Abstract
R-phycoerythrin (R-PE), a pigment complex found in red algae, was extracted and purified from a newly identified red alga, Colaconema formosanum, and its bioactivities were examined. It was revealed that R-PE treatment resulted in high cell viability (>70%) to the mammalian cell lines NIH-3T3, RBL-2H3, RAW264.7, and Hs68, and had no effect on cell morphology in NIH-3T3 cells. Its suppression effect was insignificant on the production of IL-6 and TNF-α in lipopolysaccharides-stimulated RAW264.7 cells. However, calcium ionophore A23187-induced β-hexosaminidase release was effectively inhibited in a dose-dependent manner in RBL-2H3 cells. Additionally, it was revealed to be non-irritating to bionic epidermal tissues. Notably, procollagen production was promoted in Hs68 cells. Overall, the data revealed that R-PE purified from C. formosanum exhibits anti-allergic and anti-aging bioactivities with no observed consequential toxicity on multiple mammalian cell lines as well as epidermal tissues, suggesting that this macromolecule is a novel material for potential cosmetic use.
1. Introduction
Algae are photosynthetic organisms found on land and in the ocean. As a part of the primary producers in the ocean, algae provide oxygen and nutrients for other organisms, regulating the marine ecosystem. Macroalgae (also known as seaweeds) grow in coastal areas and do not possess typical organs commonly found in terrestrial plants [1]. Macroalgae can be distinguished by their pigment and are categorized into three groups, namely Chlorophyta (green algae), Rhodophyta (red algae), and class Phaeophyceae (brown algae) of Ochrophyta [1]. The growth rate of macroalgae is fairly rapid, and it is feasible to manipulate their growth conditions to control the production of bioactive compounds such as proteins, polyphenols, and pigments [2]. Notably, as acquisition of knowledge about seaweed-derived compounds has increased, research and development for using these compounds in medical and food additive applications has subsequently increased [3,4,5,6]. Moreover, there is an increasing preference for natural ingredients over synthetic compounds in cosmetics, because natural ingredients tend to be safer compared to the latter.
Algae have been reported to be rich in bioactive substances such as unsaturated fatty acids, polyphenols, polysaccharides, amino acids, and pigments [7,8]. Some of them contain pigment complexes known as phycobiliproteins, which can be categorized into phycoerythrocyanin (PEC), phycocyanins (PC), phycoerythrin (PE), and allophycocyanins (APC) [9]. PE, the major pigment in red seaweed, can be further subcategorized into C-phycoerythrin (C-PE), B-phycoerythrin (B-PE), and R-phycoerythrin (R-PE) and according to their absorption spectra, and according to the group of photosynthetic organisms that produce them: C for cyanobacteria, B for Bangiophyceae (primitive filamentous Rhodophyta), and R for more complex Rhodophyta [10]. Among these pigments, R-PE is the most abundant phycobiliprotein in red algae. R-PE is a water-soluble oligomeric protein that is commonly used as a fluorescent tag in flow cytometry, ELISA assays, and fluorescence microscopy [11,12,13,14] as well as a photosensitizer in cancer therapy [15]. In addition, PE has been demonstrated to exhibit biological properties, including anti-viral, immunity-enhancement, anti-oxidation, anti-inflammation, and anti-tumor effects (please see the review paper in [15] to obtain more relative information), making it an ideal compound for applications in the food and biomedical industries, molecular biology research, as well as dye and cosmetic applications [11,15,16].
Aging is one of the major issues concerning the skin, especially to people exposed to sunlight (or ultraviolet rays) and polluted air for long periods of time. So, most of the skin-care related products focus on protecting the skin and slowing down the aging process. Skin is the first line of defense of the human body, and it helps to prevent the accumulation of damage from pollution, sunlight, and oxidative stress that is unavoidable in our daily lives [17]. In recent years, consumers have become skeptical about chemical ingredients in cosmetic products; therefore, there is an increasing demand for environmentally sustainable products produced using natural resources [1]. Algal extracts, such as those from Arthrospira and Chlorella vulgaris, have been reported to be beneficial by exerting a tightening effect on the skin, preventing stria formation and promoting collagen synthesis [18]. Apparently, the bioactive compounds responsible for these anti-aging effects include sulfated polysaccharides, phenolic compounds, peptides, mycosporine-like amino acids, and pigments, among others [19,20]. In addition, the bioactive substances purified from the algal extracts were examined for specific bioactivities beneficial to the skin, and the substances were scientifically proved to be relatively safe, allowing the formulation of cosmetics using the purified bioactive substances instead of the whole extracts [21]. Thus, cosmeceuticals that contain extracts or purified metabolites derived from algae are in high demand [1]. In previous studies, a technologically and economically feasible cultivation strategy for the stable biomass production of Colaconema formosanum was successfully established by Lee and Yeh in 2021 by isolating Colaconema formosanum from southern Taiwan using an indoor system [22,23]. As a parasitic alga, C. formosanum was first isolated from the macroalga Sarcodia suiae in southern Taiwan [23]. With the high protein (30% of dry weight) and high R-PE contents (5 mg g−1 dw) found for this species [22], C. formosanum exhibits immense industrial advantages for R-PE extraction, and it is expected to make the market price of R-PE more affordable. Furthermore, our group has reported a method to purify R-PE extracted from C. formosanum [24]. To our knowledge, effects of R-PE from C. formosanum on mammalian cells as well as its potential as a new biomaterial in the cosmetics industry have not yet been explored. Therefore, in this study, various in vitro analyses such as cell cytotoxicity, degranulation assays, anti-inflammatory ability, anti-allergy tests, and procollagen synthesis tests have been employed to verify these hypotheses.
