Characteristics of the Dental Pulp and Periodontal Ligament Stem Cells of the Yucatan Miniature Pig
Abstract
:1. Introduction
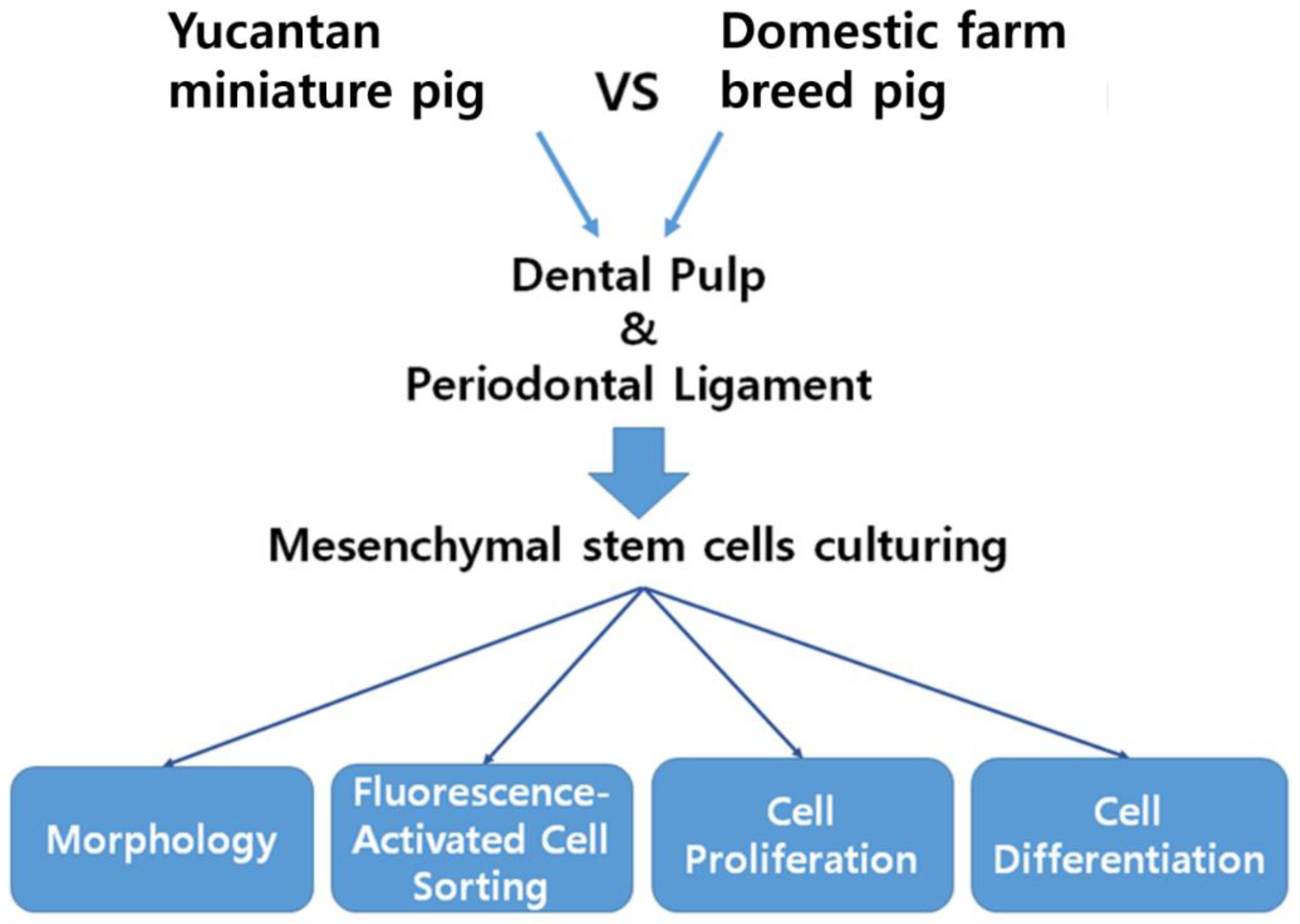
2. Materials and Methods
2.1. Animal and Cell Preparation
2.2. Fluorescence-Activated Cell Sorting (FACS) Analysis for Cell Surface Markers
2.3. Cell Proliferation
2.4. Alkaline Phosphatase Assay and Alizarin Red Staining of Osteogenic Differentiation and Mineralization
2.5. Statistical Analysis
3. Results
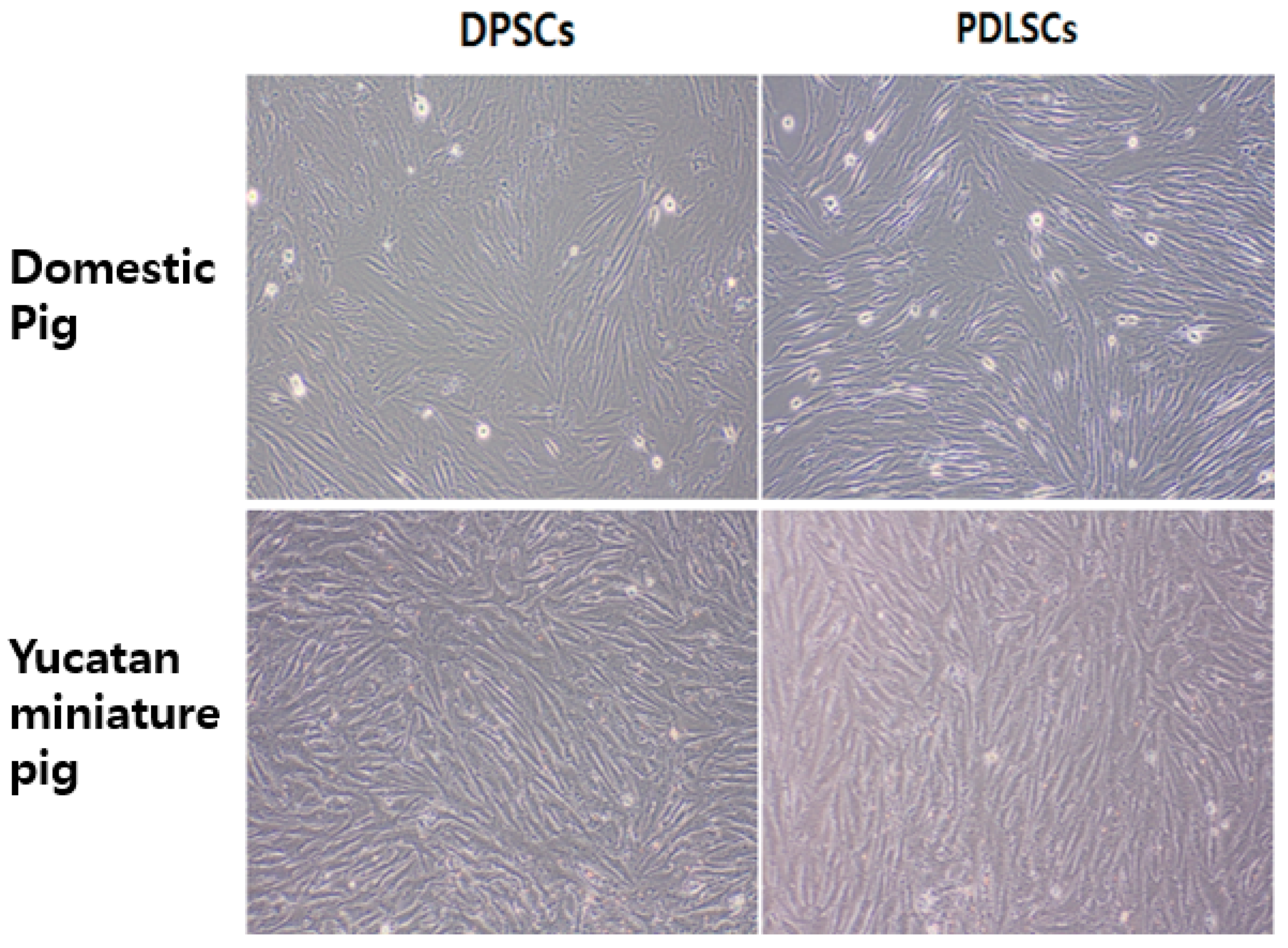
3.1. Cell Morphology
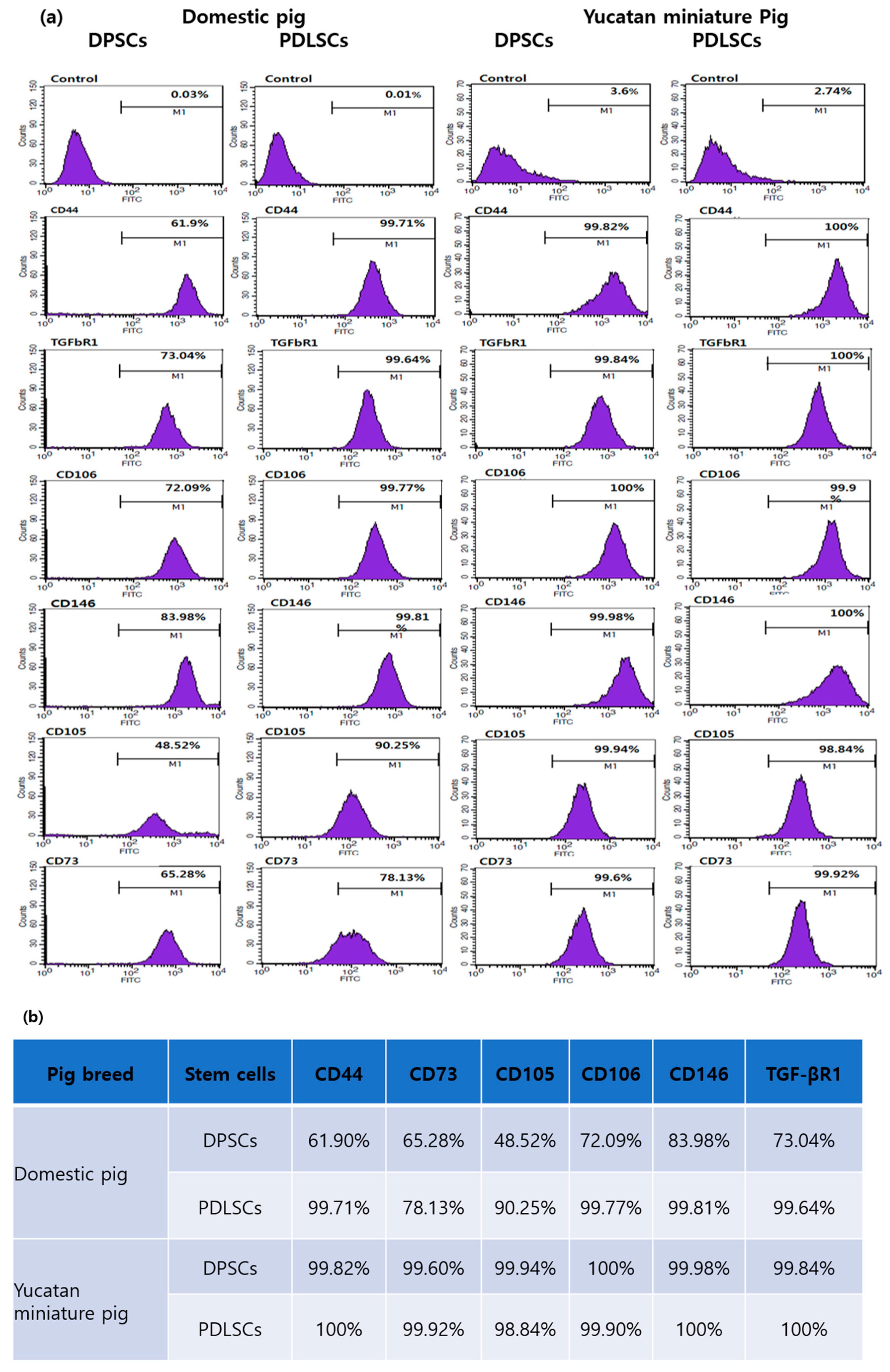
3.2. Cell Surface Marker Expression
3.3. Cell Proliferation
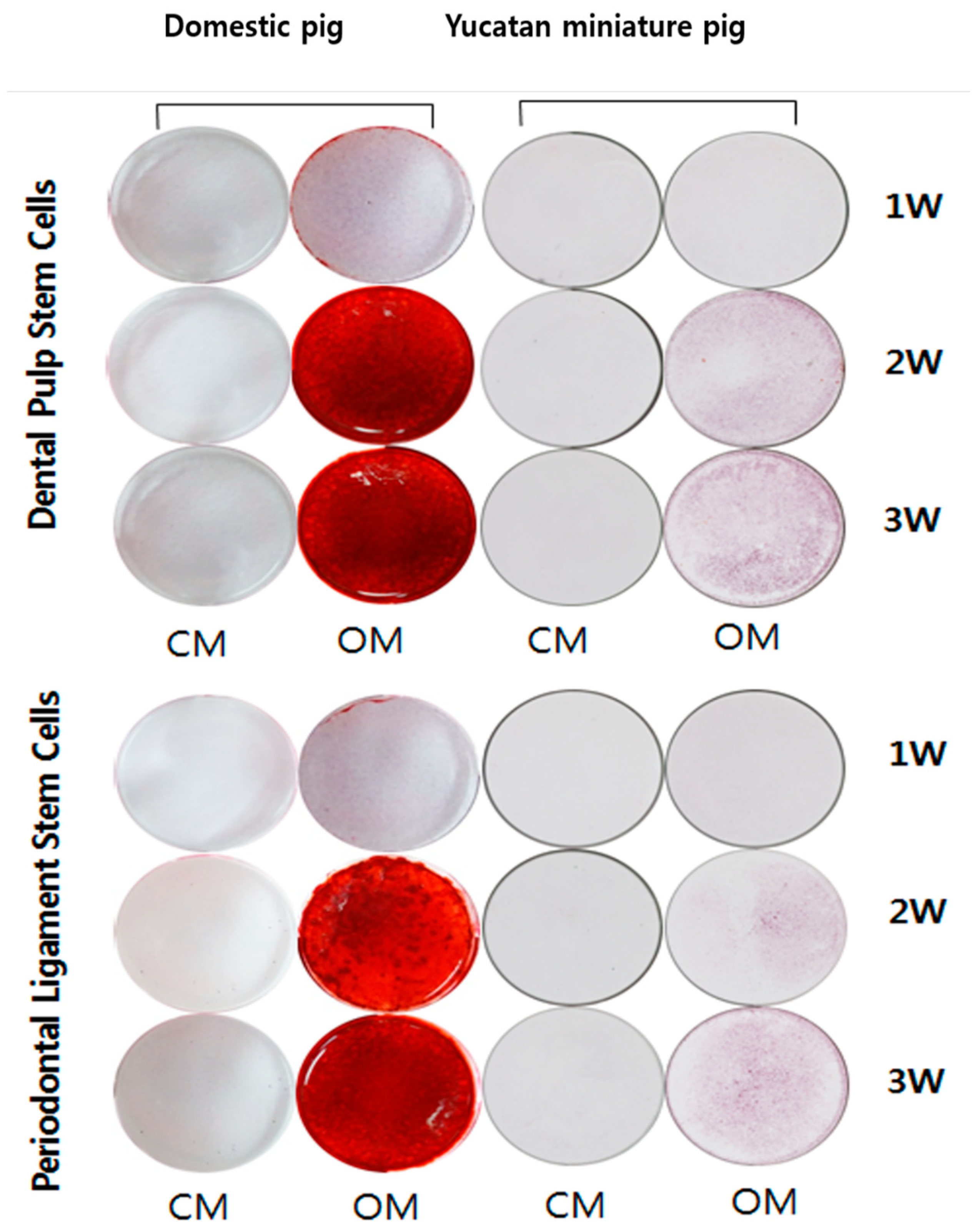
3.4. Osteogenic Differentiation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef]
- Vodička, P.; Smetana Jr, K.; Dvořánková, B.; Emerick, T.; Xu, Y.Z.; Ourednik, J.; Ourednik, V.; MotlÍk, J. The miniature pig as an animal model in biomedical research. Ann. N. Y. Acad. Sci. 2005, 1049, 161–171. [Google Scholar] [CrossRef]
- Kararli, T.T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 1995, 16, 351–380. [Google Scholar] [CrossRef]
- Swindle, M.M.; Smith, A.C.; Laber-Laird, K.; Dungan, L. Swine in biomedical research: Management and models. ILAR J. 1994, 36, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Panepinto, L.M. Miniature swine breeds used worldwide in research. In Advances in Swine in Biomedical Research; Springer: Berlin/Heidelberg, Germany, 1996; pp. 681–691. [Google Scholar]
