Phytomanagement of a Trace Element-Contaminated Site to Produce a Natural Dye: First Screening of an Emerging Biomass Valorization Chain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Device
2.2. Sediment Sampling and Analysis
2.3. Plant Sampling and TE Analysis
- (1)
- BCF ext = [TE] shoots/[TE] sediment extractable
- (2)
- BCF tot = [TE] shoots/[TE] sediment total
2.4. Dye Extraction and Dyeing Test
2.5. TE Analysis in Textile Fabrics and Dye Extract Solutions
2.6. Statistical Analysis
3. Results and Discussion
3.1. Initial Agronomic and Contamination Characteristics of the Site
3.2. Growth of M. sylvestris on the 3 Metal-Contaminated Areas
3.3. M. sylvestris Effect on TE Extractable Fraction and Mobility in the Sediment
3.4. TE Transfer in the Aerial Plant Parts of M. sylvestris
3.5. M. sylvestris Biomass Valorization Options
3.5.1. The Textile Dyeing Valorization Chain
3.5.2. Other Emerging Biomass Valorization Options for M. sylvestris
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, S.; Post, W.; Wang, D.; Nichols, J.; Bandaru, V.; West, T. Hierarchical marginal land assessment for land use planning. Land Use Policy 2013, 30, 106–113. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Negri, M.C.; Snyder, S.W. A novel framework to classify marginal land for sustainable biomass feedstock production. J. Environ. Qual. 2011, 40, 1593–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burges, A.; Alkorta, I.; Epelde, L.; Garbisu, C. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int. J. Phytoremediation 2018, 20, 384–397. [Google Scholar] [CrossRef]
- Robinson, B.H.; Banuelos, G.; Conesa, H.M.; Evangelou, M.W.; Schulin, R. The phytomanagement of Trace Elements in soil. Crit. Rev. Plant Sci. 2009, 28, 140–166. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Deram, A. Phytomanagement: A Realistic Approach to Soil remediating Phytotechnologies with New Challenges for Plant Science. Intern. J. Plant Biol. Res. 2014, 2, 1023. [Google Scholar]
- Meers, E.; Van Slycken, S.; Adriaensen, K.; Ruttens, A.; Vangronsveld, J.; Du Laing, G.; Witters, N.; Thewys, T.; Tack, F.M.G. The use of bio-energy crops (Zea mays) for ‘phytoattenuation’ of heavy metals on moderately contaminated soils: A field experiment. Chemosphere 2010, 78, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Garbisu, C.; Alkorta, I.; Kidd, P.; Epelde, L.; Mench, M. Keep and promote biodiversity at polluted sites under phytomanagement. Environ. Sci. Pollut. Res. 2020, 27, 44820–44834. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Jiang, L.; Zhang, W. A review on heavy metal contamination in the soil worldwide: Situation, impact and remediation techniques. Environ. Skept. Crit. 2014, 3, 24–38. [Google Scholar]
- Zine, H.; Midhat, L.; Hakkou, R.; El Adnani, M.; Ouhammou, A. Guidelines for a phytomanagement plan by the phytostabilization of mining wastes. Sci. Afr. 2020, 10, 2468–2476. [Google Scholar] [CrossRef]
- Angelova, V.R.; Ivanova, R.V.; Todorov, G.M.; Ivanov, K.I. Potential of Salvia sclarea L. for Phytoremediation of Soils Contaminated with Heavy Metals. Int. J. Agric. Biosyst. Eng. 2016, 10, 12. [Google Scholar]
- Deyris, P.A.; Bert, V.; Diliberto, S.; Boulanger, C.; Petit, E.; Legrand, Y.M.; Grison, C. Biosourced polymetallic catalysis: A surprising and efficient means to promote the knoevenagel condensation. Front. Chem. 2018, 6, 6–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delplanque, M.; Collet, S.; Del Gratta, F.; Schnuriger, B.; Gaucher, R.; Robinson, B.; Bert, V. Combustion of Salix used for phytoextraction: The fate of metals and viability of the process. Biomass Bioenergy 2013, 49, 160–170. [Google Scholar] [CrossRef]