2. Materials and Methods
2.1. Extraction of R-Phycoerythrin (R-PE) from Colaconema Formosanum
The alga was cultured in incubators (Tominaga, Taipei City, Taiwan) at 20 ± 1 °C, 60 ± 10 μmol photons m−2 s−1 (light-emitting diode, LED white light), and a 12:12 h light:dark photoperiod in a 5 L beaker with 4L of sterilized PES seawater medium (30 psu) for several days to obtain a working sample. Next, the method to extract R-PE from C. formosanum in this study was modified from the method reported by Lee et al. [24]. First, phycobiliproteins were extracted from the fresh alga C. formosanum (100 g wet weight) in 1 L of phosphate-buffered saline (PBS, pH 7.4) at 4°C. To prevent phycobiliprotein degradation during the experiments, 5 mM NaN3 and 4 mM EDTA were added to PBS. The fresh alga was homogenized using a FastPrep-24 homogenizer (TeenPrep, 6 cycles, 4.0 m∙s−1 for 5 s; MP Biomedicals, Solon, OH, USA). The extract was roughly filtered to remove debris, and the filtrate was subjected to centrifugation at 20,000 rpm using a barrel centrifuge (Beckman Coulter, Brea, California, USA). The red supernatant, called R-PE extract, was collected and stored at 4 °C in the dark. The R-PE extract was subsequently subjected to fractionation with (NH4)2SO4 at 20–60% (w/v) saturation at 4 °C. Solid ammonium sulfate was slowly added by gentle stirring, and the solution was left to rest for 2 h, followed by centrifugation at 10,000 rpm for 20 min at 4 °C and precipitate formation. The precipitate was dissolved in PBS (pH 7.4) and dialyzed overnight against the same buffer. The dialyzed red-colored solution was passed through a 0.22-μm membrane filter (Merck Millipore, Burlington, MA, USA).
2.2. Purification of R-PE by Ion-Exchange Chromatography Using Fast Protein Liquid Chromatography (FPLC)
The dialyzed samples of R-PE were subjected to ion-exchange chromatography in a loaded HiTrap DEAE FF column (5 mL), which was pre-equilibrated with 50 mM PBS (pH 7.4) containing 10 mM NaCl. After the samples were passed through, the column was extensively washed using equilibrium buffer. Ion-exchange chromatography was subsequently conducted at an elution rate of 5 mL/min using linear gradient elution ranging from 0.0 to 500 mM NaCl. The eluted red fractions were collected. The eluted fractions were analyzed by UV-Vis spectrophotometry using an NGC medium-pressure chromatography system (BIO-RAD, Hercules, CA, USA) at wavelengths of 280, 498, 566, and 620 nm. The obtained R-PE fractions were further analyzed using an absorption spectrum from 300 to 700 nm and were passed through a 0.22-µm filter (Merck Millipore, Burlington, MA, USA).
2.3. Cell Culture
The NIH-3T3, Hs68, RBL-2H3, and RAW264.7 cell lines were purchased from the Bioresource Collection and Research Center (BCRC, Hsinchu City, Taiwan).
As a mouse fibroblast cell line, NIH-3T3 was cultured with Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Thermo Fisher Scientific, Waltham, MA, USA) containing 10% calf serum (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), 1.5 g/L sodium bicarbonate (Sigma-Aldrich, St. Louis, MO, USA), 4 mM L-glutamine (Sigma-Aldrich, St. Louis, MO, USA), and 4.5 g/L glucose (Sigma-Aldrich, St. Louis, MO, USA).
As a human fibroblast cell line, Hs68 was cultured using DMEM containing 4 mM L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, and 10% FBS (Gibco, Thermo Fisher Scientific, Waltham, MA, USA).
The rat basophilic leukemia (RBL)-2H3 cell line was cultured using Eagle’s minimum essential medium (EMEM, Sigma-Aldrich, St. Louis, MO, USA) supplemented with 4 mM L-glutamine, 1.5 g/L sodium bicarbonate, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate, and 15% FBS.
As a murine macrophage cell line, RAW264.7 was cultured with DMEM containing 4 mM L-glutamine, 1.5 g/L sodium bicarbonate, and 10% FBS.
All cell lines were consistently maintained and kept within a humid chamber set to 37℃ with 5% CO2, which were struck twice per week using standard procedures.
2.4. Morphological Grading and Cell Viability Assay
NIH-3T3 cells were seeded in a 96-well plate (1 × 104 cells per well, Corning, New York, NY, USA) with culture medium and left to settle overnight. Cells were then treated with a culture medium containing 0 (negative control), 0.25, 0.5, 1, 2, 5, and 10 μg/mL of R-PE extract. The wells containing medium supplemented with 10% DMSO (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) were also included in the trial as a positive control group. After 24-h incubation, morphological grading for each group was analyzed using the definition from [25], with grades 0, 1, 2, 3, and 4 representing none, slight, mild, moderate, and severe reactivity, respectively. The culture medium was replaced with 1 mg/mL MTT reagent (Sigma-Aldrich, St. Louis, MO, USA) 48-h post-treatment and incubated for 3 h. Isopropanol was added into wells after the removal of the MTT reagent to dissolve formazan, and plates were analyzed using a spectrophotometer (Jasco Spectrophotometer V-630) at a wavelength of 570 nm [26]. If the level of cell viability for the highest R-PE extract dose was less than 70% of the control group, then it was considered to be cytotoxic [25]. The percentage of cell viability was calculated using the following equation:
Cell viability (optical density, OD) = (OD of exposed cells/OD of control cells) × 100
2.5. Anti-Inflammation Test
To determine the anti-inflammation ability of R-PE, interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNFα) were detected in this study following the protocols as described in [27,28]. RAW264.7 cells were seeded in a 24-well plate (5 × 105 cells/well) (Corning, New York, NY, USA) and left to settle overnight at 37℃. On the next day, cells were co-treated with lipopolysaccharides (LPS, 1 μg/mL, Sigma-Aldrich, St. Louis, MO, USA) and increasing concentrations of R-PE (0 (negative control), 1.25, 2.5, 5, 10, and 20 μg/mL). Concurrently, an additional cell group that was co-treated with LPS and the p38 MAPK inhibitor SB203580 (3 μM, Sigma-Aldrich, St. Louis, MO, USA) was established as a positive control group. After 24 h, the supernatant for each group was collected for the detection of the produced IL-6 and TNF-α. Supernatant samples were applied to the Quantikine® Colorimetric Sandwich ELISA Kits (R&D Systems, Minneapolis, MN, USA). Percentage of inhibition (%) = 100 × (sample/control). In addition, the cells were subjected to an MTT assay to evaluate cell viability after treatments.
