Dialdehyde cellulose crosslinked poly(vinyl alcohol) hydrogels: Influence of catalyst and crosslinker shelf life. Carbohydr. Polym. 2018, 198, 181–190. [Google Scholar] [CrossRef]
- Liu, P.; Xu, H.; Mi, X.; Xu, L.; Yang, Y. Oxidized Sucrose: A Potent and Biocompatible Crosslinker for Three-Dimensional Fibrous Protein Scaffolds. Macromol. Mater. Eng. 2015, 300, 414–422. [Google Scholar] [CrossRef]
- Muhammad, M.; Willems, C.; Rodriguez-Fernandez, J.; Gallego-Ferrer, G.; Groth, T. Synthesis and Characterization of Oxidized Polysaccharides for In Situ Forming Hydrogels. Biomolecules 2020, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Yin, Y.; Shen, Z.; Bu, X.; Zhang, F. Oxidation of primary hydroxyl groups in chitooligomer by a laccase-TEMPO system and physico-chemical characterisation of oxidation products. Carbohydr. Polym. 2016, 135, 234–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Tao, W.; Lickfield, G.C. Molecular modeling of cellulose in amorphous state. III. An innovative elastomeric crosslink system. J. Polym. Sci. Part B Polym. Phys. 2010, 45, 1821–1833. [Google Scholar]
- Chen, W.; Lickfield, G.C.; Yang, C.Q. Molecular modeling of cellulose in amorphous state. Part I: Model building and plastic deformation study. Polymer 2004, 45, 1063–1071. [Google Scholar] [CrossRef]
- Wu, C.; Xu, W. Atomistic molecular modelling of crosslinked epoxy resin. Polymer 2006, 47, 6004–6009. [Google Scholar] [CrossRef]
- Yarovsky, I.; Evans, E. Computer simulation of structure and properties of crosslinked polymers: Application to epoxy resins. Polymer 2002, 43, 963–969. [Google Scholar] [CrossRef]