- Bert, V.; Allemon, J.; Sajet, P.; Dieu, S.; Papin, A.; Collet, S.; Gaucher, R.; Chalot, M.; Michiels, B.; Raventos, C. Torrefaction and pyrolysis of metal-enriched poplars from phytotechnologies: Effect of temperature and biomass chlorine content on metal distribution in end-products and valorization options. Biomass Bioenergy 2017, 96, 1–11. [Google Scholar] [CrossRef]
- Grignet, A.; De Vaufleury, A.; Papin, A.; Bert, V. Urban soil phytomanagment for Zn and Cd in situ removal, greening, and Zn-rich biomass production taking care of snail exposure. Environ. Sci. Pollut. Res. 2020, 27, 3187–3201. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Devin, I.; Menana, Z.; Chrusciel, L.; Chalot, M.; Bert, V.; Brosse, N. Steam explosion pretreatment of willow grown on phytomanaged soils for bioethanol production. Ind. Crop. Prod. 2019, 140. [Google Scholar] [CrossRef]
- Asad, M.; Menana, Z.; Ziegler-Devin, I.; Bert, V.; Chalot, M.; Herzig, R.; Mench, M.; Brosse, N. Pretreatment of trace element-enriched biomasses grown on phytomanaged soils for bioethanol production. Ind. Crop. Prod. 2017, 107, 63–72. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Craker, L.E.; Xing, B.; Nielsen, N.E.; Wilcox, A. Aromatic plant production on metal contaminated soils. Sci. Total Environ. 2008, 395, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Perlein, A.; Bert, V.; Desannaux, O.; Fernandes de Souza, M.; Papin, A.; Gaucher, R.; Zdanevitch, I.; Meers, E. The use of Sorghum in a phytoattenuation strategy: A field experiment on a TE contaminated site. Appl. Sci. 2021, 11, 3471. [Google Scholar] [CrossRef]
- Perlein, A.; Zdanevitch, I.; Gaucher, R.; Robinson, B.; Papin, A.; Sahraoui, A.L.H.; Bert, V. Phytomanagement of a metal(loid)-contaminated agricultural site using aromatic and medicinal plants to produce essential oils: Analysis of the metal(loid) fate in the value chain. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Spekreijse, J.; Lammens, T.; Parisi, C.; Ronzon, T.; Vis, M. Insights into the European Market. of Bio-Based Chemicals. Analysis Based on Ten Key Product Categories; Publications Office of the European Union: Luxembourg, 2019; ISBN 9789279984204. [Google Scholar]
- Shahid, M.; Islam, S.U.; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Maurya, I.C.; Singh, S.; Srivastava, P.; Maiti, B.; Bahadur, L. Natural dye extract from Cassia fistula and its application in dye-sensitized solar cell: Experimental and density functional theory studies. Opt. Mater. 2019, 90, 273–280. [Google Scholar] [CrossRef]
- Rovira, J.; Domingo, J.L. Human health risks due to exposure to inorganic and organic chemicals from textiles: A review. Environ. Res. 2019, 168, 62–69. [Google Scholar] [CrossRef]
- Repon, M.R.; Islam, M.T.; Mamun, M.A.A. Ecological risk assessment and health safety speculation during color fastness properties enhancement of natural dyed cotton through metallic mordants. Fash Text. 2017, 4, 1–17. [Google Scholar] [CrossRef]
- Gasparetto, J.C.; Martins, C.A.F.; Hayashi, S.S.; Otuky, M.F.; Pontarolo, R. Ethnobotanical and scientific aspects of Malva sylvestris L.: A millennial herbal medicine. J. Pharm. Pharmacol. 2011, 64, 172–189. [Google Scholar] [CrossRef]
- Azab, A. Malva: Food, medicine and chemistry. Eur. Chem. Bull. 2017, 6, 295–320. [Google Scholar] [CrossRef] [Green Version]
