Probiotic Characteristics of Ligilactobacillus salivarius AS22 Isolated from Sheep Dung and Its Application in Corn-Fox Tail Millet Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Isolation of LAB Strains
2.3. Determination of Organic Acid and Enzyme Activies in the Cell-Free Supernatant
2.4. Characterization of LAB
2.5. Growth Profile of Isolated Strain
2.6. Bile and Acid Tolerance Test of L. salivarius AS22
2.7. Analysis of Haemolytic Activity
2.8. Antibacterial Activity of L. salivarius AS22
2.9. Antifungal Activity Analysis by Pour Plate Method
2.10. Antioxidant Properties
2.11. Bacterial Cultivation and Enumeration
2.12. Silage Preparation
2.13. Microbial Population and Lactic Acid Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Characterization of Ligilactobacillus sp. from the Sheep Dung
3.2. Growth Kinetics of LAB
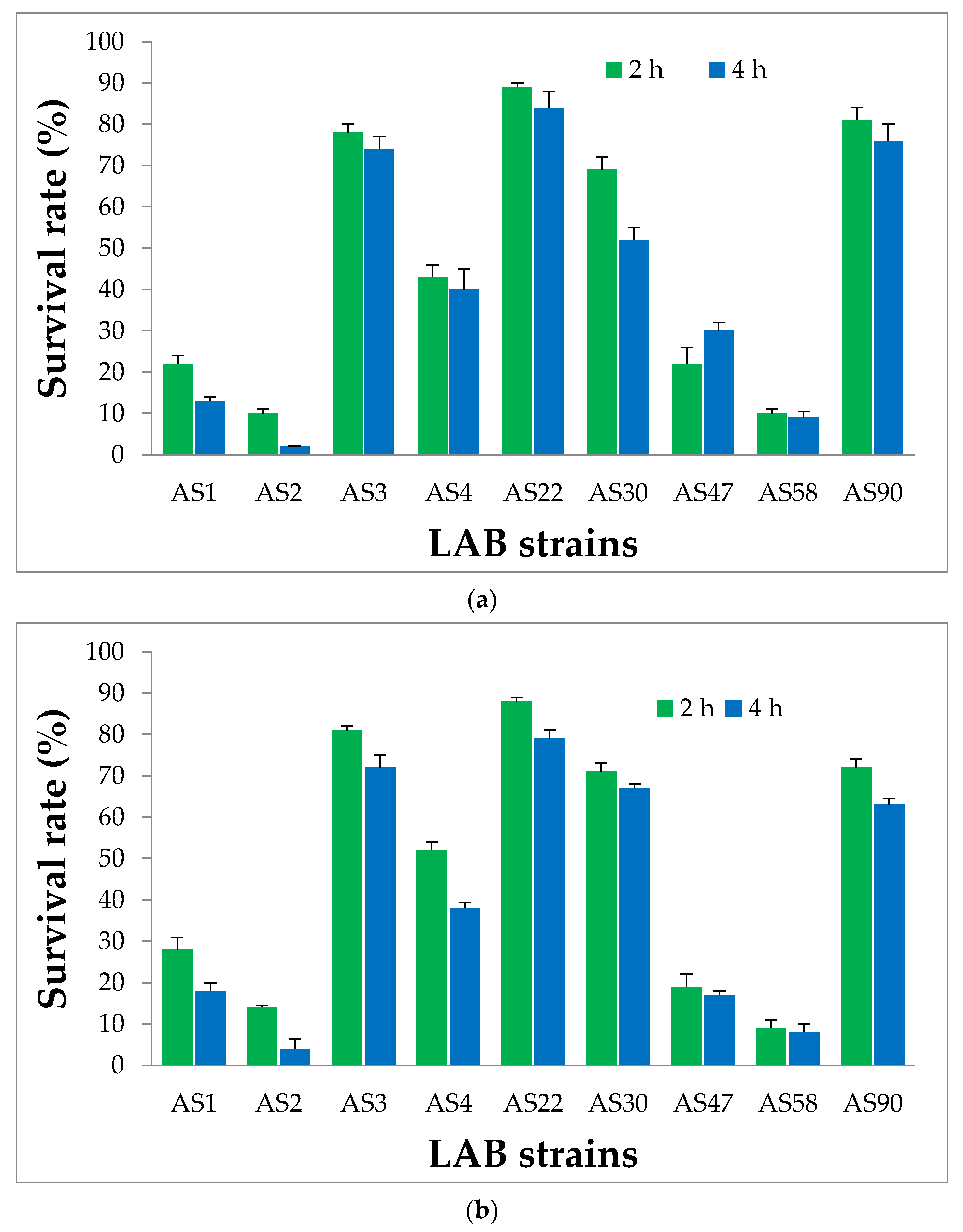
3.3. Probiotic Properties of LAB
3.4. Hemolytic Property of Probiotics
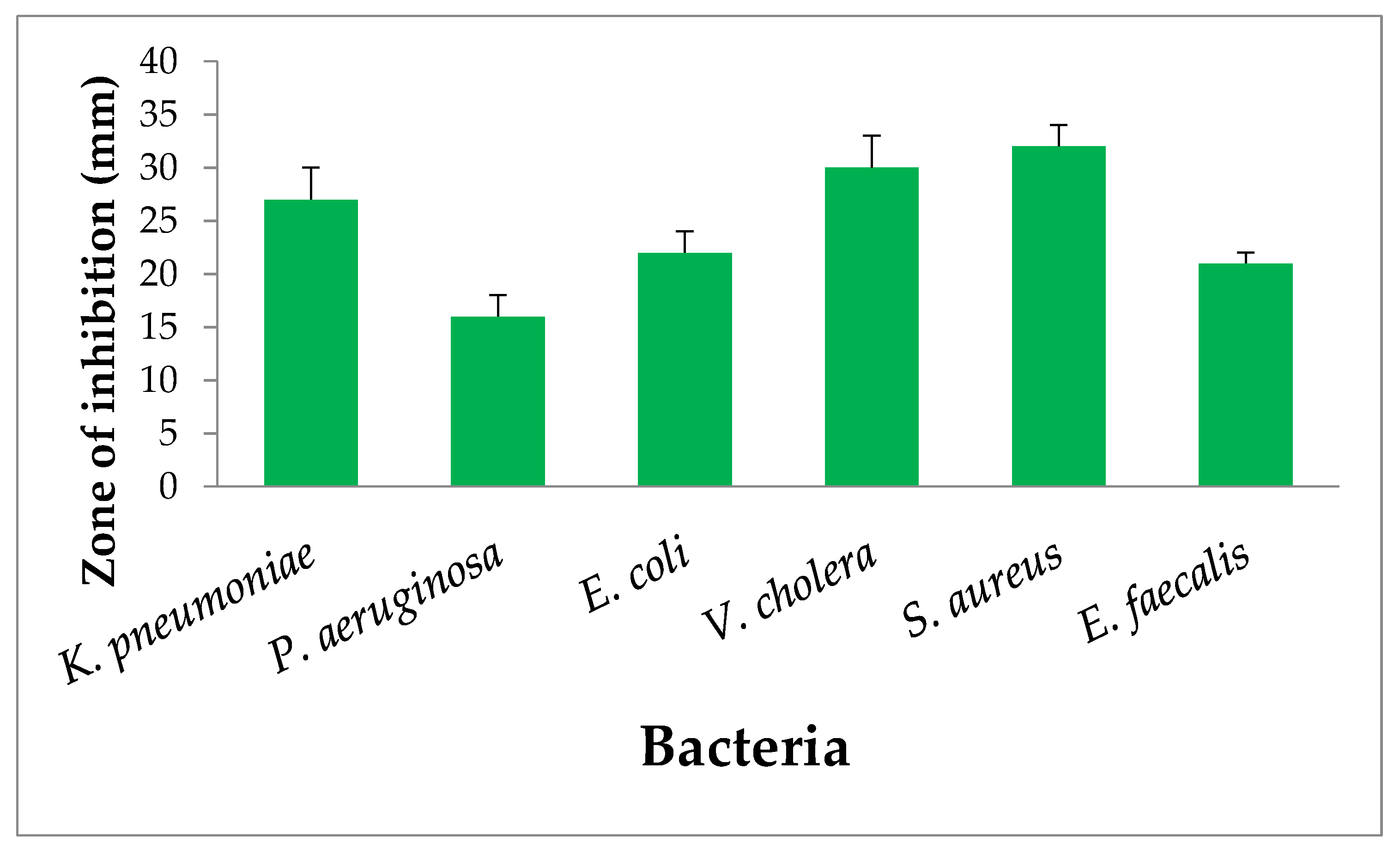
3.5. Antibacterial Activity
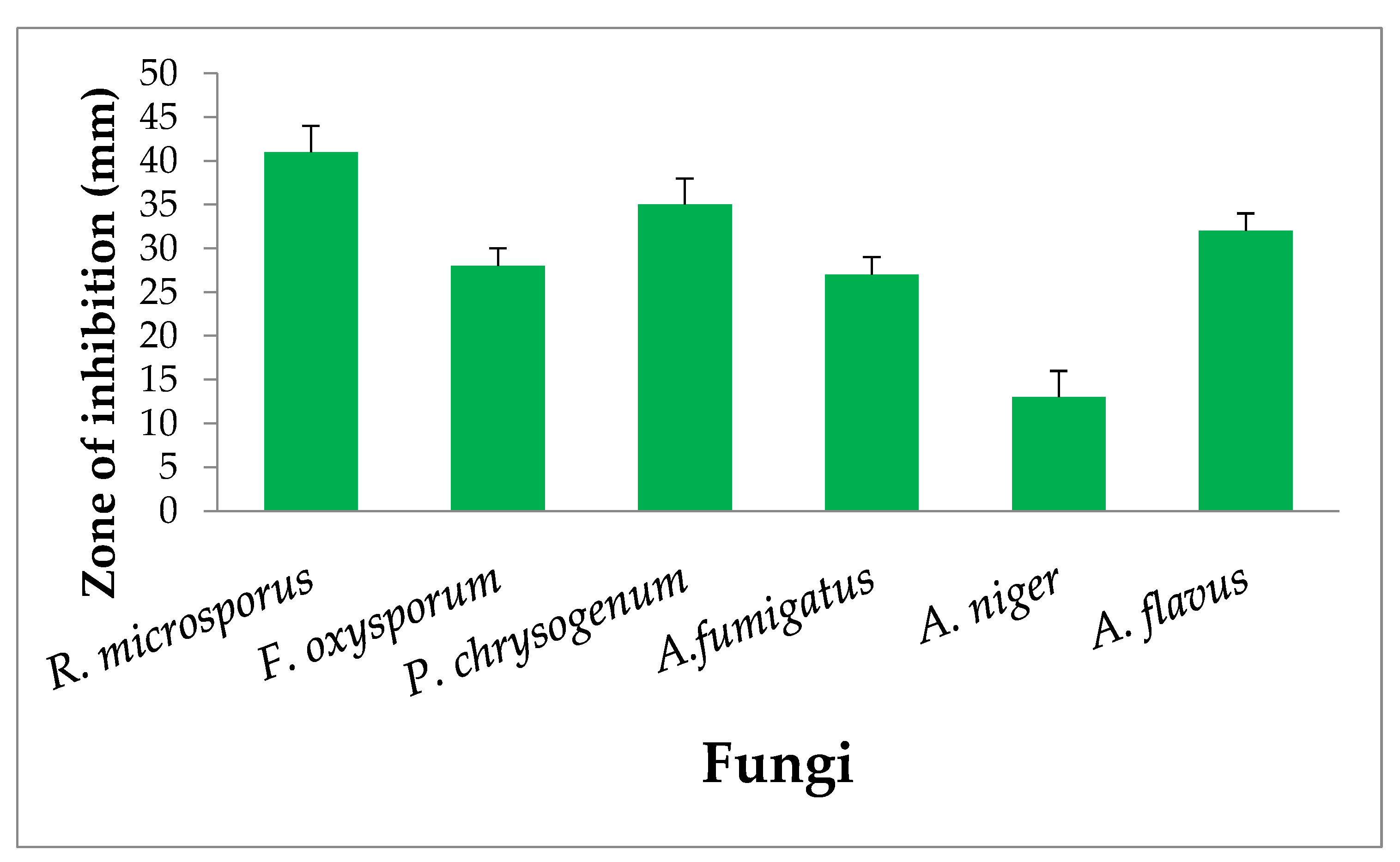
3.6. Antifungal Activity of Cell-Free Extract of L. salivarius AS22
3.7. Antioxidant Activity of L. salivarius AS22
3.8. Lactic Acid Content and Microbial Profiles of Silages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffith, M.; Mclntyre, H.C.H. The interrelationship of growth and frost tolerance in winter rye. Physiol. Plant. 1993, 87, 335–344. [Google Scholar] [CrossRef]
- Weinberg, Z.; Muck, R.; Chen, Y.; Gamburg, M. Lactic Acid Bacteria Used in Inoculants for Silage as Probiotics for Ruminants. Appl. Biochem. Biotechnol. 2004, 118, 1–9. [Google Scholar] [CrossRef]
- Tamang, J.P.; Sarkar, P.K. Microbiology of mesu, a traditional fermented bamboo shoot product. Int. J. Food Microbiol. 1996, 29, 49–58. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Ben-Dov, E.; Shapiro, O.H.; Siboni, N.; Kushmaro, A. Advantage of using inosine at the 3′ termini of 16S rRNA gene universal primers for the study of microbial diversity. Appl. Environ. Microbiol. 2006, 72, 6902–6906. [Google Scholar] [CrossRef]
