A Novel Vascular Intervention Surgical Robot Based on Force Feedback and Flexible Clamping
Abstract
1. Introduction
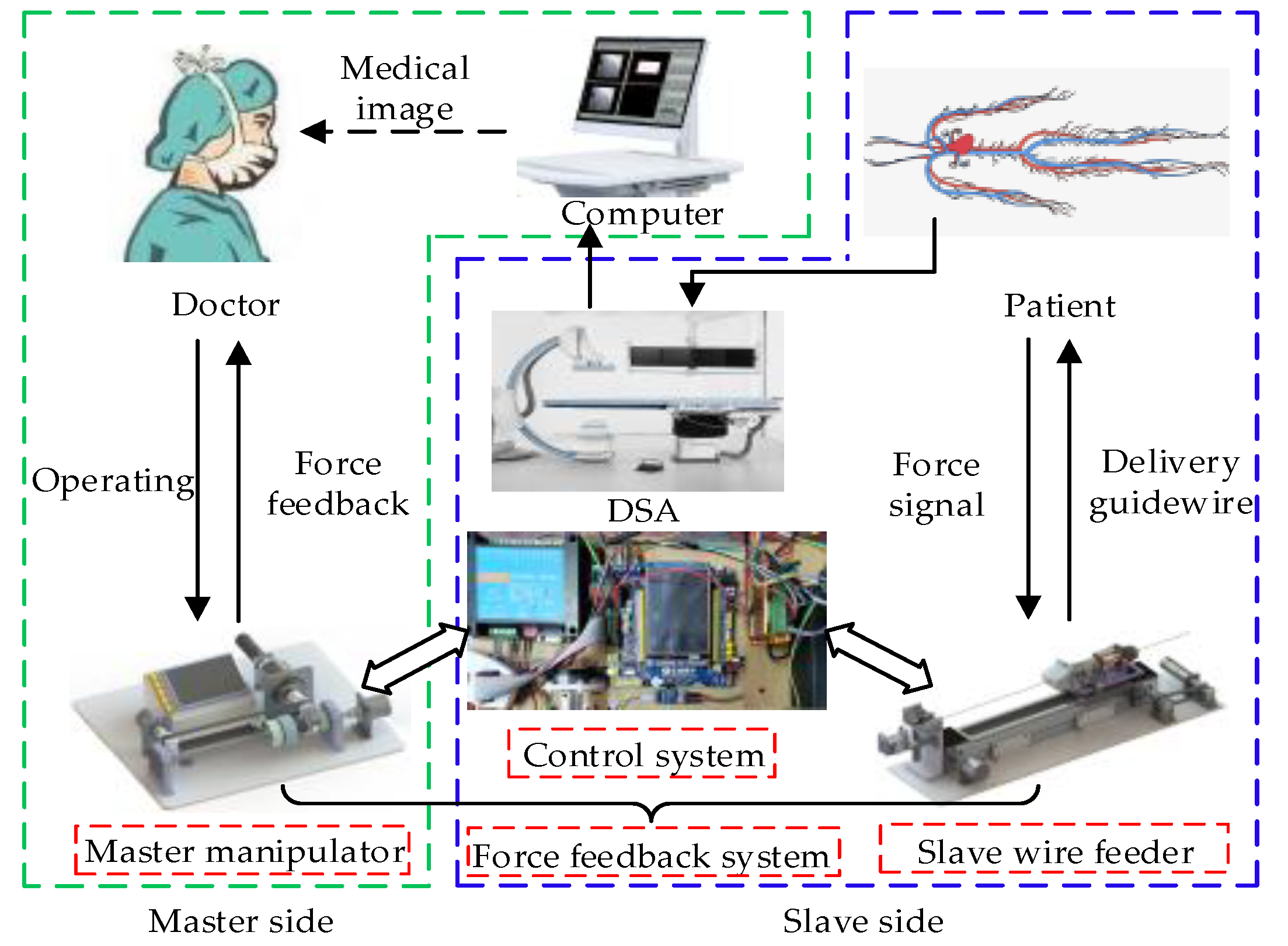
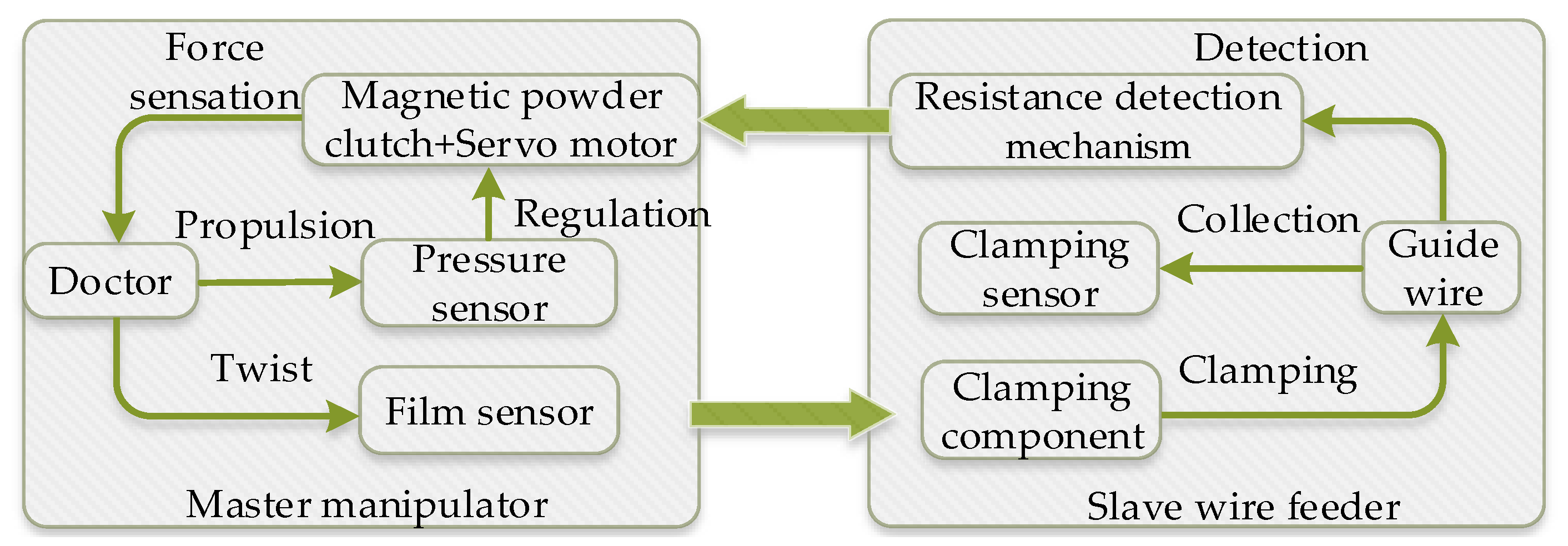
2. System Description
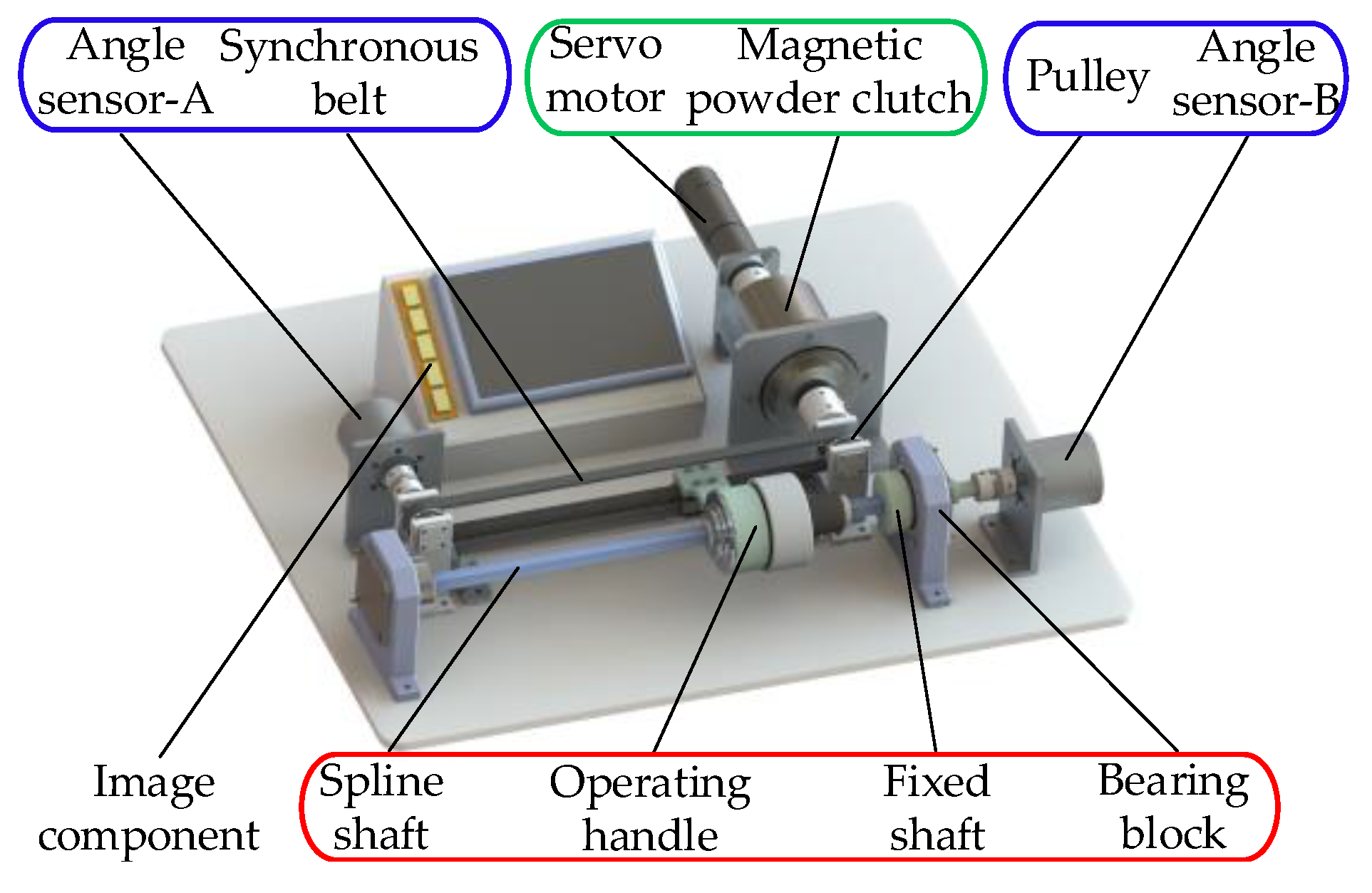