- Guinot, P.; Rogé, A.; Gargadennec, A.; Garcia, M.; Dupont, D.; Lecoeur, E.; Candelier, L.; Andary, C. Dyeing plants screening: An approach to combine past heritage and present development. Color. Technol. 2006, 122, 93–101. [Google Scholar] [CrossRef]
- Despy, J.; Wymeersch, N.; Bouchat, I.; Destree, C.; Burette, A.; Richet, A.; Olive, G. Old inks: Pigments extracted from plants. In Proceedings of the 19e Symposium National on Applied Biological Sciences, Gembloux, Belgium, 7 February 2014. [Google Scholar]
- Montiel-Rozas, M.M.; Madejón, E.; Madejón, P. Evaluation of phytostabilizer ability of three ruderal plants in mining soils restored by application of organic amendments. Ecol. Eng. 2015, 83, 431–436. [Google Scholar] [CrossRef]
- Montiel-Rozas, M.M.; Madejón, E.; Madejón, P. Effect of heavy metals and organic matter on root exudates (low molecular weight organic acids) of herbaceous specie: An assessment in sand and soil condition under different levels of contamination. Environ. Pollut. 2016, 216, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Madejón, E.; Pérez de Mora, A.; Felipe, E.; Burgo, P.; Cabrera, F. Soil amendments reduce trace element solubility in a contaminated soil and allow regrowth of natural vegetation. Environ. Pollut. 2006, 139, 40–52. [Google Scholar] [CrossRef]
- Ramavandi, B.; Rahbar, A.; Sahebi, S. Effective removal of Hg2+ from aqueous solutions and seawater by Malva sylvestris. Desalination Water Treat. 2016, 57, 23814–23826. [Google Scholar] [CrossRef]
- Ramavandi, B.; Asgari, G. Comparative study of sun-dried and oven-dried Malva sylvestris biomass for high-rate Cu(II° removal from wastewater. Process. Saf. Environ. Prot. 2018, 116, 61–73. [Google Scholar] [CrossRef]
- Delfine, S.; Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Menichini, F.; Senatore, F. Variation of Malva sylvestris essential oil yield, chemical composition and biological activity in response to different environments across Southern Italy. Ind. Crop. Prod. 2017, 98, 29–37. [Google Scholar] [CrossRef]
- Phanthavongsa, P. Etude de deux Modalités de Phytomanagement Testées sur un Terrain de Gestion de Sédiments Contaminés par des Métaux et Métalloïdes. Ph.D. Thesis, Ecole Doctorale n°554 Environnements—Santé, Université de Bourgogne Franche-Comté, Besançon, France, 9 April 2018. [Google Scholar]
- Infoclimat.fr. Available online: https://www.infoclimat.fr/climatologie/annee/2019/lille-lesquin/valeurs/07015.html (accessed on 30 June 2020).
- Graines de Semences. Available online: https://www.graines-semences.com (accessed on 15 February 2021).
- Plante Aromatique. Available online: https://www.plantearomatique.com/nos-plantes/188-mauve-sylvestre-4.html (accessed on 15 February 2021).
- ISO. Soil quality—Extraction of Trace Elements from Soil Using Ammonium Nitrate Solution; ISO 19730:2008; ISO: Geneva, Switzerland, 2008. [Google Scholar]
- Komárek, M.; Chrastný, V.; Štíchová, J. Metal/metalloid contamination and isotopic composition of lead in edible mushrooms and forest soils originating from a smelting area. Environ. Int. 2007, 33, 677–684. [Google Scholar] [CrossRef]
- AIDA INERIS. Arrêté du 30/06/20 Modifiant l’arrêté du 9 août 2006 Relatif aux Niveaux à Prendre en Compte lors d’une Analyse de Rejets Dans les Eaux de Surface ou de Sédiments Marins, Estuariens ou Extraits de Cours d’eau ou Canaux Relevant Respectivement des Rubriques 2.2.3.0, 3.2.1.0 et 4.1.3.0 de la Nomenclature Annexée à L’article R. 214-1 du Code de L’environnement. Available online: https://aida.ineris.fr/consultation_document/43365 (accessed on 1 February 2021).