- Scudamore, K.A.; Livesey, C.T. Occurrence and significance of mycotoxins in forage crops and silage: A review. J. Sci. Food Agric. 1998, 77, 1–17. [Google Scholar] [CrossRef]
- Yiannikouris, A.; Jouany, J.-P. Mycotoxins in feeds and their fate in animals: A review. Anim. Res. 2002, 51, 81–99. [Google Scholar] [CrossRef]
- Speijers, G.J.A.; Speijers, M.H.M. Combined toxic effects of mycotoxins. Toxicol. Lett. 2004, 153, 91–98. [Google Scholar] [CrossRef]
- Magnusson, J.; Schnürer, J. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 2001, 67, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Donaghy, J.; Kelly, P.F.; McKay, A.M. Detection of ferulic acid esterase production by Bacillus spp. and lactobacilli. Appl. Microbiol. Biotechnol. 1998, 50, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Meeske, R. Silage additives: Do they make a difference? S. Afr. J. Anim. Sci. 2005, 6, 49–55. [Google Scholar]
- Filya, I. The effect of Lactobacillus buchneri, with or without homofermentative lactic acid bacteria, on the fermentation, aerobic stability and ruminal degradability of wheat, sorghum and maize silages. J. Appl. Microbiol. 2003, 95, 1080–1086. [Google Scholar] [CrossRef]
- Valan Arasu, M.; Kim, D.; Kim, P.; Jung, M.; Soundharrajan, I.; Jane, M.; Lee, K.; Al-Dhabi, N.; Choi, K. In vitro antifungal, probiotic and antioxidant properties of novel Lactobacillus plantarum K46 isolated from fermented sesame leaf. Ann. Microbiol. 2013, 64, 1333–1346. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef]
- Fabiszewska, A.U.; Zielińska, K.J.; Wróbel, B. Trends in designing microbial silage quality by biotechnological methods using lactic acid bacteria inoculants: A minireview. World J. Microbiol. Biotechnol. 2019, 35, 76. [Google Scholar] [CrossRef]