2.6. Degranulation Assay
As a rat basophilic leukemia cell line, the RBL-2H3 cell line is used as a model for mast cells. RBL-2H3 cells exhibit phenotypes of mucosal mast cells and are recognized as a brilliant tool for investigating the regulation of mast cell responses [29]. Thus, the RBL-2H3 cell line was used in this test to investigate the potential effect of R-PE on the degranulation of mast cells. β-hexosaminidase was used as a marker to monitor the degranulation of mast cell [30]. The β-hexosaminidase detection method was modified from reference [31]. RBL-2H3 cells were seeded at 1 × 105 cells per well in 24-well cell culture plates (Corning, New York, NY, USA). Cells were first treated with 1 µM calcium ionophore A23187 (Sigma-Aldrich, St. Louis, MO, USA) followed by the addition of differing dosages of R-PE (0 (negative control), 1.25, 2.5, 5, 10, and 20 μg/mL) and incubated for 2 h. Concurrently, cells that were incubated with 100 μM quercetin (Sigma-Aldrich, St. Louis, MO, USA) were used as a positive control. The methodology used for β-hexosaminidase quantification was obtained from previously published studies [32,33]. After treatment, the supernatant (30 μL) from each well was collected and transferred to a new 96-well plate prior to the addition of 50 μL of 4-nitrophenyl N-acetyl-β-D-glucosaminide (NP-GlcNAc, 1.3 mg/mL in citrate buffer (pH 4.5), Sigma-Aldrich, St. Louis, MO, USA). The plate was left to stay at 37℃ for 1 h, and the reaction was terminated by the addition of 80 μL of 0.5 M NaOH. The plate was then analyzed for any formation of p-nitrophenolate using a spectrophotometer (Jasco, Halifax, NS, Canada) at a wavelength of 405 nm. Cells left from the original culture plate were used in an MTT assay to examine cell viability after treatments.
2.7. Skin Irritation Test
To verify that topical application would not cause an irritation to the skin, an epidermal tissue was used to mimic a scenario where R-PE was applied on the tissue level. An epidermal irritation assessment is a key test for any medical device or cosmetic product, so that only products considered safe to the skin would be provided to consumers. Traditional animal tests have been reported to not only cause pain and discomfort for test animals, but such testing has also not always been representative of the effects observed in humans; thus, it is regarded that using a reconstructed bionic tissue is advantageous as they possess numerous cell types surrounded by a local microenvironment, simulating human skin in vivo [34]. Effects of R-PE on skin irritation were evaluated by using the bionic epidermal tissue EpiDerm™ (MatTek LIFE SCIENCES, Ashland, MA, USA) according to manufacturer’s instructions and with experiments following the methodology modified from references [35,36,37]. The inserts containing the EpiDerm sample were inserted into a 6-well plate (Corning, New York, NY, USA) containing pre-warmed DMEM. An amount of 100 μL DMEM containing either 0 (negative control) or 1 mg/mL purified R-PE (final concentration 0.1 mg/mL) was added into the cell culture insert atop the EpiDerm sample. Tissue that was treated with 5% sodium dodecyl sulfate (SDS) served as a positive control. After 1-h incubation in a humidified environment of 37 °C, 5% CO2 incubator, the inserts were removed and were washed twice with PBS (pH 7.4), followed by placement into a 24-well plate containing 300 μL of a 1 mg/mL MTT solution (in DMEM). After 3-h incubation, isopropanol was applied to dissolve MTT formazan crystals. The supernatant was transferred to a 96-well plate, and the sample measured at an OD of 570 nm using spectrophotometry. Relative viability was calculated following the equation described in Section 2.4. Chemicals are considered irritants when they decrease cell viability to less than 50% (Category 2), and chemicals that result in cell viabilities of greater than 50% are considered to be non-irritants (No Category) [37].
2.8. Procollagen Synthesis Test
Hs68 cells were inoculated in a 24-well plate (2 × 105 cells/well) and left to settle overnight. Cells were subsequently treated with a culture medium containing 0, 0.625, 1.25, 2.5, 5, and 10 μg/mL R-PE extract. The positive control group was treated with 100 ng/mL of transforming growth factor (TGF)-β1 (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) [38,39]. Next, 72-h post-treatment, the supernatant from each sample was collected and subjected to procollagen detection. Monoclonal anti-human procollagen Type I C-Peptide (PIP) (Takara Bio, Shiga, Japan). (MK101) was used according to the manufacturer’s instructions for the detection of procollagen. In addition, the MTT assay was conducted to evaluate cell viability after treatment.
2.9. Statistical Analysis
Data were analyzed using IBM SPSS Statistics 22.0 (IBM Corp, Armonk, NY, USA). We first subjected the data for each group to the Kolmogorov–Smirnov test to verify that they were normally distributed (α > 0.05) [40]. Student’s t-test was used to analyze cell viability, IL-6, TNF-α, β-hexosaminidase inhibition, epidermal tissues, and procollagen production. All data are presented as the means ± standard deviation (SD), with each experiment performed in quintuplicate. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Purification of R-Phycoerythrin (R-PE) from Colaconema Formosanum
The absorption spectrum of purified R-PE from C. formosanum revealed characteristic peaks at 280, 498, and 566 nm, but not at 620 nm (Figure 1). The scan wavelength was in the range of 200 to 700 nm. Absorption peaks were observed at 498, 539, and 565 nm in the absorption spectrum, and the fluorescence emission maximum was at 580 nm. In addition, the purity of R-PE (A566/A280 ratio) reached 5.79, and the absorbance ratios of A620/A566 and A566/A498 were 0.003 and 1.40, respectively (Figure 2).
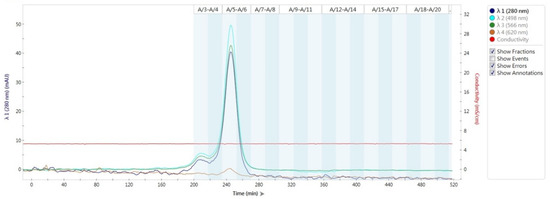
Figure 1.
Absorption spectrum of R-phycoerythrin (R-PE) purified by ion exchange chromatography in FPLC using a HiTrap DEAE FF column. The elution was monitored using an UV-Vis spectrophotometer at wavelengths of 280, 498, 566, and 620 nm.

Figure 2.