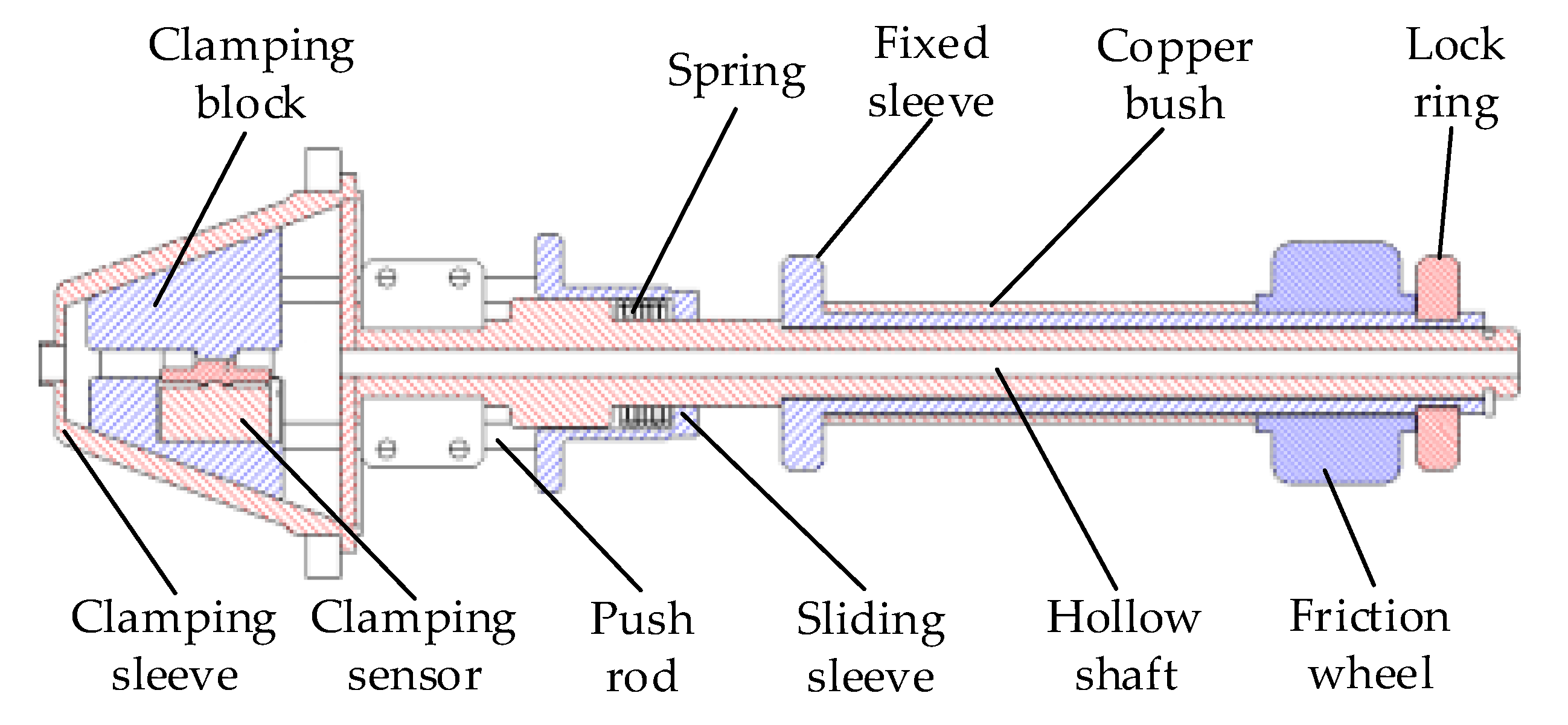
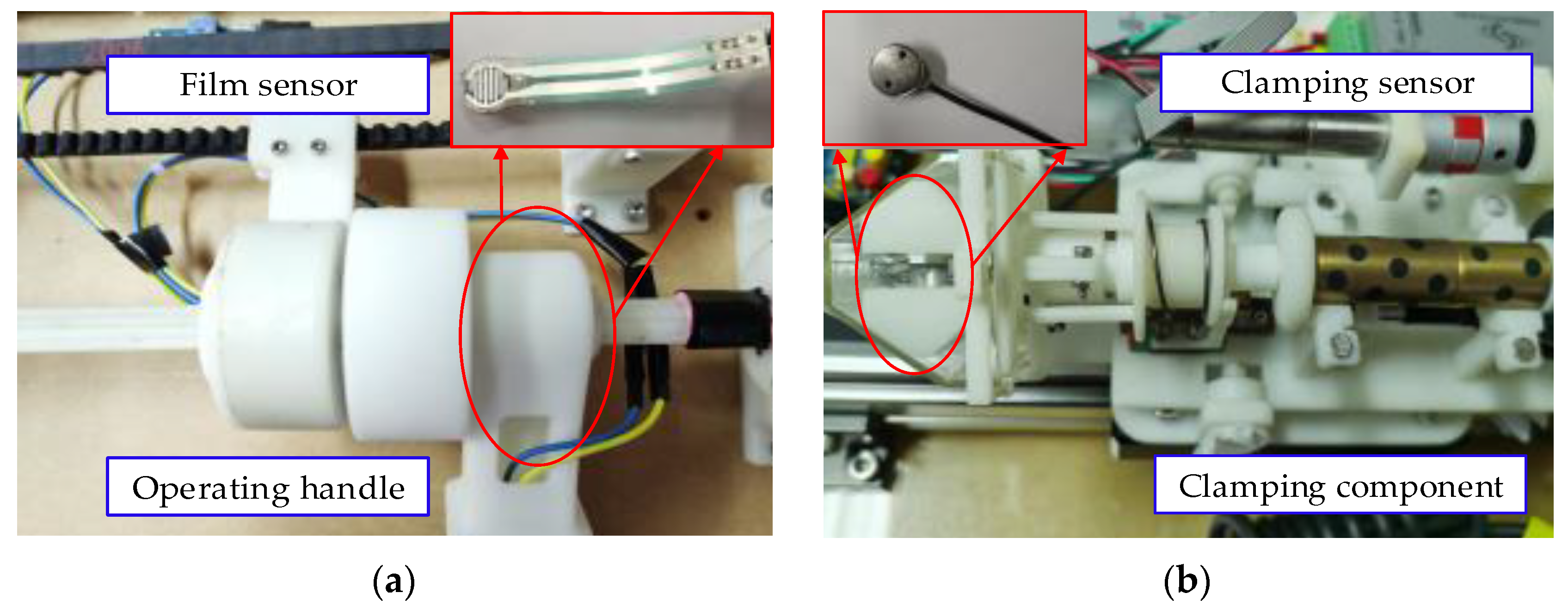
2.1. Master Manipulator