- Arasu, M.V.; Jung, M.W.; Kim, D.H.; Ilavenil, S.; Jane, M.; Park, H.S.; Al-Dhabi, N.A.; Jeon, B.T.; Choi, K.C. Enhancing Nutritional Quality of Silage by Fermentation with Lactobacillus plantarum. Indian J. Microbiol. 2014, 54, 396–402. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Lee, Y.H.; Lee, W.H.; Oh, Y.-K.; Park, K.; Kwak, W.S. Long-term anaerobic conservation of fruit and vegetable discards without or with moisture adjustment after aerobic preservation with sodium metabisulfite. Waste Manag. 2019, 87, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.C.; Ilavenil, S.; Kuppusamy, P. Probiotic Potential of Lactic Acid Bacteria and its Role on Triticale Silage Fermentation. In Proceedings of the 2018 Korean Society of Grassland Forage and Korean Society of Livestock and Environment Joint Symposium and Academic Presentation, Jeju, Korea, 6–7 September 2018; pp. 214–215. [Google Scholar]
- Timmerman, H.; Veldman, A.; Elsen, E.; Rombouts, F.; Beynen, A. Mortality and Growth Performance of Broilers Given Drinking Water Supplemented with Chicken-Specific Probiotics. Poult. Sci. 2006, 85, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Martine, C.; Copani, G.; Niderkorn, V. Impacts of forage legumes on intake, digestion and methane emissions in ruminants. Legume Perspect. 2016, 12, 24–25. [Google Scholar]
- Jung, H.-J.G. Nutritional Ecology of the Ruminant, 2nd Ed. J. Nutr. 1995, 125, 1025. [Google Scholar] [CrossRef]
- Yitbarek, M.; Tamir, B. Silage Additives: Review. Open J. Appl. Sci. 2013, 4, 17. [Google Scholar] [CrossRef]
- Ho, J.C.K.; Yin Sze, L. Isolation, identification and characterization of enzyme-producing lactic acid bacteria from traditional fermented foods. Biosci. Horiz. Int. J. Stud. Res. 2018, 11. [Google Scholar] [CrossRef]
- Reijniemon, T.S.; Raishy, R.; Hussain, R.R.; Rajamani, B. In-vitro Functional Properties of Lactobacillus plantarum Isolated from Fermented Ragi Malt. South Ind. J. Biol. Sci. 2015, 1, 15–23. [Google Scholar] [CrossRef]
- Soundharrajan, I.; Muthusamy, K.; Han, O.-K.; Lee, H.J.; Purushothaman, S.; Kim, D.; Choi, K.C. Effects of Microbial Inoculants on the Fermentation and Preservation of Triticale Silages at High and Low Moisture Levels. Appl. Sci. 2020, 10, 7855. [Google Scholar] [CrossRef]