- Yo, A.; Mn, A.; Ks, A.; Yh, A.; Ki, A.; Gk, B.; Jing, L.A.; Rk, C.; Nk, D.; Hw, D. Molecular dynamics simulation of cross-linking processes and material properties for epoxy resins using first-principle calculation combined with global reaction route mapping algorithms. Chem. Phys. Lett. 2020, 762, 138104. [Google Scholar]
- Zhang, W.; Wang, Z.; Lv, S.; Zhan, W.; Yang, X. Molecular simulation of different structure dopamine-modified graphene oxide and its effects on thermal and mechanical properties of the epoxy resin system. Polymer 2020, 212, 123120. [Google Scholar] [CrossRef]
- Hai, B.F.; Yuen, M. Material properties of the cross-linked epoxy resin compound predicted by molecular dynamics simulation. Polymer 2007, 48, 2174–2178. [Google Scholar]
- Nan, T.; Ning, R.; Jie, K. Self-toughening of epoxy resin through controlling topology of cross-linked networks. Polymer 2016, 99, 376–385. [Google Scholar]
- Hsiung, H.H.; Huang, H.Y.; Wang, Y.H.; Wang, C.; Chen, C.C. Physical properties and reaction kinetics of cotton cellulose crosslinked with dimethyloldihydroxyethyleneurea-maleic acid. J. Appl. Polym. Sci. 2010, 92, 3886–3893. [Google Scholar] [CrossRef]
- Park, C.; Kim, G.; Jung, J.; Krishnakumar, B.; Ranac, S.; Yun, G.J. Enhanced self-healing performance of graphene oxide/vitrimer nanocomposites: A molecular dynamics simulations study. Polymer 2020, 206, 122862. [Google Scholar] [CrossRef]
- Madan, G.L.; Patel, S.B.; Baddi, N.T.; Mehta, P.C. Physical Chemistry of Crosslinking: Part I: Thermodynamics of Cellulose in Aqueous Solution of Crosslinking Agents. Text. Res. J. 2016, 46, 329–342. [Google Scholar] [CrossRef]