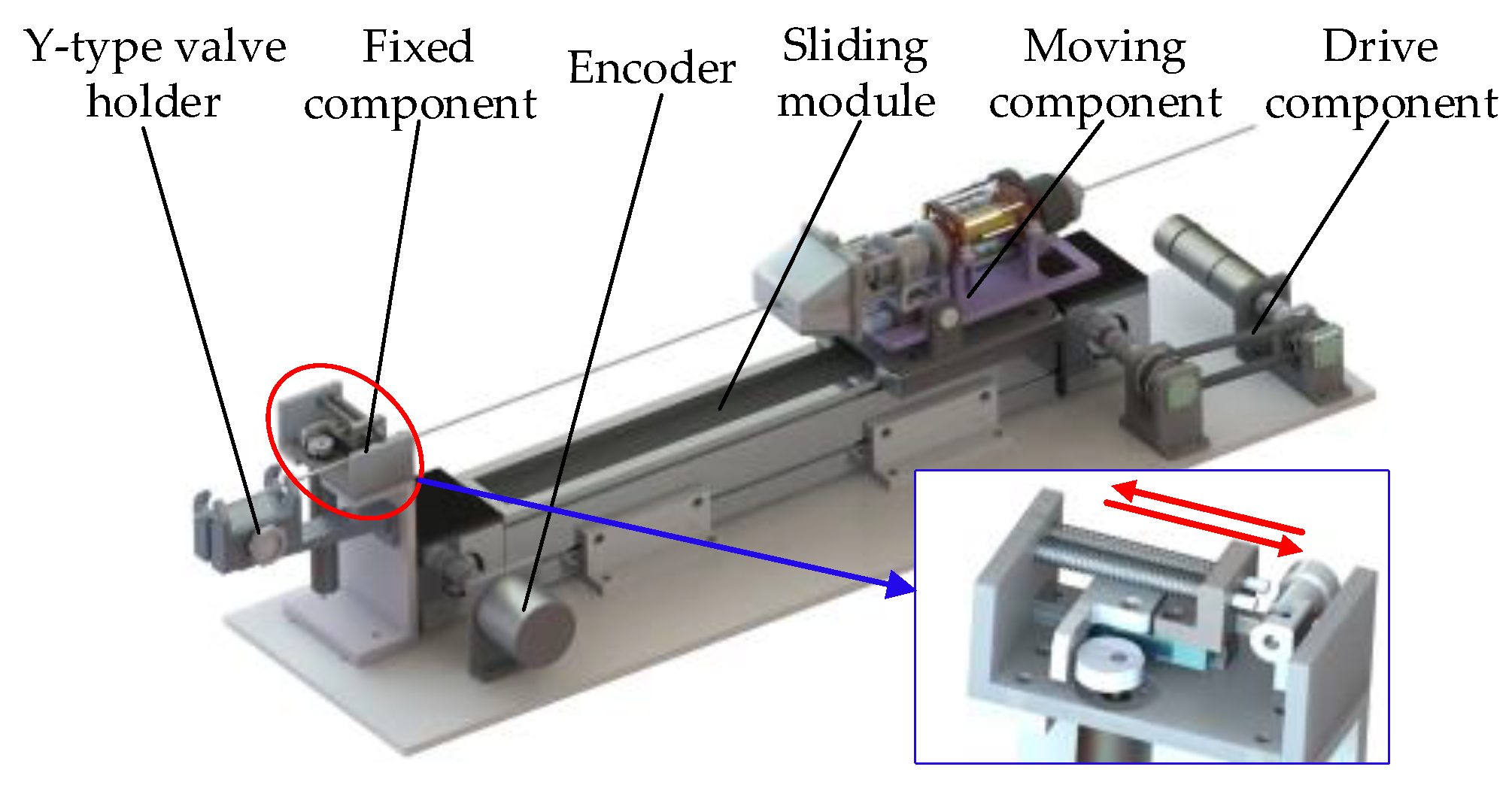
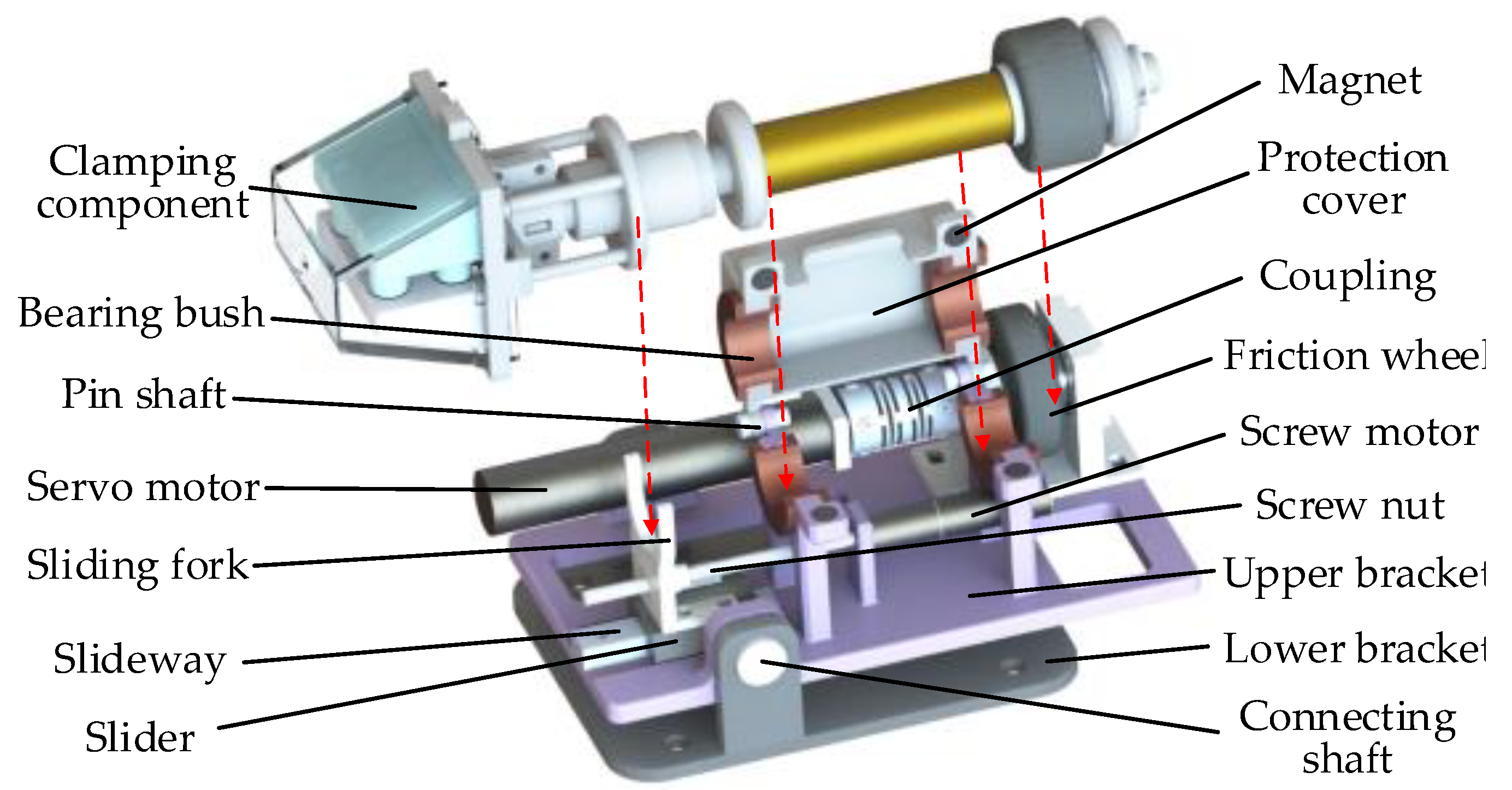
2.2. Slave Wire Feeder
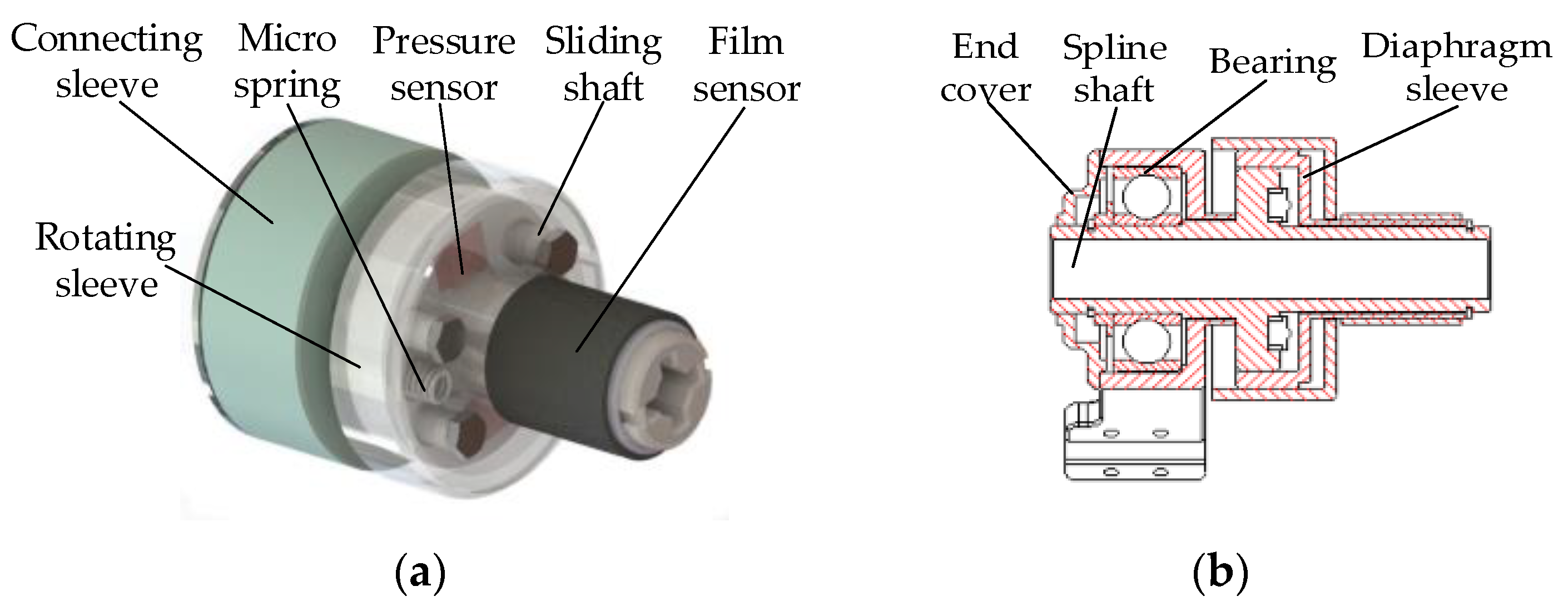