- Soundharrajan, I.; Kim, D.; Kuppusamy, P.; Muthusamy, K.; Lee, H.J.; Choi, K.C. Probiotic and Triticale Silage Fermentation Potential of Pediococcus pentosaceus and Lactobacillus brevis and Their Impacts on Pathogenic Bacteria. Microorganisms 2019, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tian, W.; Jiang, Z.; Liang, Z.; Wu, X.; Du, B. Genomic Characterization and Probiotic Potency of Bacillus sp. DU-106, a Highly Effective Producer of L-Lactic Acid Isolated From Fermented Yogurt. Front. Microbiol. 2018, 9, 2216. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of Probiotic Lactobacilli in Acidic Environments Is Enhanced in the Presence of Metabolizable Sugars. Appl. Environ. Microbiol. 2005, 71, 3060. [Google Scholar] [CrossRef]
- Quilodrán-Vega, S.; Albarracin, L.; Mansilla, F.; Arce, L.; Zhou, B.; Islam, M.A.; Tomokiyo, M.; Al Kassaa, I.; Suda, Y.; Kitazawa, H.; et al. Functional and Genomic Characterization of Ligilactobacillus salivarius TUCO-L2 Isolated From Lama glama Milk: A Promising Immunobiotic Strain to Combat Infections. Front. Microbiol. 2020, 11, 608752. [Google Scholar] [CrossRef]
- Messaoudi, S.; Madi, A.; Prévost, H.; Feuilloley, M.; Manai, M.; Dousset, X.; Connil, N. In vitro evaluation of the probiotic potential of Lactobacillus salivarius SMXD51. Anaerobe 2012, 18, 584–589. [Google Scholar] [CrossRef]
- Sanhueza, E.; Paredes-Osses, E.; González, C.L.; García, A. Effect of pH in the survival of Lactobacillus salivarius strain UCO_979C wild type and the pH acid acclimated variant. Electron. J. Biotechnol. 2015, 18, 343–346. [Google Scholar] [CrossRef]
- Collins, J.K.; Thornton, G.; Sullivan, G.O. Selection of probiotic strains for human applications. Int. Dairy. J. 1998, 8, 487–490. [Google Scholar] [CrossRef]
- Oh, Y.J.; Jung, D.S. Evaluation of probiotic properties of Lactobacillus and Pediococcus strains isolated from Omegisool, a traditionally fermented millet alcoholic beverage in Korea. LWT—Food Sci. Technol. 2015, 63, 437–444. [Google Scholar] [CrossRef]
- Bujnakova, D.; Strakova, E. Safety, probiotic and technological properties of Lactobacilli isolated from unpasteurised ovine and caprine cheeses. Ann. Microbiol. 2017, 67, 813–826. [Google Scholar] [CrossRef]