The absorption spectrum and fluorescence emission spectrum of purified R-phycoerythrin. The absorption spectrum and the purity indexes of purified R-phycoerythrin were determined using an UV-Vis spectrophotometer. RFU = relative fluorescence unit.
3.2. Morphological Grading and Cell Viability Assay
Seven treatment concentrations (up to 10 μg/mL) of the R-PE extract were applied to murine NIH-3T3 fibroblast cells (Figure 3), and all tested groups did not exhibit cytotoxicity or morphological changes (grade 0). In contrast, a clear morphological change was observed in 10% DMSO-treated cells (grade 4). The cell viability of NIH-3T3 fibroblast cells seemed to be dose-dependent on R-PE. As the concentration of R-PE rises, the cell viability decreases. Although the results from the MTT assay revealed that cells that had received 1, 2, 5, and 10 μg/mL of R-PE exhibit significantly reduced cell viability at 92.1 ± 1.03%, 90.9 ± 1.11%, 89.2 ± 1.41%, and 82.4 ± 1.21%, respectively, compared to the vehicle control group (100 ± 1.37%), but still higher than 70% and no obvious morphological changes was seen from the cells, indicating R-PE treatment is not toxic to NIH-3T3 cells. However, the DMSO-treated cells exhibited the lowest cell viability rate (13.4 ± 1.42%), which is under 70%; therefore, the said treatment is determined to be toxic to NIH-3T3 cells.

Figure 3.
Cell viability of NIH-3T3 cells treated to different concentrations of R-phycoerythrin (R-PE) purified from Colaconema formosanum. Data are shown as the mean ± standard deviation (n = 5). Significant differences from the control group were denoted by asterisks (paired sample t-test, p < 0.05).
3.3. Anti-Inflammation Test Using R-Phycoerythrin
The production rate of the two pro-inflammatory cytokines measured, IL-6 and TNF-α, did not exhibit significant differences between RAW264.7 cells that were treated with or without the R-PE extract in the presence of LPS (Figure 4). Although production rates of the two pro-inflammatory cytokines were apparently lowered, this was statistically insignificant. Therefore, R-PE could not be proved effective in anti-inflammation. However, compared to the vehicle control cells, the addition of SB203580 significantly inhibited the production of TNF-α (production rate: 52.81 ± 0.32%) and IL-6 (production rate: 15.32 ± 0.27%) in RAW264.7 cells in the presence of LPS. No significant reduction in the cell viability of RAW264.7 cells after R-PE treatments was observed.

Figure 4.
Effect of different concentrations of R-phycoerythrin on production of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) and cell viability in lipopolysaccharides-treated RAW264.7 cells. Data are shown as the mean ± standard deviation (n = 5). For TNF-α and IL-6 production rates, significant differences from the control group were denoted by asterisks (paired sample t-test, p < 0.05).
3.4. Degranulation Assay
To verify the anti-allergic properties of R-PE, the rat basophilic cell line RBL-2H3 was treated with calcium ionophore A23187 in the absence or presence of different concentrations of R-PE. The results revealed that the inhibition of β-hexosaminidase release is successfully arrested by R-PE in a dose-dependent manner, with 20 μg/mL of R-PE exhibiting the lowest β-hexosaminidase release rate (82.4 ± 6.43%) (Figure 5). However, the average inhibitory effect of 100 μM quercetin (positive control) was stronger than that of R-PE at all tested concentrations. Therefore, 20 μg mL−1 R-PE could be considered effective in its anti-allergic property, but not as effective as 100 μM quercetin. No dose-dependent effect was observed in the cell viability of RBL-2H3 cells, and the cell viabilities did not differ significantly between the control and experimental groups.
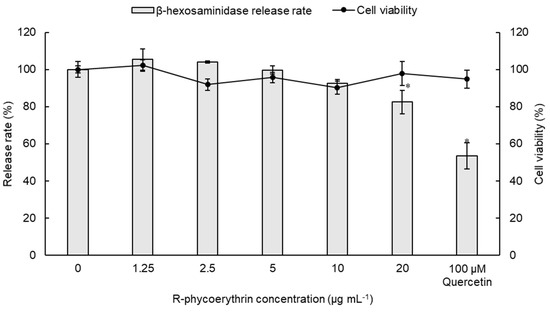
Figure 5.
Concentration-dependent inhibition of β-hexosaminidase release and cell viability of RBL-2H3 cells by R-phycoerythrin purified from Colaconema formosanum. Data are shown as the mean ± standard deviation (n = 5). For β-hexosaminidase release rate, significant differences from the control group were denoted by asterisks (paired sample t-test, p < 0.05).
3.5. Skin Irritancy Test
The skin irritancy test was conducted using bionic epidermal tissue (EpiDerm). The results revealed that the relative viability for the tissue treated with 0.1 mg/mL R-PE is 105.01 ± 4.34% (Figure 6), and R-PE was determined as a non-irritant. Cell viability values for the negative (vehicle) and positive (5% SDS) controls are 100 ± 3.32% and 0.76 ± 0.08%, respectively.
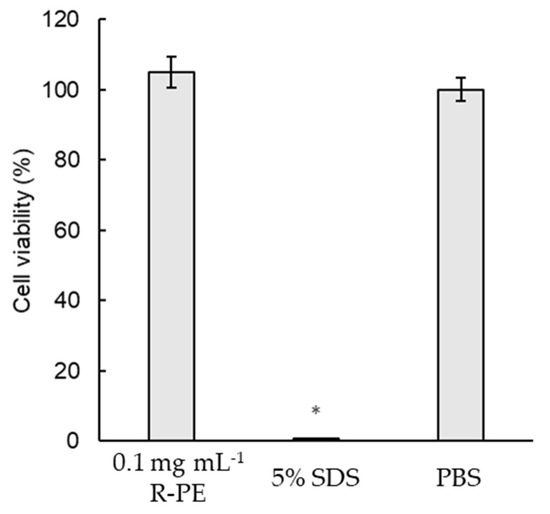
Figure 6.
In vitro skin irritancy test on bionic epidermal tissue. Bionic epidermal tissue (EpiDerm) was treated with medium containing PBS, or medium containing 0.1 mg/mL of R-phycoerythrin (R-PE) purified from Colaconema formosanum, or 5% SDS, for 1 h. The skin irritancy degree was judged by relative cell viability compare to the control group. Data are shown as the mean ± standard deviation (n = 3). Significant differences from the control group were denoted by asterisks (Student’s t-test, p < 0.05).