- McAnulty, P.A.; Dayan, A.D.; Ganderup, N.-C.; Hastings, K.L. The Minipig in Biomedical Research; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Kobayashi, H.; Tai, T.; Nagao, K.; Nakajima, K. The Minipig—A New Tool in Stem Cell Research. Pluripotent Stem Cell Biol. Adv. Mech. Methods Models 2014, 197, 431–444. [Google Scholar]
- Lei, M.; Li, K.; Li, B.; Gao, L.-N.; Chen, F.-M.; Jin, Y. Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials 2014, 35, 6332–6343. [Google Scholar] [CrossRef]
- Huang, G.-J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Mantesso, A.; Sharpe, P. Dental stem cells for tooth regeneration and repair. Expert Opin. Biol. Ther. 2009, 9, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Sun, H.-H.; Lu, H.; Yu, Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012, 33, 6320–6344. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Alencar, A.H.G.d.; Kitten, G.T.; Vencio, E.F.; Gava, E. Mesenchymal stem cells in the dental tissues: Perspectives for tissue regeneration. Braz. Dent. J. 2011, 22, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, R.; Ding, G. Swine dental pulp stem cells inhibit T-cell proliferation. Transplant. Proc. 2011, 43, 3955–3959. [Google Scholar] [CrossRef]
- Wada, N.; Menicanin, D.; Shi, S.; Bartold, P.M.; Gronthos, S. Immunomodulatory properties of human periodontal ligament stem cells. J. Cell. Physiol. 2009, 219, 667–676. [Google Scholar] [CrossRef]
- Ding, G.; Liu, Y.; Wang, W.; Wei, F.; Liu, D.; Fan, Z.; An, Y.; Zhang, C.; Wang, S. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells 2010, 28, 1829–1838. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zheng, Y.; Ding, G.; Fang, D.; Zhang, C.; Bartold, P.M.; Gronthos, S.; Shi, S.; Wang, S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 2008, 26, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-Y.; Jeon, S.H.; Choung, P.-H. Efficacy of periodontal stem cell transplantation in the treatment of advanced periodontitis. Cell Transplant. 2011, 20, 271–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Shi, S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, H.; Mir, L.M.; Andre, F.M. In vitro osteoblastic differentiation of mesenchymal stem cells generates cell layers with distinct properties. Stem Cell Res. Ther. 2018, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Bobis, S.; Jarocha, D.; Majka, M. Mesenchymal stem cells: Characteristics and clinical applications. Folia Histochem. Et Cytobiol. 2006, 44, 215–230. [Google Scholar]
- Osipova, E.; Shamanskaya, T.; Kurakina, O.; Nikitina, V.; Purbueva, B.; Ustugov, A.; Kachanov, D.; Skorobogatova, E.; Dishlevaya, Z.; Roumiantsev, S. Biological characteristics of mesenchymal stem cells during ex vivo expansion. J. Adv. Med. Med. Res. 2011, 1, 85–95. [Google Scholar] [CrossRef]