- Nami, Y.; Haghshenas, B.; Vaseghi Bakhshayesh, R.; Mohammadzadeh Jalaly, H.; Lotfi, H.; Eslami, S.; Hejazi, M.A. Novel autochthonous lactobacilli with probiotic aptitudes as a main starter culture for probiotic fermented milk. LWT 2018, 98, 85–93. [Google Scholar] [CrossRef]
- Tarrah, A.; da Silva Duarte, V.; de Castilhos, J.; Pakroo, S.; Lemos Junior, W.J.F.; Luchese, R.H.; Fioravante Guerra, A.; Rossi, R.C.; Righetto Ziegler, D.; Corich, V.; et al. Probiotic potential and biofilm inhibitory activity of Lactobacillus casei group strains isolated from infant feces. J. Funct. Foods 2019, 54, 489–497. [Google Scholar] [CrossRef]
- Murry, A.C.; Hinton, A.; Morrison, H. Inhibition of Growth of Escherichia coli, Salmonella typhimurium, and Clostridia perfringens on Chicken Feed Media by Lactobacillus salivarius and Lactobacillus plantarum. Int. J. Poult. Sci. 2004, 3, 603–607. [Google Scholar] [CrossRef]
- Kang, C.-H.; Han, S.H.; Kim, Y.; Paek, N.-S.; So, J.-S. In Vitro Probiotic Properties of Lactobacillus salivarius MG242 Isolated from Human Vagina. Probiotics Antimicrob. Proteins 2018, 10, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Rondón, A.; del Valle, A.; Milián, G.; Arteaga, F.; Rodríguez, M.; Valdivia, A.; Martínez, M.; Rondón, A.; del Valle, A.; Milián, G.; et al. Production of a Symbiotic Biopreparation (Mixture of Agave fourcroydes Lem. Pulp and PROBIOLACTIL®) for Use in Calves. Agrisost 2019, 25, 1–9. [Google Scholar]
- Rondón, A.J.; Samaniego, L.M.; Bocourt, R.; Rodríguez, S.; Milián, G.; Ranilla, J.; Laurencio, M. Isolation, identification and partial characterization of the probiotic properties of Lactobacillus sp. strains obtained from the gastrointestinal tract of broilers. Cienc. Y Tecnol. Aliment. 2008, 6, 56–63. [Google Scholar] [CrossRef][Green Version]
- Moradi, M.; Tajik, H.; Mardani, K.; Ezati, P. Efficacy of lyophilized cell-free supernatant of Lactobacillus salivarius (Ls-BU2) on Escherichia coli and shelf life of ground beef. Vet. Res. Forum Int. Q. J. 2019, 10, 193–198. [Google Scholar] [CrossRef]