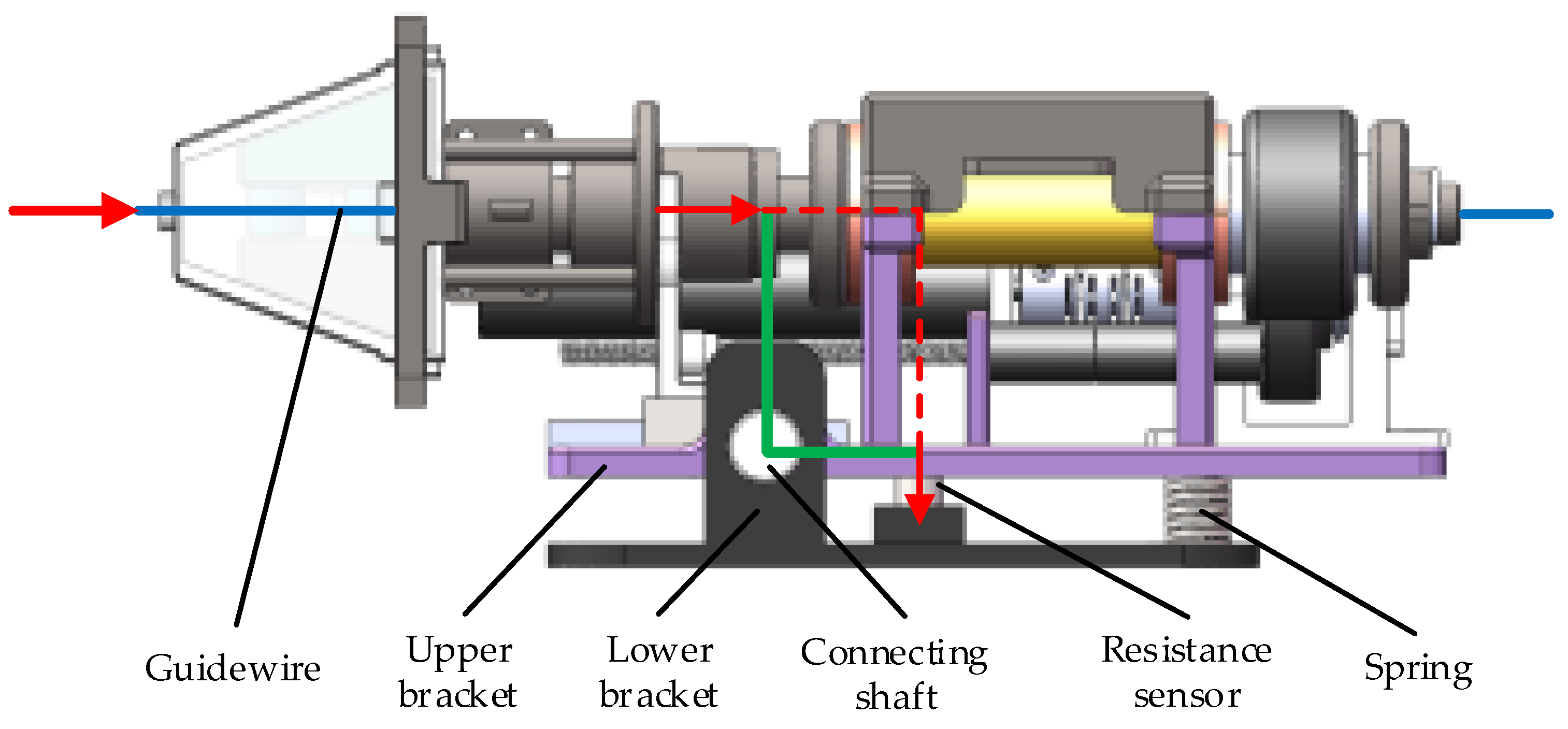
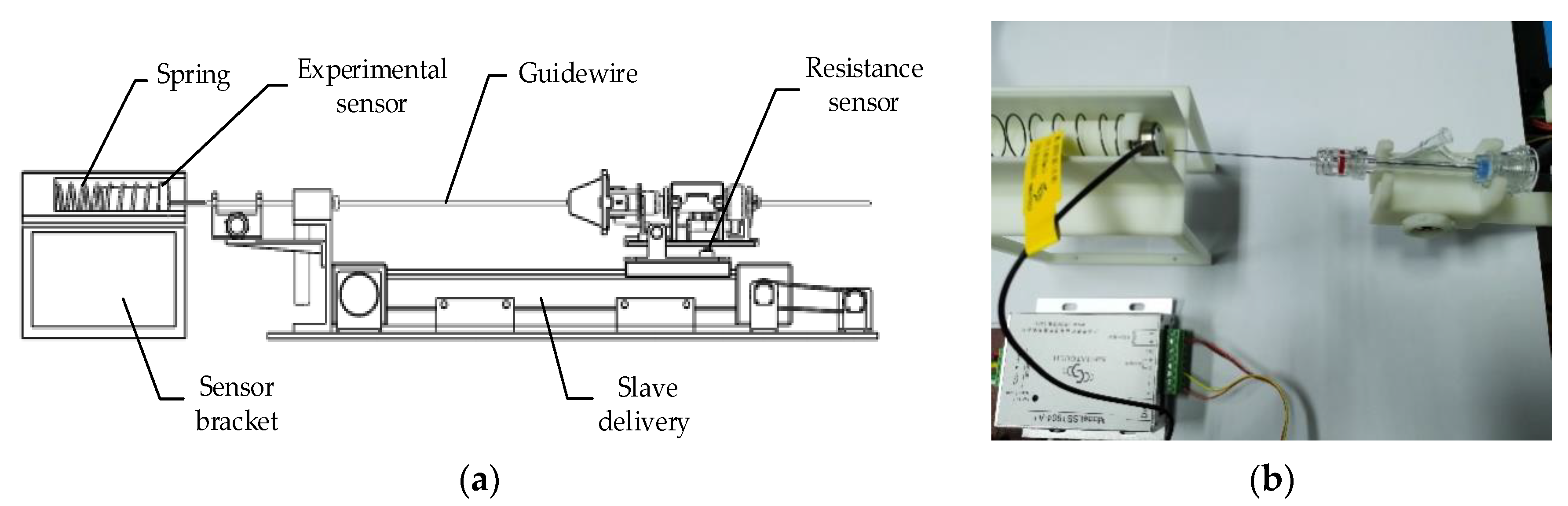
2.3. Force Feedback System