- Wagner, W.; Wein, F.; Seckinger, A.; Frankhauser, M.; Wirkner, U.; Krause, U.; Blake, J.; Schwager, C.; Eckstein, V.; Ansorge, W. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 2005, 33, 1402–1416. [Google Scholar] [CrossRef]
- Holan, V.; Trosan, P.; Cejka, C.; Javorkova, E.; Zajicova, A.; Hermankova, B.; Chudickova, M.; Cejkova, J. A comparative study of the therapeutic potential of mesenchymal stem cells and limbal epithelial stem cells for ocular surface reconstruction. Stem Cells Transl. Med. 2015, 4, 1052–1063. [Google Scholar] [CrossRef]
- Hui, J.H.; Li, L.; Teo, Y.-H.; Ouyang, H.-W.; Lee, E.-H. Comparative study of the ability of mesenchymal stem cells derived from bone marrow, periosteum, and adipose tissue in treatment of partial growth arrest in rabbit. Tissue Eng. 2005, 11, 904–912. [Google Scholar] [CrossRef]
- Johnstone, S.A. A Comparative Study of the Biological and Molecular Properties of Mesenchymal Stem Cells Isolated from Bone Marrow and the Olfactory System. Ph.D. Thesis, University of Glasgow, Scotland, UK, 2015. [Google Scholar]
- Heino, T.J.; Alm, J.J.; Moritz, N.; Aro, H.T. Comparison of the osteogenic capacity of minipig and human bone marrow-derived mesenchymal stem cells. J. Orthop. Res. 2012, 30, 1019–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, C.; Xiong, F.; Yu, M.