3.6. Procollagen Synthesis Test
The bioactivity of R-PE in promoting type-I procollagen synthesis was examined in the Hs68 human fibroblast cell line via treatment of various concentrations. The results indicated that R-PE facilitates the synthesis of type-I procollagen in a dose-dependent manner (Figure 7). The 5 and 10 μg/mL R-PE treatments, compared to the negative control, significantly raised the procollagen synthesis rate. The treatment of 10 μg/mL R-PE led to the highest type-I procollagen synthesis rate (130.7 ± 4.2%). This value was even greater than that of the positive control group (TGF-β1, 121.3 ± 4.6%) as well as other experimental groups treated with lower doses of R-PE. No negative effect on the cell viability of Hs68 cells was observed in all groups.
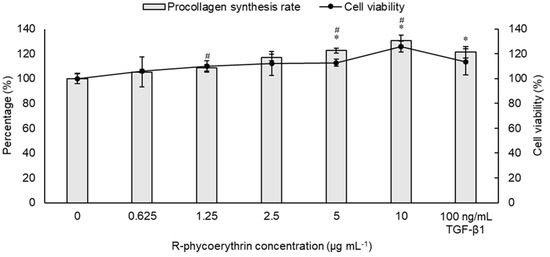
Figure 7.
Concentration-dependent promotion of type-I procollagen synthesis rate and cell viability of Hs68 cells by R-phycoerythrin purified from Colaconema formosanum. TGF-β1 stands for transforming growth factor-β1. Data are shown as the mean ± standard deviation (n = 5). For procollagen synthesis rate and cell viability rate, significant differences from the control group were denoted by asterisks and number signs, respectively (paired sample t-test, p < 0.05).
4. Discussion
Purified R-PE was obtained from C. formosanum, and similar to the R-PE extracted from the Mediterranean red algae Corallina elongata [41], characteristic peaks were observed at 280, 498, and 566 nm, but not 620 nm, indicating that the purified R-PE exhibits high purity and a low PC content (λ: 610–620 nm) [42]. In addition, R-PE purified from C. formosanum was a classical “three-peak” phycoerythrin with maximum absorption observed at 498, 539, and 565 nm, respectively (Figure 2). This is a typical absorption spectrum of R-PE, as described in a previous study [43]. The maximum fluorescence emission of R-PE obtained from C. formosanum was observed at 580 nm, which was consistent with a previous study related to the fluorescence spectrum of PE [44]. Therefore, using the above-described spectral parameters, the purified PE in this study was confirmed to be typical R-PE. Generally, the purity of R-PE is estimated using three indexes: A565/A280 > 5.3, A565/A495 < 1.5, and A620/A566 < 0.005 [16,41,43]. A565/A280 represents the purity of the product as a result of the preparation protocol with respect to contaminating proteins, and A565/A495 is an indicator of the property of the purified pigment. A pigment is considered to not be overly contaminated with B-phycoerythrin (B-PE) when A565/A495 < 1.5. The high ratio of A620/A566 indicates the contamination of the product with PC [41]. As a reference, purified PE with purity ratios of 0.7, 3.9, and >4.0 is classified as food-grade, reactive-grade, and analytical-grade PE, respectively [45]. The purity ratio (A566/A280) of purified R-PE in this study reached 5.79 (Figure 2), far exceeding the standard for food-grade PE. The absorbance ratio of A620/A566 was 0.003, and A566/A498 was 1.40, which demonstrated the absence of PC and B-PE contaminants, respectively.
In this study, the potential bioactivities and functions of R-PE purified from C. formosanum were analyzed by testing the pigment in several cell lines and bionic epidermal tissues by using multiple functional models. The morphological grading and cell viability assay results indicated that purified R-PE does not exert significant toxic effects on NIH-3T3 cells and that morphological changes are not observed, indicating that R-PE does not exhibit significant cytotoxic effects on the examined cell lines.
Previous studies reported that PE extract from the red alga Porphyra vietnamensis had the potential anti-inflammatory effect on paw edema inhibition in the in vivo system using Wistar rats [46], this effect above be regarded as the term ’soothing effect’ in folk medicine [47]. In addition, PE extract from cyanobacterium Pseudanabanea tenuis showed the potential anti-oxidant effects on mercury chloride-caused oxidative stress and renal damage inhibition in the in vivo system using male mice [48]. The release of pro-inflammatory cytokines leads to the inflammation of the skin, and these cytokines include IL-6 and tumor necrosis factor α (TNF-α). IL-6 exhibits a pro-inflammatory effect when it comes into contact with outer and inner stimulants, while TNF-α regulates multiple critical functions including apoptosis, proliferation, and inflammatory cytokine production. In this study, the anti-inflammatory effects of R-PE were determined by assessing its ability to inhibit IL-6 and TNF-α. Compared to the vehicle control cells, the production rate of these two pro-inflammatory cytokines did not differ between RAW264.7 cell samples that were treated with or without R-PE in the presence of LPS, suggesting that R-PE purified from C. formosanum per se may not exhibit inhibitory effects on IL-6 and TNF-α production. This result was supported by a past research using macrophages [49]. In this past research, though the production IL-6 and TNF-α in LPS treated macrophages were not affected by 10 μg/mL R-PE, the production of IL-1β was lowered, suggesting an anti-inflammation effect. In addition, another past study showed that the phycobiliproteins of Palmaria palmata, as 100 μg/mL phycobilin, inhibited the production of IL-6 and TNF-α [50]. The possibility of not using the optimal concentration of R-PE in this assay may have constrained the results; therefore, the potential anti-inflammatory effects of R-PE extracted from C. formosanum might be discovered using higher concentrations of R-PE, or through assays on other pro-inflammatory cytokines.