- Ziifle, H.M.; Benerito, R.R.; Gonzales, E.J.; Berni, R.J. Kinetics of the Reactions of Ethyleneurea Derivatives with Cotton Cellulose. Text. Res. J. 2016, 38, 925–930. [Google Scholar] [CrossRef]
- Dang, X.; Liu, P.; Yang, M.; Deng, H.; Shan, Z.; Zhen, W. Production and characterization of dialdehyde cellulose through green and sustainable approach. Cellulose 2019, 26, 9503–9515. [Google Scholar] [CrossRef]


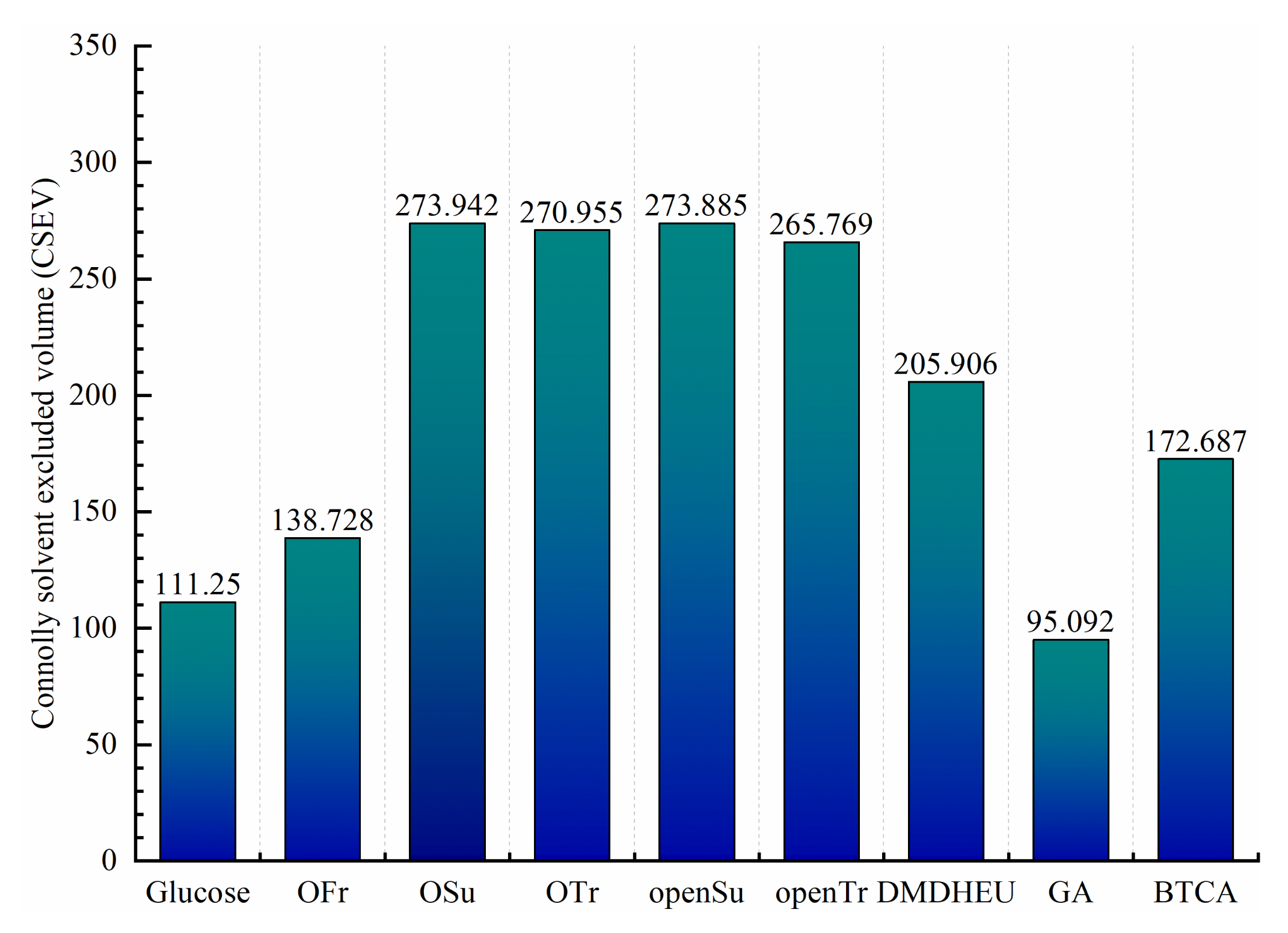


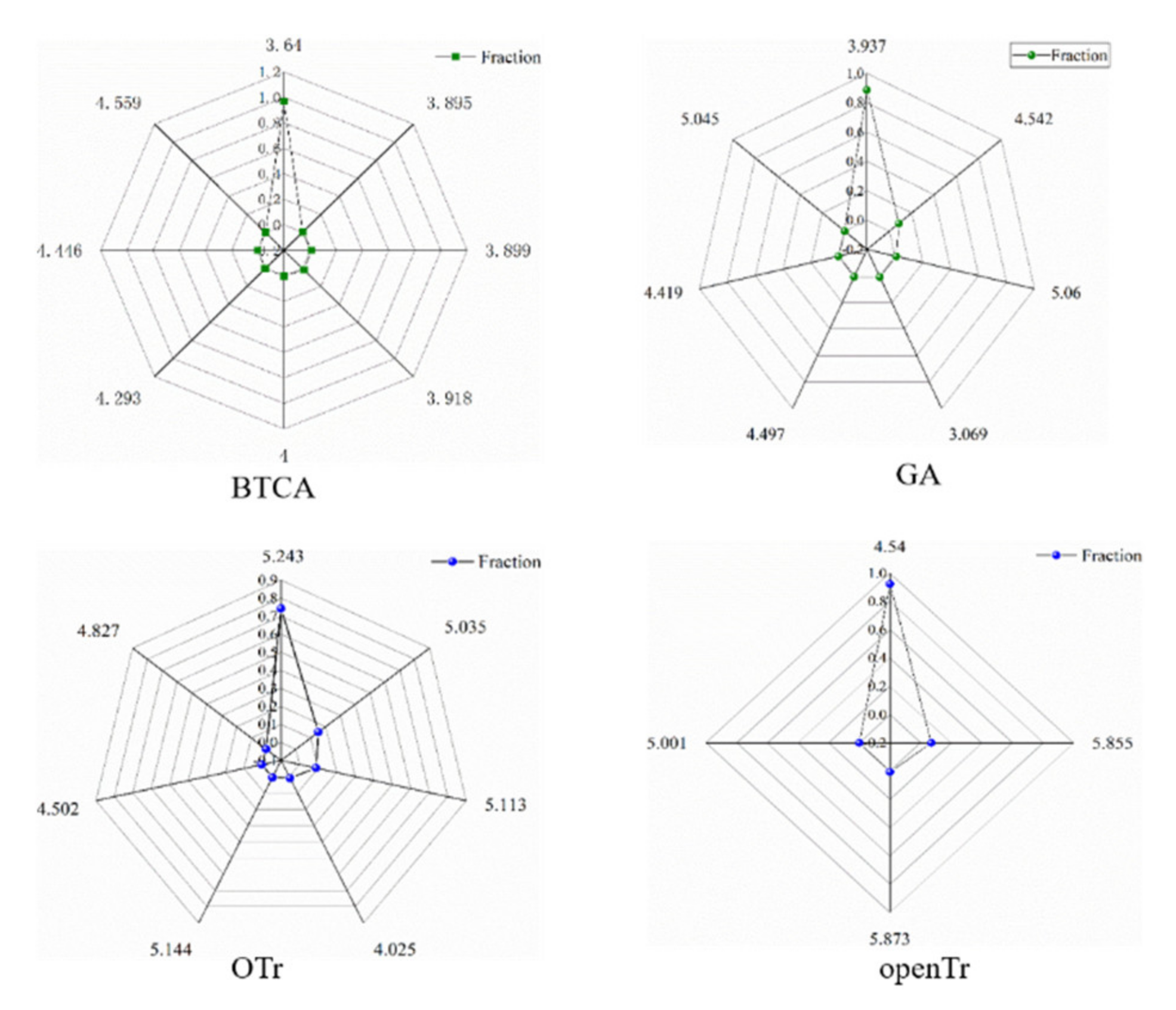
| Finishing Agents | Molecular Weight | PCG | NRS | RBN Value |
|---|---|---|---|---|
| Glucose | 180.156 | 1 | 2 | 5 |
| OFr | 178.140 | 2 | 4 | 6 |
| OSu | 354.264 | 4 | 8 | 14 |
| OTr | 338.265 | 4 | 8 | 14 |
| openSu | 396.213 | 7 | 9,10 | 14 |
| openTr | 366.231 | 6 | 9 | 14 |
| DMDHEU | 324.252 | 2 | 2 | 6 |
| GA | 100.117 | 2 | 4 | 4 |
| BTCA | 234.160 | 4 | 2,3 | 7 |
| Molecular Conformations | Molecular Conformational Energy (a.u.) | δE (kcal/mol) | Proportion |
|---|---|---|---|
| BTCA-1 | −911.72876 | 0 | 96.87% |
| BTCA-2 | −911.724877 | 2.44 | 1.58% |
| BTCA-3 | −911.724415 | 2.73 | 0.97% |
| BTCA-4 | −911.723258 | 3.45 | 0.28% |
| BTCA-5 | −911.722893 | 3.68 | 0.19% |
| BTCA-6 | −911.721756 | 4.4 | 0.06% |
| BTCA-7 | −911.721025 | 4.85 | 0.03% |
| BTCA-8 | −911.720344 | 5.28 | 0.01% |
| GA-1 | −345.36023 | 0 | 88.56% |
| GA-2 | −345.358668 | 0.98 | 8.46% |
| GA-3 | −345.356575 | 2.29 | 0.92% |
| GA-4 | −345.35652 | 2.33 | 0.87% |
| GA-5 | −345.355609 | 2.9 | 0.66% |
| GA-6 | −345.355221 | 3.14 | 0.44% |
| GA-7 | −345.354292 | 3.73 | 0.08% |
| OTr-1 | −1294.015689 | 0 | 74.23% |
| OTr-2 | −1294.014191 | 0.94 | 15.17% |