-J.; Peng, F.-L.; Shang, Y.-C.; Zhao, C.-P.; Xu, Y.-F.; Liu, Z.-S.; Zhou, C. Comparative study of mesenchymal stem cells from C57BL/10 and mdx mice. BMC Cell Biol. 2008, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R. (Ed.) Biomarkers in Toxicology, 1st ed.; Academic Press: New York, NY, USA, 2014. [Google Scholar]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Gay, I.C.; Chen, S.; MacDougall, M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod. Craniofac. Res. 2007, 10, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, L.; Bonsi, L.; Calvitti, M.; Rondelli, D.; Arpinati, M.; Chirumbolo, G.; Becchetti, E.; Marchionni, C.; Alviano, F.; Fossati, V. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 2005, 80, 836–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.-Y.; Lee, J.; Kim, C.-L.; Lee, K.S.; Lee, S.-H.; Gu, N.-Y.; Kim, J.-M.; Lee, B.C.; Koo, O.J.; Song, J.-Y. Comparative studies on proliferation, molecular markers and differentiation potential of mesenchymal stem cells from various tissues (adipose, bone marrow, ear skin, abdominal skin, and lung) and maintenance of multipotency during serial passages in miniature pig. Res. Vet. Sci. 2015, 100, 115–124. [Google Scholar] [PubMed]
- Fish, R.; Danneman, P.J.; Brown, M.; Karas, A. Anesthesia and Analgesia in Laboratory Animals; Academic Press: New York, NY, USA, 2011. [Google Scholar]