Mast cells play significant roles in type I allergic reactions. Histamine, which is mainly stored in mast cells, is released during degranulation; degranulation is induced by allergens binding to IgE to deal with invading pathogens or allergens in downstream allergic reaction signaling pathways. An increase in the concentration of intracellular calcium cations (Ca2+) has also been reported to possibly lead to the degranulation of mast cells [51]. A calcium ionophore, A23187, can carry extracellular Ca2+ across the plasma membrane into mast cells, thereby initiating degranulation [52]. Therefore, the suppression of degranulation via target extracts or compounds facilitates the alleviation of allergic reactions. As histamine is easily degraded by histamine N-methyltransferase as well as other related enzymes after being secreted into the extracellular fluid, the detection of β-hexosaminidase becomes the primary route to evaluate mast cell degranulation levels [53]. Phycobiliproteins are known to have anti-allergic effects. R-PC was reported to have an inhibitory effect on IgE induced mast cell degranulation [54], while PE and phycoerythrobilin (PEB) extracted from Porphyra yezoensis has been reported to decrease the release of β-hexosaminidase induced by A23187, possibly via the chelation of extracellular Ca2+, which then leads to the suppression of Ca2+ influx into cells [29]. Similarly, data within this study revealed that R-PE purified from C. formosanum may inhibit the release of β-hexosaminidase induced by A23187 in a dose-dependent manner (Figure 5), indicating that R-PE purified from C. formosanum is an effective macromolecule with anti-allergy properties when used in suitable concentrations. On the other hand, the inhibitory effect of PEB seems to be stronger than that of PE [29]. Thus, digesting R-PE to obtain R-phycoerythrobilin would be a way to enhance the inhibitory effect on mast cell degranulation.
Although the bioactive substances obtained from algae are generally safer compared to synthetic compounds, assessments on their safety upon application is still necessary [21]. In the current study, compared with that of the control group, the R-PE-treated samples did not exhibit a difference regarding cell viability; therefore, this compound is recognized as a non-irritant according to the OECD 493 testing guideline [37]. The results in this study indicate that R-PE does not cause skin irritation when used on the tissue surface in the trial dose; therefore, its safety for application on human skin in the tested doses is guaranteed.
Collagen is the most abundant protein in the human body, and it supports the maintenance of tissue structure and elasticity [55]. Skin ages when reactive oxygen species (ROS) trigger the downstream mitogen-activated protein kinase (MAPK) signal transduction pathways, resulting in the induction of metalloproteinase (MMP) expression [19]. MMPs degrade collagen, elastic fiber, and other extracellular compounds, causing wrinkles and aging. To prevent the loss of collagen, efforts have been focused on the search for novel marine-sourced bioactive compounds with properties related to skin-care applications. As such, red algae extracts have become one of the major subjects of interest for study [56,57]. Notably, in this present study, type-I procollagen synthesis rates were significantly increased in sample groups treated with R-PE to a level even greater than that observed in the positive control group (TGF-β1) (5 and 10 μg/mL of R-PE, Figure 7). The detection of procollagens is a key index in terms of assessing collagen synthesis levels [58]. So, the results indicated that R-PE purified from C. formosanum could stimulate the secretion of type-I collagen by the human fibroblast cells, resulting in an anti-aging effect. Aside from collagen synthesis, the anti-aging effect could also be achieved by inhibiting MMP expression [59]. C-PC at 20 μg/mL was shown to be effective in inhibiting MMP-1 and MMP-9 in human keratinocytes (HaCaT cells), and the effect strengthens with increasing concentrations [60]. As mentioned above, MMPs degrade extracellular compounds, leading to the aging of the skin and the formation of wrinkles. The inhibitory effect of C-PC on MMPs would slow down the aging process. Hence, further studies could be done to determine the effect of R-PE on the inhibition of MMPs.
5. Conclusions
The controlled experiment of this study showed that R-phycoerythrin (R-PE) purified from C. formosanum had anti-allergy and anti-aging properties, and without cell toxicity by series of in vitro studies. These findings provide useful information for the cosmetic and personal-care product industries and present an important step in valuating and identifying potential effect of this species for commercial applications. Given these results, we believe that the PE applications will be introduced in the cosmetic market in the future. A recent study has shown that incorporating R-PE in a solid matrix of gelatin could greatly enhance its photochemical stability and storage for up to 8 months under ambient conditions [61], further support its applications in cosmetic sector. As a further consideration, we therefore highlight support in the development of further research into areas such as standardized extract systems, quality assurance, PE activity maintenance and storage, and quality control of extract production to assess sustainable and large-scale commercial applications in the future.
Author Contributions