- Adetoye, A.; Pinloche, E.; Adeniyi, B.A.; Ayeni, F.A. Characterization and anti-salmonella activities of lactic acid bacteria isolated from cattle faeces. BMC Microbiol. 2018, 18, 96. [Google Scholar] [CrossRef]
- Mayakrishnan, V.; Soundharrajan, I.; Kim, D.; Priya, K.; Choi, K. In-vitro assessment of the probiotic potential of Lactobacillus plantarum KCC-24 isolated from Italian rye-grass (Lolium multiflorum) forage. Anaerobe 2015, 32, 90–97. [Google Scholar] [CrossRef]
- Ilavenil, S.; Vijayakumar, M.; Kim, D.H.; Valan Arasu, M.; Park, H.S.; Ravikumar, S.; Choi, K.C. Assessment of probiotic, antifungal and cholesterol lowering properties of Pediococcus pentosaceus KCC-23 isolated from Italian ryegrass. J. Sci. Food Agric. 2016, 96, 593–601. [Google Scholar] [CrossRef]
- Schillinger, U.; Villarreal, J.V. Inhibition of Penicillium nordicum in MRS medium by lactic acid bacteria isolated from foods. Food Control 2010, 21, 107–111. [Google Scholar] [CrossRef]
- Sun, Z.; Li, H.; Li, Y.; Qiao, J. Lactobacillus salivarius, a Potential Probiotic to Improve the Health of LPS-Challenged Piglet Intestine by Alleviating Inflammation as Well as Oxidative Stress in a Dose-Dependent Manner During Weaning Transition. Front. Vet. Sci. 2020, 7, 547425. [Google Scholar] [CrossRef] [PubMed]
- Barrera, C.; Burca, C.; Betoret, E.; García-Hernández, J.; Hernández, M.; Betoret, N. Improving antioxidant properties and probiotic effect of clementine juice inoculated with Lactobacillus salivarius spp. salivarius (CECT 4063) by trehalose addition and/or sublethal homogenisation. Int. J. Food Sci. Technol. 2019, 54, 2109–2122. [Google Scholar] [CrossRef]
- Lin, X.; Xia, Y.; Wang, G.; Yang, Y.; Xiong, Z.; Lv, F.; Zhou, W.; Ai, L. Lactic Acid Bacteria With Antioxidant Activities Alleviating Oxidized Oil Induced Hepatic Injury in Mice. Front. Microbiol. 2018, 9, 2684. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Kim, K.T.; Kim, T.Y.; Paik, H.D. Probiotic Properties and Antioxidant Activities of Pediococcus pentosaceus SC28 and Levilactobacillus brevis KU15151 in Fermented Black Gamju. Foods 2020, 9, 1154. [Google Scholar] [CrossRef]