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root-mediated pH change in the rhizosphere and their responses to environmental constraints: A review. Plant. Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; Taylor and Francis Group: Oxford, UK, 2011. [Google Scholar]
- Jogawat, A.; Yadav, B.; Narayan, O.M. Metal transporter in organelles and their roles in heavy metal transportation and sequestration mechanisms in plants. Physiol. Plant 2021, 173, 259–275. [Google Scholar] [CrossRef]
- Singh, B.R.; Gupta, S.K.; Azaizeh, H.; Shilev, S.; Sudre, D.; Song, W.Y.; Martinoia, E.; Mench, M. Safety of food crops on land contaminated with trace elements (review). J. Sci. Food Agric. 2011, 91, 1349–1366. [Google Scholar] [CrossRef] [Green Version]
- Unver, M.C.; Ugulu, I.; Durkan, N.; Baslar, S.; Dogan, Y. Heavy metal contents of Malva sylvestris sold as edible greens in the local markets of Izmir. Ekoloji 2015, 96, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Hiçsönmez, Ü.; Ereeş, F.S.; Özdemir, C.; Özdemir, A.; Ҫam, S. Determination of major and minor elements in the Malva sylvestris L. from Turkey using ICP-OES techniques. Biol. Trace Elem. Res. 2009, 128, 248–257. [Google Scholar] [CrossRef]
- Tokalioğlu, Ş. Determination of trace elements in commonly consumed medicinal herbs by ICP-MS and multivariate analysis. Food Chem. 2012, 134, 2504–2508. [Google Scholar] [CrossRef]
- Kostic, D.; Arsić, B.; Randelović, S.; Pavlović, A.; Tošić, S.; Stojanović, G. Correlation analysis of heavy metal contents of Malva sylvestris L. plant and its extracts from polluted and non-polluted locations in Niš, Republic of Serbia. Water Air Soik. Pollut. 2019, 230, 98. [Google Scholar] [CrossRef]
- Desideri, D.; Assunta Meli, M.; Roselli, C. Determination of essential and non-essential elements in some medicinal plants by polarized X ray fluorescence spectrometer (EDPXRF). Microchem. J. 2010, 95, 174–180. [Google Scholar] [CrossRef]
- Khan, S.; Rehman, S.; Khan, A.Z.; Khan, M.A.; Shah, M.T. Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotoxicol. Environ. Saf. 2010, 73, 1820–1827. [Google Scholar] [CrossRef]
- Abe, T.; Fukami, M.; Ogasawara, M. Cadmium accumulation in the shoots and roots of 93 weed species. Soil Sci. Plant Nutr. 2008, 54, 566–573. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, D.S.L.M.; Martins, M.; Pisani, L.P.; Ventura, S.P.M.; de Rosso, V.V. Insight on the use of alternative solvents and technologies to recover bio-based food pigments. Compr. Rev. Food Sci. Food Saf. 2021, 20, 787–818. [Google Scholar] [CrossRef]
- Prabhu, K.H.; Teli, M.D. Eco-dyeing using Tamarindus indica L. seed coat tannin as a natural mordant for textiles with antibacterial activity. J. Saudi Chem. Soc. 2014, 18, 864–872. [Google Scholar] [CrossRef] [Green Version]
- Rezić, I.; Steffan, I. ICP-OES determination of metals present in ttile materials. Microchem. J. 2007, 85, 46–51. [Google Scholar] [CrossRef]
- OEKO-Tex. International Association for Research and Testing in the Field of Textile and Leather Ecology. Eco Passport by OEKO-Tex. 2021. Available online: https://www.oeko-tex.com/en/apply-here/eco-passport-by-oeko-tex (accessed on 25 August 2021).
- Sungur, Ş.; Gülmez, F. Determination of metal contents of various fibers used in textile industry by MP-AES. J. Spectrosc. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- OEKO-Tex. International Association for Research and Testing in the Field of Textile and Leather Ecology. Standards 100 by OEKO-Tex. 2021. Available online: https://www.oeko-tex.com/en/downloads (accessed on 11 August 2021).