Conceptualization, M.-C.L.; methodology, P.-T.L., W.-Q.-C.L., J.H., Y.-J.C., B.C. and F.-H.N.; formal analysis, W.-Q.-C.L. and F.-H.N.; investigation, B.C. and F.-H.N.; resources, H.-Y.Y. and J.H.; writing—original draft preparation, P.-T.L.; writing—review and editing, M.-C.L.; funding acquisition, M.-C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology (MOST, Taiwan), grant number MOST 108-2823-8-019-001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors especially thank the editors and anonymous reviewers for their thoughtful comments. The authors thank Szu-Hsiu Liu, Yu-Chen Kao (Research assistant), and Shu-I Jen (Research assistant) from Dermatologic Skin Care and Cosmetics Technology, Industrial Technology Research Institute, Hsinchu, Taiwan, for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Wang, H.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Gallagher, E.; Tasdemir, D.; Hayes, M. Heart health peptides from macroalgae and their potential use in functional foods. J. Agric. Food Chem. 2011, 59, 6829–6836. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Amarante, S.J.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Brown algae phlorotannins: A marine alternative to break the oxidative stress, inflammation and cancer network. Foods 2021, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. An overview to the health benefits of seaweeds consumption. Mar. Drugs 2021, 19, 341. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-N.; Nabende, W.Y.; Jeong, H.; Hahn, D.; Jeong, G.-S. The marine-derived natural product epiloliolide isolated from Sargassum horneri regulates NLRP3 via PKA/CREB, promoting proliferation and anti-inflammatory effects of human periodontal ligament cells. Mar. Drugs 2021, 19, 388. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva, R.V.; Belik, A.A.; Kusaykin, M.I.; Malyarenko, O.S.; Zvyagintseva, T.N.; Ermakova, S.P. Laminarans and 1,3-β-D-glucanases. Int. J. Biol. Macromol. 2020, 163, 1010–1025. [Google Scholar] [CrossRef]
- Ismail, M.M.; Alotaibi, B.S.; EL-Sheekh, M.M. Therapeutic uses of red macroalgae. Molecules 2020, 25, 4411. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Kawsar, S.M.A. Protein, R-phycoerythrin from marine red alga Amphiroa anceps (Lamarck): Extraction, purification and characterization. Phytol. Balc. 2011, 17, 347–354. [Google Scholar]
- Kronick, M.N. The use of phycobiliproteins as fluorescent labels in immunoassay. J. Immunol. Methods 1986, 92, 1–13. [Google Scholar] [CrossRef]
- Glazer, A.N.; Stryer, L. Fluorescent tandem phycobiliprotein conjugates. Emission wavelength shifting by energy transfer. Biophys. J. 1983, 43, 383–386. [Google Scholar] [CrossRef][Green Version]
- Pereira, T.; Barroso, S.; Mendes, S.; Amaral, R.A.; Dias, J.R.; Baptista, T.; Saraiva, J.A.; Alves, N.M.; Gil, M.M. Optimization of phycobiliprotein pigments extraction from red algae Gracilaria gracilis for substitution of synthetic food colorants. Food Chem. 2020, 321, 126688. [Google Scholar] [CrossRef] [PubMed]
- Deepika, C. Extraction and spectral characterization of R-phycoerythrin from macroalgae-Kappaphycus alvarezii. Int. Res. J. Eng. Technol. 2018, 5, 1181. [Google Scholar]
- Li, S.; Ji, L.; Shi, Q.; Wu, H.; Fan, J. Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Bioresour. Technol. 2019, 292, 122048. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Hou, Y. Efficient purification of R-phycoerythrin from marine algae (Porphyra yezoensis) based on a deep eutectic solvents aqueous two-phase system. Mar. Drugs 2020, 18, 618. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transpl. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Stolz, P.; Obermayer, B. Manufacturing microalgae for skin care. Cosmet. Toilet. 2005, 120, 99–106. [Google Scholar]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef]
- Guillerme, J.-B.; Couteau, C.; Coiffard, L. Applications for marine resources in cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds compounds: An ecosustainable source of cosmetic ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Lee, M.C.; Yeh, H.Y.; Jhang, F.J.; Lee, P.T.; Lin, Y.K.; Nan, F.H. Enhancing growth, phycoerythrin production, and pigment composition in the red alga Colaconema sp. through optimal environmental conditions in an indoor system. Bioresour. Technol. 2021, 333, 125199. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Yeh, H.-Y. Molecular and morphological characterization of Colaconema formosanum sp. nov. (Colaconemataceae, Rhodophyta)—A new endophytic filamentous red algal species from Taiwan. J. Mar. Sci. Eng. 2021, 9, 809. [Google Scholar] [CrossRef]
- Lee, P.-T.; Huang, J.; Huang, C.-Y.; Liu, Z.-X.; Yeh, H.-Y.; Huang, H.-T.; Chen, L.-L.; Nan, F.-H.; Lee, M.-C. Phycoerythrin from Colaconema sp. has immunostimulatory effects on the whiteleg shrimp Litopenaeus vannamei and increases resistance to Vibrio parahaemolyticus and white spot syndrome virus. Animals 2021, 11, 2371. [Google Scholar] [CrossRef] [PubMed]
- ISO. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylostalo, J.H. Macrophage inflammatory assay. Bio-Protocol 2014, 4, e1108. [Google Scholar] [CrossRef]
- Lee, C.W.; Kim, S.C.; Kwak, T.W.; Lee, J.R.; Jo, M.J.; Ahn, Y.-T.; Kim, J.M.; An, W.G. Anti-inflammatory effects of bangpungtongsung-San, a traditional herbal prescription. Evid. -Based Complement. Altern. Med. 2012, 2012, 892943. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Komura, Y.; Nishimura, Y.; Sugawara, T.; Hirata, T. Inhibition of mast cell degranulation by phycoerythrin and its pigment moiety phycoerythrobilin, prepared from Porphyra yezoensis. Food Sci. Technol. Res. 2011, 17, 171–177. [Google Scholar] [CrossRef]
- Guo, R.H.; Park, J.U.; Jo, S.J.; Ahn, J.H.; Park, J.H.; Yang, J.Y.; Lee, S.S.; Park, M.J.; Kim, Y.R. Anti-allergic inflammatory effects of the essential oil from fruits of Zanthoxylum coreanum Nakai. Front. Pharmacol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Tanaka, Y.; Takagaki, Y.; Nishimune, T. Effects of metal elements on β-hexosaminidase release from rat basophilic leukemia cells (RBL-2H3). Chem. Pharm. Bull. 1991, 39, 2072–2076. [Google Scholar] [CrossRef][Green Version]
- Quah, Y.; Lee, S.-J.; Lee, E.-B.; Birhanu, B.T.; Ali, M.S.; Abbas, M.A.; Boby, N.; Im, Z.-E.; Park, S.-C. Cornus officinalis ethanolic extract with potential anti-allergic, anti-inflammatory, and antioxidant activities. Nutrients 2020, 12, 3317. [Google Scholar] [CrossRef] [PubMed]