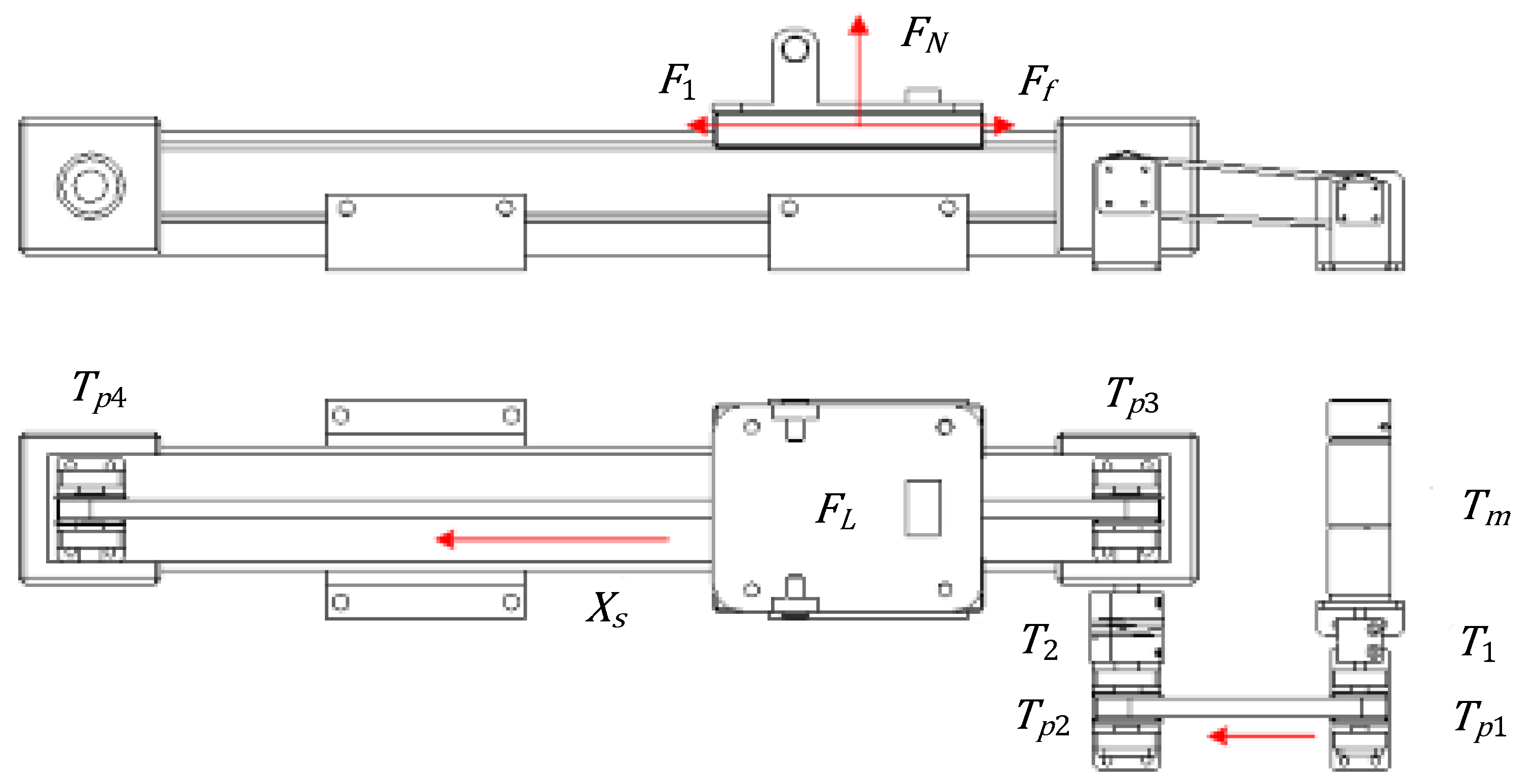
3. Dynamic Analysis
3.1. Dynamical Model of Propulsion
3.2. Dynamical Model of Rotation