- Rather, L.J.; Islam, S.U.; Shabbir, M.; Bukhari, M.N.; Shahid, M.; Khan, M.A.; Mohammad, F. Ecological dyeing of Woolen yarn with Adhatod vasica natural dye in the presence of biomordants as an alternative copartner to metal mordants. J. If. Environ. Chem. Eng. 2016, 4, 3041–3049. [Google Scholar] [CrossRef]
- European Parliament. Regulation (Eu) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003; European Parliament: Strasbourg, France, 2019. [Google Scholar]
- Elallem, K.A.; Sobeh, M.; Boularbah, A.; Yasri, A. Chemically degraded soil rehabilitation process using medicinal and aromatic plants: Review. Environ. Sci. Pollut. Res. 2020, 28, 73–93. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, S.; Wang, T.; Chang, Z.; Shen, Z.; Chen, Y. Using contaminated plants involved in phytoremediation for anaerobic digestion. Int. J. Phytoremediation 2015, 17, 201–207. [Google Scholar] [CrossRef]
- Wong, M.H.; Cheung, Y.H. Gas production and digestion efficiency of sewage sludge containing elevated toxic metals. Bioressource Technol. 1995, 54, 261–268. [Google Scholar] [CrossRef]
- Thewys, T.; Witters, N.; Van Slycken, S.V.; Ruttens, A.; Meers, E.; Tack, F.M.G.; Vangronsveld, J. Economic viability of phytoremediation of a cadmium contaminated agricultural area using energy maize. Part I: Effect on the farmer’s income. Int. J. Phytoremediation 2010, 12, 650–662. [Google Scholar] [CrossRef] [Green Version]
- Thewys, T.; Witters, N.; Meers, E.; Vangronsveld, J. Economic viability of phytoremediation of a cadmium contaminated agricultural area using energy maize. Part II: Economics of anaerobic digestion of metal contaminated maize in Belgium. Int. J. Phytoremediation 2010, 12, 663–679. [Google Scholar] [CrossRef] [Green Version]
- Witters, N.; Mendelsohn, R.O.; Van Slycken, S.V.; Weyens, N.; Schreurs, E.; Meers, E.; Tack, F.; Carleer, R.; Vangronsveld, J. Phytoremediation, a sustainable remediation technology? Conclusions from a case study. I: Energy production and carbon dioxide abatement. Biomass Bioenergy 2012, 39, 454–469. [Google Scholar] [CrossRef]
- Souza, M.F.; Devriendt, N.; Willems, B.; Guisson, R.; Biswas, J.K.; Meers, E. Techno-economic feasibility of extrusion as a pretreatment step for biogas production from grass. BioEnergy Res. 2021. [Google Scholar] [CrossRef]