| OTr-3 | −1294.01368 | 1.26 | 8.83% |
| OTr-4 | −1294.011451 | 2.66 | 0.83% |
| OTr-5 | −1294.010956 | 2.97 | 0.49% |
| OTr-6 | −1294.01078 | 3.08 | 0.41% |
| OTr-7 | −1294.008548 | 4.48 | 0.04% |
| openTr-1 | −1441.946577 | 0 | 92.31% |
| openTr-2 | −1441.944137 | 1.53 | 6.95% |
| openTr-3 | −1441.941999 | 2.87 | 0.72% |
| openTr-4 | −1441.938257 | 5.22 | 0.01% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, J.; Wang, D.; Yuan, J.; Fan, X. Relationship between Anti-Wrinkle Property of Cotton Fabrics and Crosslinking Properties of Glycosyl Polyaldehydes and Polyuronic Acids Finishing Agents: A Molecular Simulation Study. Appl. Sci. 2021, 11, 10994. https://doi.org/10.3390/app112210994
Lou J, Wang D, Yuan J, Fan X. Relationship between Anti-Wrinkle Property of Cotton Fabrics and Crosslinking Properties of Glycosyl Polyaldehydes and Polyuronic Acids Finishing Agents: A Molecular Simulation Study. Applied Sciences. 2021; 11(22):10994. https://doi.org/10.3390/app112210994
Chicago/Turabian StyleLou, Jiangfei, Dan Wang, Jiugang Yuan, and Xuerong Fan. 2021. "Relationship between Anti-Wrinkle Property of Cotton Fabrics and Crosslinking Properties of Glycosyl Polyaldehydes and Polyuronic Acids Finishing Agents: A Molecular Simulation Study" Applied Sciences 11, no. 22: 10994. https://doi.org/10.3390/app112210994
APA StyleLou, J., Wang, D., Yuan, J., & Fan, X. (2021). Relationship between Anti-Wrinkle Property of Cotton Fabrics and Crosslinking Properties of Glycosyl Polyaldehydes and Polyuronic Acids Finishing Agents: A Molecular Simulation Study. Applied Sciences, 11(22), 10994. https://doi.org/10.3390/app112210994