- Passante, E.; Ehrhardt, C.; Sheridan, H.; Frankish, N. RBL-2H3 cells are an imprecise model for mast cell mediator release. Inflamm. Res. 2009, 58, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-M.; Chou, Y.-T.; Wen, Z.-H.; Wang, Z.-R.; Chen, C.-H.; Ho, M.-L. Novel biodegradable porous scaffold applied to skin regeneration. PLoS ONE 2013, 8, e56330. [Google Scholar] [CrossRef] [PubMed]
- Netzlaff, F.; Lehr, C.M.; Wertz, P.W.; Schaefer, U.F. The human epidermis models EpiSkin®, SkinEthic® and EpiDerm®: An evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur. J. Pharm. Biopharm. 2005, 60, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Faller, C.; Bracher, M.; Dami, N.; Roguet, R. Predictive ability of reconstructed human epidermis equivalents for the assessment of skin irritation of cosmetics. Toxicol. Vitr. 2002, 16, 557–572. [Google Scholar] [CrossRef]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD: Paris, France, 2021. [Google Scholar]
- Massagué, J. TGF-β signal transduction. Annu. Rev. Biochem. 1998, 67, 753–791. [Google Scholar] [CrossRef]
- Lee, H.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Protective effects of unsaponifiable matter from perilla seed meal on UVB-induced damages and the underlying mechanisms in human skin fibroblasts. Antioxidants 2019, 8, 644. [Google Scholar] [CrossRef] [PubMed]
- Massey, F.J. The Kolmogorov-Smirnov Test for Goodness of Fit. J. Am. Stat. Assoc. 1951, 46, 68–78. [Google Scholar] [CrossRef]
- Rossano, R.; Ungaro, N.; D’Ambrosio, A.; Liuzzi, G.; Riccio, P. Extracting and purifying R-phycoerythrin from Mediterranean red algae Corallina elongata Ellis & Solander. J. Biotechnol. 2003, 101, 289–293. [Google Scholar]
- MacColl, R.; Guard-Friar, D. Phycobiliproteins; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Liu, L.N.; Chen, X.L.; Zhang, X.Y.; Zhang, Y.Z.; Zhou, B.C. One-step chromatography method for efficient separation and purification of R-phycoerythrin from Polysiphonia urceolata. J. Biotechnol. 2005, 116, 91–100. [Google Scholar] [CrossRef]
- Bermejo Román, R.; Alvárez-Pez, J.M.; Acién Fernández, F.G.; Molina Grima, E. Recovery of pure B-phycoerythrin from the microalga Porphyridium cruentum. J. Biotechnol. 2002, 93, 73–85. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Sinha, R.P. Thermokinetic stability of phycocyanin and phycoerythrin in food-grade preservatives. J. Appl. Phycol. 2016, 28, 1063–1070. [Google Scholar] [CrossRef]
- Bhatia, S.; Sharma, K.; Sharma, A.; Nagpal, K.; Bera, T. Anti-inflammatory, analgesic and antiulcer properties of Porphyra vietnamensis. Avicenna J. Phytomed. 2015, 5, 69–77. [Google Scholar]
- Couteau, C.; Coiffard, L. Phycocosmetics and other marine cosmetics, specific cosmetics formulated using marine resources. Mar. Drugs 2020, 18, 322. [Google Scholar] [CrossRef] [PubMed]
- Cano-Europa, E.; Ortiz-Butron, R.; Gallardo-Casas, C.; Blas-Valdivia, V.; Pineda-Reynoso, M.; Olvera-Ramírez, R.; Franco-Colin, M. Phycobiliproteins from Pseudanabaena tenuis rich in c-phycoerythrin protect against HgCl2-caused oxidative stress and cellular damage in the kidney. J. Appl. Phycol. 2010, 22, 495–501. [Google Scholar] [CrossRef]
- Cian, R.E.; Martínez-Augustin, O.; Drago, S.R. Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Res. Int. 2012, 49, 364–372. [Google Scholar] [CrossRef]
- Lee, D.; Nishizawa, M.; Shimizu, Y.; Saeki, H. Anti-inflammatory effects of dulse (Palmaria palmata) resulting from the simultaneous water-extraction of phycobiliproteins and chlorophyll a. Food Res. Int. 2017, 100, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Woo, C.H.; Yoon, S.B.; Kim, J.H. Protein kinase Cδ functions downstream of Ca2+ mobilization in FcεRI signaling to degranulation in mast cells. J. Allergy Clin. Immunol. 2004, 114, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Oliver, J.M. Roles for Ca2+ stores release and two Ca2+ influx pathways in the FcεR1-activated Ca2+ responses of RBL-2H3 mast cells. Mol. Biol. Cell 1995, 6, 825–839. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Torres-Atencio, I.; Ainsua-Enrich, E.; de Mora, F.; Picado, C.; Martín, M. Prostaglandin E2 prevents hyperosmolar-induced human mast cell activation through prostanoid receptors EP2 and EP4. PLoS ONE 2014, 9, e110870. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Cao, M.; Pan, T.; Yang, Y.; Mao, H.; Sun, L.; Liu, G. Anti-allergic activity of R-phycocyanin from Porphyra haitanensis in antigen-sensitized mice and mast cells. Int. Immunopharmacol. 2015, 25, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Kim, G. Chapter 53—Collagen. High Yield Orthop. 2010, 107–109. [Google Scholar]
- Resende, D.I.S.P.; Ferreira, M.; Magalhães, C.; Sousa Lobo, J.M.; Sousa, E.; Almeida, I.F. Trends in the use of marine ingredients in anti-aging cosmetics. Algal Res. 2021, 55, 102273. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, S.; Chen, J.; Li, C.; Zhou, C.; Hong, P.; Sun, S.; Qian, Z.-J. Trehalose against UVB-induced skin photoaging by suppressing MMP expression and enhancing procollagen I synthesis in HaCaT cells. J. Funct. Foods 2020, 74, 104198. [Google Scholar] [CrossRef]
- Seo, W.-Y.; Kim, J.-H.; Baek, D.-S.; Kim, S.-J.; Kang, S.; Yang, W.S.; Song, J.-A.; Lee, M.-S.; Kim, S.; Kim, Y.-S. Production of recombinant human procollagen type I C-terminal propeptide and establishment of a sandwich ELISA for quantification. Sci. Rep. 2017, 7, 15946. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, H.; Choi, S.; Pandey, L.K.; Depuydt, S.; De Saeger, J.; Park, J.T.; Han, T. Extracts of red seaweed, Pyropia yezoensis, inhibit melanogenesis but stimulate collagen synthesis. J. Appl. Phycol. 2021, 33, 653–662. [Google Scholar] [CrossRef]
- Jang, Y.A.; Kim, B.A. Protective effect of spirulina-derived C-phycocyanin against ultraviolet B-induced damage in HaCaT cells. Medicina 2021, 57, 273. [Google Scholar] [CrossRef] [PubMed]
- Bharmoria, P.; Correia, S.F.H.; Martins, M.; Hernández-Rodríguez, M.A.; Ventura, S.P.M.; Ferreira, R.A.S.; Carlos, L.D.; Coutinho, J.A.P. Protein Cohabitation: Improving the Photochemical Stability of R-Phycoerythrin in the Solid State. J. Phys. Chem. Lett. 2020, 11, 6249–6255. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).