| Parameters | Area 1 | Area 2 | Area 3 | S1 * | |
|---|---|---|---|---|---|
| Clay (%) | 7.7 | 10.7 | 11.69 | - | |
| Silt (%) | 69 | 70.5 | 78.52 | - | |
| Sand (%) | 23.3 | 18.8 | 9.79 | - | |
| Sediment texture (USDA texture triangle) | Fine silt | Fine silt | Fine silt | - | |
| Carbon (g kg−1) | 76.3 | 82.4 | 96.8 | - | |
| Organic matter (g kg−1) | 131.3 | 141.7 | 166.5 | - | |
| C/N ** | 28.4 | 28 | 31.5 | - | |
| CEC (meq 100 g−1) + | 17.6 | 17.9 | 21.2 | - | |
| CaO g kg−1 | 11.7 | 11.5 | 12.5 | - | |
| TKN (g kg−1) ++ | 2.69 | 2.94 | 3.07 | - | |
| Phosphorus Olsen (g kg−1) P2O5 | 0.216 | 0.259 | 0.258 | - | |
| pH-H2O | 8.08 | 8.06 | 8.12 | - | |
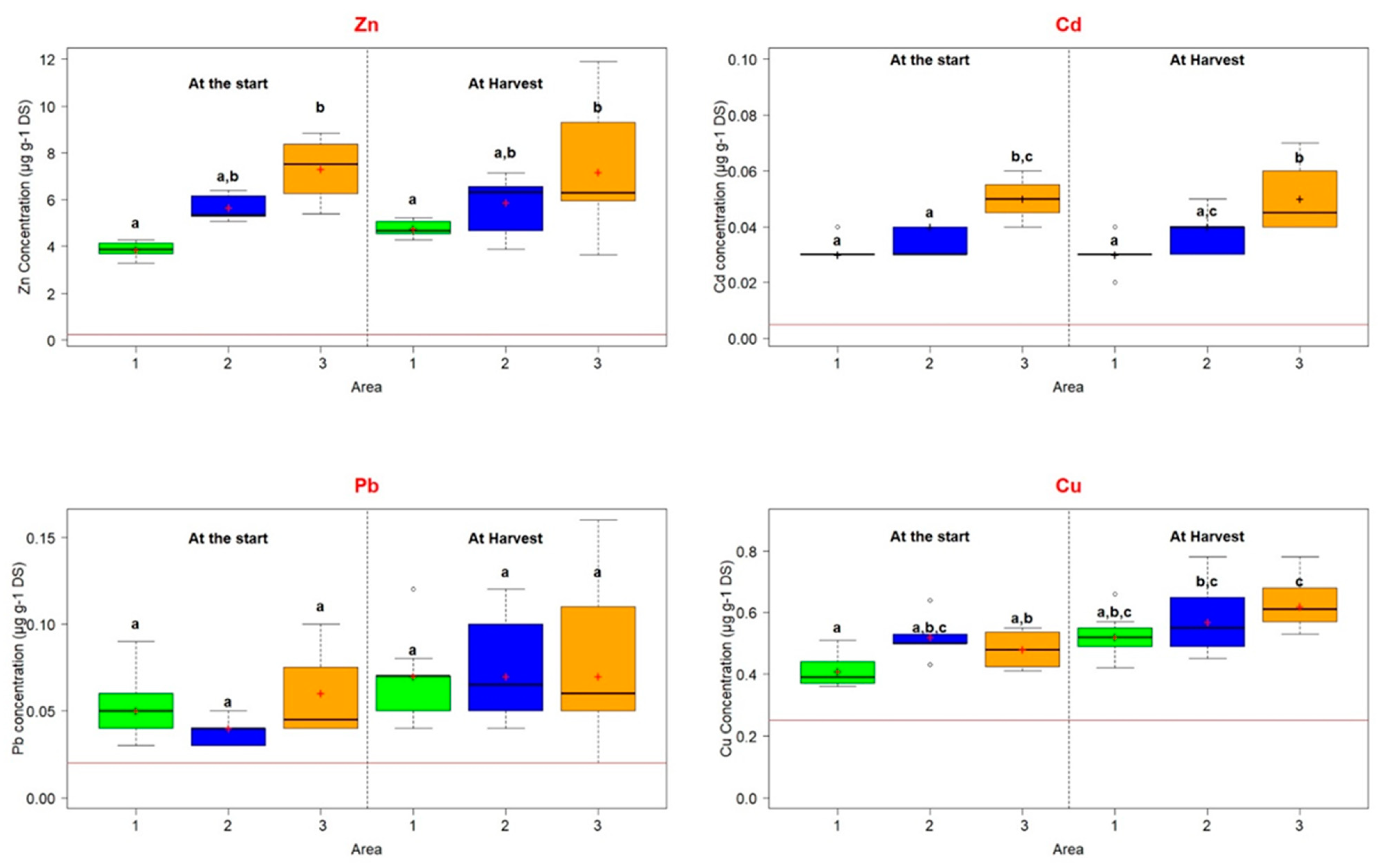
| Total TE (mg kg−1) (TE extractable concentration (mg kg−1)) | Zn | 6685 ± 509 (3.86 ± 0.39) | 6084 ± 132 (5.66 ± 0.59) | 8980 ± 340 (7.32 ± 1.46) | 300 |
| Pb | 774 ± 18 (0.05 ± 0.02) | 592 ± 12 (0.04 ± 0.008) | 1043 ± 12 (0.06 ± 0.03) | 100 | |
| Cd | 6.3 ± 0.14 (0.03 ± 0.005) | 5 ± 0.08 (0.04 ± 0.003) | 9 ± 0.13 (0.05 ± 0.008) | 2 | |
| Cu | 87 ± 2.3 (0.41 ± 0.06) | 76 ± 1.5 (0.52 ± 0.08) | 101 ± 1.3 (0.48 ± 0.07) | 100 | |
| Area 1 | Area 2 | Area 3 | |
|---|---|---|---|
| Mean dry weight (g plant−1) | 81.8 (±23.9) a | 89.8 (±23.2) a | 126.5 (±28.5) b |
| Yield (Kg 25 m−2 FW) | 31.6 | 26.3 | 38.9 |
| Yield extrapolated (ton ha−1 FW) | 12.6 | 10.5 | 15.5 |
| Yield extrapolated (ton ha−1 DW) | 2.3 | 2.2 | 2.9 |
| Mean flower % by plant (FW) | 10.3 (±2.6) | 8.4 (±1.9) | 8.4 (±1.6) |
| Flower Yield extrapolated (ton ha−1 FW) | 1.3 | 0.9 | 1.3 |
| Flower Yield extrapolated (ton ha−1 DW) | 0.241 | 0.145 | 0.226 |
| Cd | Cu | Pb | Zn | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flower | Stem | Leaf | Flower | Stem | Leaf | Flower | Stem | Leaf | Flower | Stem | Leaf | |
| Area 1 | 0.94 (±0.21) A | 1.08 (±0.22) A | 2.15 (±0.41) B | 14.81 (±4.64) a | 8.68 (±1.61) b | 13.18 (±2.85) a | 9.54 (±5.45) 1 | 1.52 (±1.13) 2 | 5.67 (±4.33) 1,2 | 186.04 (±49.41) 1 | 188.35 (±37.14) 1 | 465.33 (±96.92) 2 |
| Area 2 | 0.93 (±0.2) A | 1.08 (±0.25) A | 2.07 (±0.47) B | 11.65 (±0.87) a,b | 7.73 (±1.56) b | 11.11 (±2.72) a,b | 9.88 (±4.63) 1 | 1.28 (±0.34) 2 | 7.02 (±2.79) 1,2 | 175.03 (±40.5) 1 | 175.32 (±29.78) 1 | 453.79 (±92.69) 2 |