- Christopher, A.; Sarkar, D.; Shetty, K. Improving Phenolic-Linked Antioxidant, Antihyperglycemic and Antibacterial Properties of Emmer and Conventional Wheat Using Beneficial Lactic Acid Bacteria. Appl. Microbiol. 2021, 1, 270–288. [Google Scholar] [CrossRef]
- Nascimento Agarussi, M.C.; Gomes Pereira, O.; Paula, R.A.D.; Silva, V.P.D.; Santos Roseira, J.P.; Fonseca, E.; Silva, F. Novel lactic acid bacteria strains as inoculants on alfalfa silage fermentation. Sci. Rep. 2019, 9, 8007. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yao, D.; Li, D.; Lin, Y.; Bureenok, S.; Ni, K.; Yang, F. Effects of Lactic Acid Bacteria Isolated From Rumen Fluid and Feces of Dairy Cows on Fermentation Quality, Microbial Community, and in vitro Digestibility of Alfalfa Silage. Front. Microbiol. 2020, 10, 2998. [Google Scholar] [CrossRef]
- Guan, H.; Shuai, Y.; Yan, Y.; Ran, Q.; Wang, X.; Li, D.; Cai, Y.; Zhang, X. Microbial Community and Fermentation Dynamics of Corn Silage Prepared with Heat-Resistant Lactic Acid Bacteria in a Hot Environment. Microorganisms 2020, 8, 719. [Google Scholar] [CrossRef]
- Muck, R.E. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]


| Strain Number | Lactic Acid (µg/mL) | Acetic Acid (µg/mL) | Succinic Acid (µg/mL) |
|---|---|---|---|
| AS1 | 22.4 ± 3.2 | 2.3 ± 0.32 | 1.20 ± 0.04 |
| AS2 | 32.1 ± 1.9 | 5.7 ± 0.87 | 1.08 ± 0.32 |
| AS3 | 40.2 ± 2.2 | ND | ND |
| AS4 | 30.3 ± 5.2 | 4.4 ± 1.2 | 0.52 ± 0.05 |
| AS22 | 83.2 ±1.8 | ND | ND |
| AS30 | 29.2 ± 2.1 | 5.2 ± 0.82 | 0.209 ± 0.109 |
| AS47 | 5.2 ± 1.1 | 4.1 ± 0.32 | 1.98 ± 0.28 |
| AS58 | 28.1 ± 2.3 | 8.2 ± 0.49 | 0.272 ± 0.41 |
| AS90 | 76.0 ± 4.3 | ND | ND |
| Characters | Result |
|---|---|
| Gram staining | Positive |
| Shape | Rod |
| Gas production | Negative |
| Catalase | Negative |
| Growth in NaCl | |
| 2% NaCl | ++ |
| 4% NaCl | ++ |
| 6% NaCl | ++ |
| Growth at temperature | |
| 15 °C | + |
| 20 °C | ++ |
| 25 °C | ++ |