4. Design of the Control System
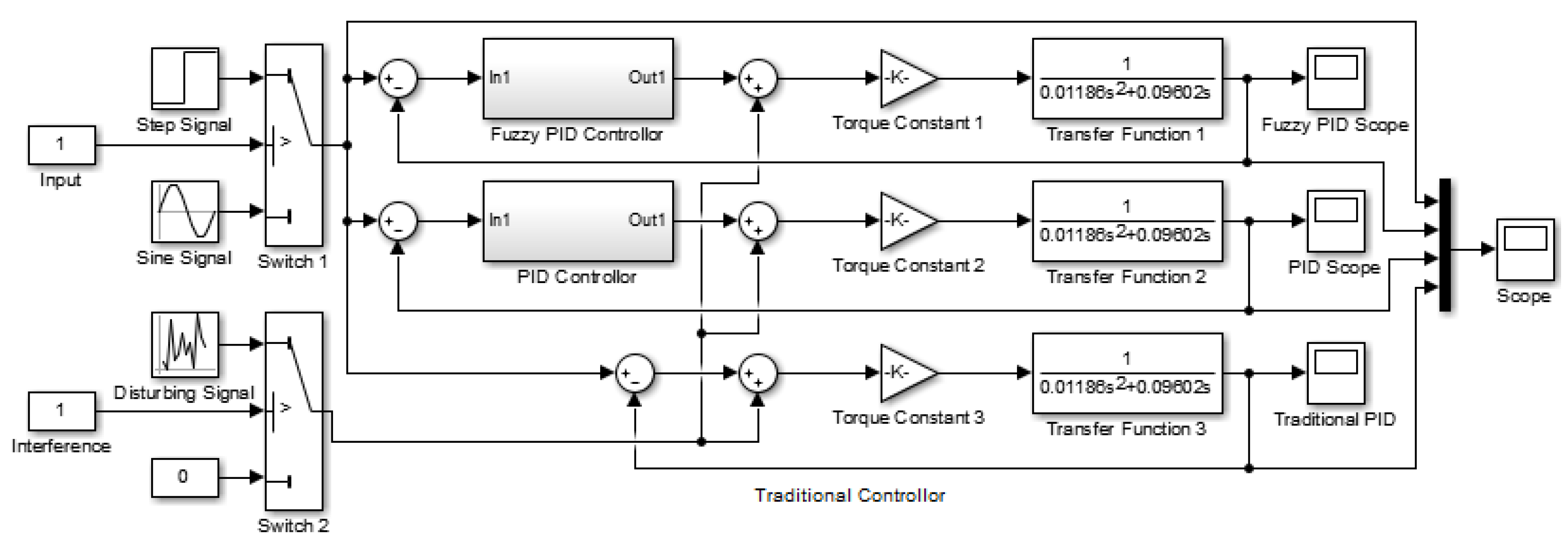
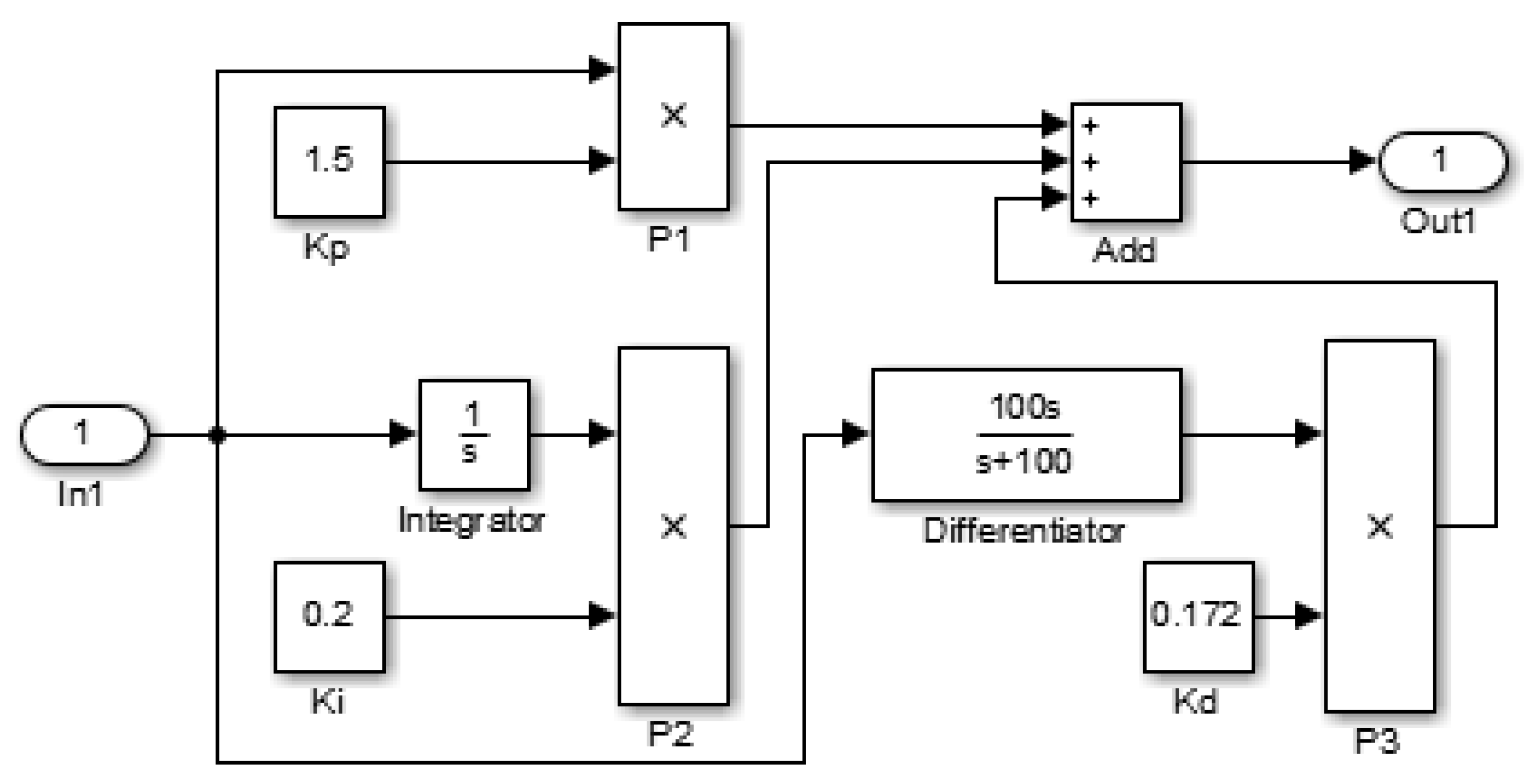
4.1. Design of the