| Area 3 | 0.95 (±0.16) A | 1.1 (±0.28) A | 2.18 (±0.45) B | 12.79 (±1.15) a,b | 8.05 (±4.23) b | 12.05 (±3.38) a,b | 11.28 (±10.79) 1 | 1.36 (±0.38) 2 | 7.3 (±5.18) 1,2 | 201.85 (±66.76) 1 | 227.09 (±54.47) 1 | 567.72 (±95.73) 3 |
| Cd | Cu | Pb | Zn | ||
|---|---|---|---|---|---|
| Area 1 | (Shoots) | 1.54 (0.27) | 11.6 (2.06) | 4.37 (2.9) | 315.38 (72.98) 1 |
| BCF tot | 0.246 (0.044) A | 0.133 (0.024) A | 0.006 (0.004) | 0.047 (0.011) | |
| BCF ext | 50.62 (12.32) a | 22.49 (5.13) a | 70.47 (45.41) | 66.31 (14.96) | |
| Area 2 | (Shoots) | 1.55 (0.35) | 9.63 (1.52) | 4.72 (1.72) | 310.43 (61.08) 1 |
| BCF tot | 0.297 (0.068) B | 0.126 (0.02) A | 0.008 (0.003) | 0.051 (0.01) | |
| BCF ext | 45.04 (13.2) a | 17.49 (4.28) b | 78.53 (45.44) | 53.91 (11.35) | |
| Area 3 | (Shoots) | 1.6 (0.27) | 10.40 (2.94) | 5.16 (3.43) | 386.37 (56.51) 2 |
| BCF tot | 0.181 (0.03) C | 0.103 (0.029) B | 0.005 (0.003) | 0.043 (0.004) | |
| BCF ext | 33.54 (8.78) b | 16.74 (4.49) b | 93.52 (69.51) | 58.54 (17.56) | |
| M. sylvestris control | (Shoots) | 0.25 (0.07) | 14.1 (3.3) | 0.42 (0.09) | 84 (29) |
| (Flower) | 0.44 | 7.8 | 0.22 | 47 | |
| Dye Extract Area 1 | Dye Extract Area 2 | Dye Extract Area 3 | Dye Extract RT * | Dye Extract 10 °C | Usual Concentration in Dye ** | Eco Passport Threshold Value *** | ||
|---|---|---|---|---|---|---|---|---|
| Cd | µg L−1 | 0.96 | 0.87 | 0.88 | 1.08 | 0.66 | - | - |
| µg g−1 | 0.0001 | 0.0009 | 0.0009 | 0.001 | 0.0007 | 1 | 20 | |
| Cu | µg L−1 | 105.3 | 69.8 | 74.7 | 45.1 | 47.3 | - | - |
| µg g−1 | 0.105 | 0.07 | 0.075 | 0.045 | 0.047 | 33–110 | 250 | |
| Pb | µg L−1 | 3.99 | 4.86 | 6.36 | 3.02 | 2.81 | - | - |
| µg g−1 | 0.004 | 0.005 | 0.006 | 0.003 | 0.003 | 6–52 | 90 | |
| Zn | µg L−1 | 1108 | 1960 | 1945 | 584 | 683 | ||
| µg g−1 | 1.1 | 1.96 | 1.95 | 0.58 | 0.68 | 3–32 | 1500 | |
| Cd | Cu | Pb | Zn | ||
|---|---|---|---|---|---|
| Before dyeing | Cotton unpretreated | 0.01 * | 0.16 | 0.04 | 2.67 |
| Silk unpretreated | 0.01 * | 0.59 | 0.11 | 5.80 | |
| Cotton alum treated | 0.01 * | 1.08 | 0.06 | 1.67 | |
| Silk alum treated | 0.01 * | 4.18 | 0.11 | 2.60 | |
| Cotton iron treated | 0.01 * | 1.14 | 0.10 | 3.02 | |
| Silk iron treated | 0.01 * | 8.72 | 0.19 | 8.71 | |
| After dyeing Area 1 | Cotton untreated | 0.01 * | 1.67 | 0.20 | 10.28 |