| 30 °C | ++ |
| 35 °C | + |
| Enzyme activity | |
| Protease | ++ |
| Cellulase | + |
| Fibrolytic activity | ++ |
| Carbohydrate fermentation | |
| Sorbitol | + |
| Maltose | + |
| Esculin | + |
| Salicin | + |
| Glucose | + |
| Parameters | Control | AS22 Treated | p-Value |
|---|---|---|---|
| LAB (Log CFU/g) | 5.3 ± 0.29 | 6.80 ± 0.14 | 0.0005 ** |
| Fungi (Log CFU/g) | 1.2 ± 0.36 | 0.90 ± 0.29 | 0.0156 * |
| Actinomycetes(Log CFU/g) | 0.72 ± 0.28 | 0.43 ± 0.19 | 0000 **** |
| pH | 5.29 ± 0.03 | 3.84 ± 0.07 | 0.00262 ** |
| Lactic acid (µg/g) | 22.3 ± 3.9 | 38.7 ± 1.40 | 0.0002 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balasubramanian, B.; Soundharrajan, I.; Al-Dhabi, N.A.; Vijayaraghavan, P.; Balasubramanian, K.; Valan Arasu, M.; Choi, K.C. Probiotic Characteristics of Ligilactobacillus salivarius AS22 Isolated from Sheep Dung and Its Application in Corn-Fox Tail Millet Silage. Appl. Sci. 2021, 11, 9447. https://doi.org/10.3390/app11209447
Balasubramanian B, Soundharrajan I, Al-Dhabi NA, Vijayaraghavan P, Balasubramanian K, Valan Arasu M, Choi KC. Probiotic Characteristics of Ligilactobacillus salivarius AS22 Isolated from Sheep Dung and Its Application in Corn-Fox Tail Millet Silage. Applied Sciences. 2021; 11(20):9447. https://doi.org/10.3390/app11209447
Chicago/Turabian StyleBalasubramanian, Balamuralikrishnan, Ilavenil Soundharrajan, Naif Abdullah Al-Dhabi, Ponnuswamy Vijayaraghavan, Kaleeswaran Balasubramanian, Mariadhas Valan Arasu, and Ki Choon Choi. 2021. "Probiotic Characteristics of Ligilactobacillus salivarius AS22 Isolated from Sheep Dung and Its Application in Corn-Fox Tail Millet Silage" Applied Sciences 11, no. 20: 9447. https://doi.org/10.3390/app11209447
APA StyleBalasubramanian, B., Soundharrajan, I., Al-Dhabi, N. A., Vijayaraghavan, P., Balasubramanian, K., Valan Arasu, M., & Choi, K. C. (2021). Probiotic Characteristics of Ligilactobacillus salivarius AS22 Isolated from Sheep Dung and Its Application in Corn-Fox Tail Millet Silage. Applied Sciences, 11(20), 9447. https://doi.org/10.3390/app11209447