- Maleki, M.; Ghanbarvand, F.; Behvarz, M.R.; Ejtemaei, M.; Ghadirkhomi, E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int. J. Stem Cells 2014, 7, 118. [Google Scholar] [CrossRef] [Green Version]
- Lv, F.-J.; Tuan, R.S.; Cheung, K.M.; Leung, V.Y. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef]
- Harkness, L.; Zaher, W.; Ditzel, N.; Isa, A.; Kassem, M. CD146/MCAM defines functionality of human bone marrow stromal stem cell populations. Stem Cell Res. Ther. 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, S.-J.; Jang, S.; Rah, H.; Choi, S. Characteristics of the Dental Pulp and Periodontal Ligament Stem Cells of the Yucatan Miniature Pig. Appl. Sci. 2021, 11, 9461. https://doi.org/10.3390/app11209461
Son S-J, Jang S, Rah H, Choi S. Characteristics of the Dental Pulp and Periodontal Ligament Stem Cells of the Yucatan Miniature Pig. Applied Sciences. 2021; 11(20):9461. https://doi.org/10.3390/app11209461
Chicago/Turabian StyleSon, Soo-Jin, SeokJin Jang, HyungChul Rah, and SeokHwa Choi. 2021. "Characteristics of the Dental Pulp and Periodontal Ligament Stem Cells of the Yucatan Miniature Pig" Applied Sciences 11, no. 20: 9461. https://doi.org/10.3390/app11209461
APA StyleSon, S.-J., Jang, S., Rah, H., & Choi, S. (2021). Characteristics of the Dental Pulp and Periodontal Ligament Stem Cells of the Yucatan Miniature Pig. Applied Sciences, 11(20), 9461. https://doi.org/10.3390/app11209461