| Cotton alum treated | 0.01 * | 2.35 | 0.10 | 6.17 | |
| Cotton iron treated | 0.01 * | 1.90 | 0.19 | 19.98 | |
| Silk untreated | 0.07 | 10.57 | 0.19 | 24.75 | |
| Silk alum treated | 0.06 | 9.74 | 0.31 | 29.27 | |
| Silk iron treated | 0.10 | 12.79 | 0.69 | 50.10 | |
| After dyeing Area 2 | Cotton untreated | 0.03 | 0.21 | 0.45 | 6.44 |
| Cotton alum treated | 0.02 | 0.28 | 0.38 | 5.86 | |
| Cotton iron treated | 0.03 | 0.29 | 0.56 | 8.56 | |
| Silk untreated | 0.10 | 0.51 | 0.36 | 11.45 | |
| Silk alum treated | 0.08 | 0.52 | 0.69 | 16.33 | |
| Silk iron treated | 0.09 | 1.03 | 1.34 | 18.48 | |
| After dyeing Area 3 | Cotton untreated | 0.01 * | 1.44 | 0.17 | 9.57 |
| Cotton alum treated | 0.01 * | 1.11 | 0.07 | 3.64 | |
| Cotton iron treated | 0.03 | 1.96 | 0.26 | 17.40 | |
| Silk untreated | 0.07 | 10.33 | 0.22 | 21.30 | |
| Silk alum treated | 0.07 | 8.65 | 0.43 | 30.10 | |
| Silk iron treated | 0.08 | 10.09 | 0.89 | 32.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perlein, A.; Bert, V.; Fernandes de Souza, M.; Gaucher, R.; Papin, A.; Geuens, J.; Wens, A.; Meers, E. Phytomanagement of a Trace Element-Contaminated Site to Produce a Natural Dye: First Screening of an Emerging Biomass Valorization Chain. Appl. Sci. 2021, 11, 10613. https://doi.org/10.3390/app112210613
Perlein A, Bert V, Fernandes de Souza M, Gaucher R, Papin A, Geuens J, Wens A, Meers E. Phytomanagement of a Trace Element-Contaminated Site to Produce a Natural Dye: First Screening of an Emerging Biomass Valorization Chain. Applied Sciences. 2021; 11(22):10613. https://doi.org/10.3390/app112210613
Chicago/Turabian StylePerlein, Alexandre, Valérie Bert, Marcella Fernandes de Souza, Rodolphe Gaucher, Arnaud Papin, Jeroen Geuens, Annelore Wens, and Erik Meers. 2021. "Phytomanagement of a Trace Element-Contaminated Site to Produce a Natural Dye: First Screening of an Emerging Biomass Valorization Chain" Applied Sciences 11, no. 22: 10613. https://doi.org/10.3390/app112210613
APA StylePerlein, A., Bert, V., Fernandes de Souza, M., Gaucher, R., Papin, A., Geuens, J., Wens, A., & Meers, E. (2021). Phytomanagement of a Trace Element-Contaminated Site to Produce a Natural Dye: First Screening of an Emerging Biomass Valorization Chain. Applied Sciences, 11(22), 10613. https://doi.org/10.3390/app112210613