Traditional Controller and the PID Controller
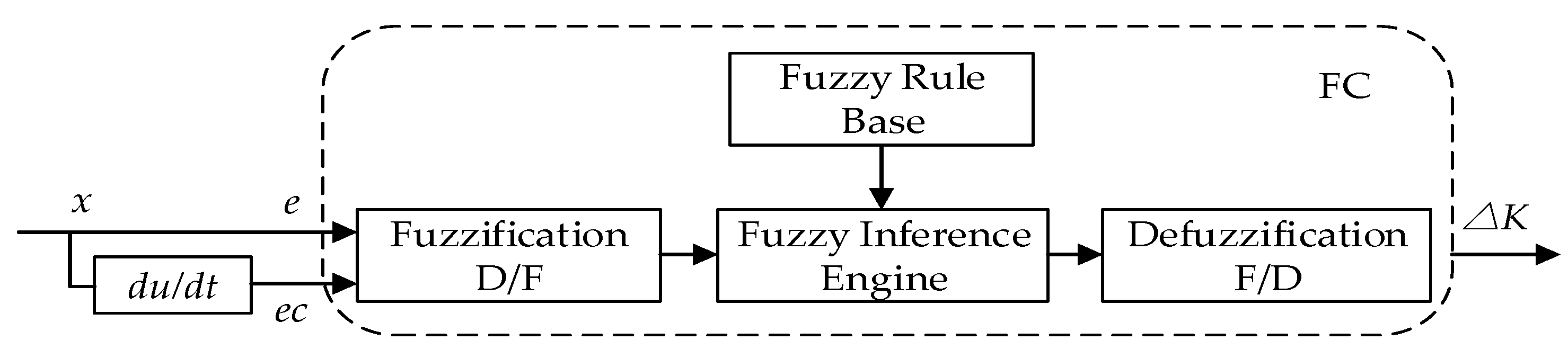
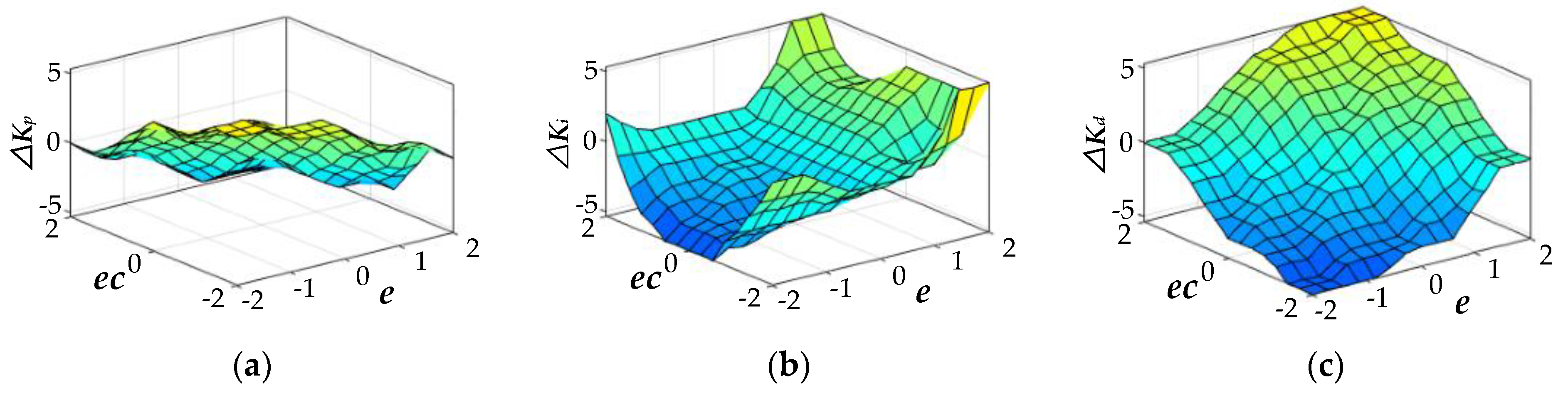
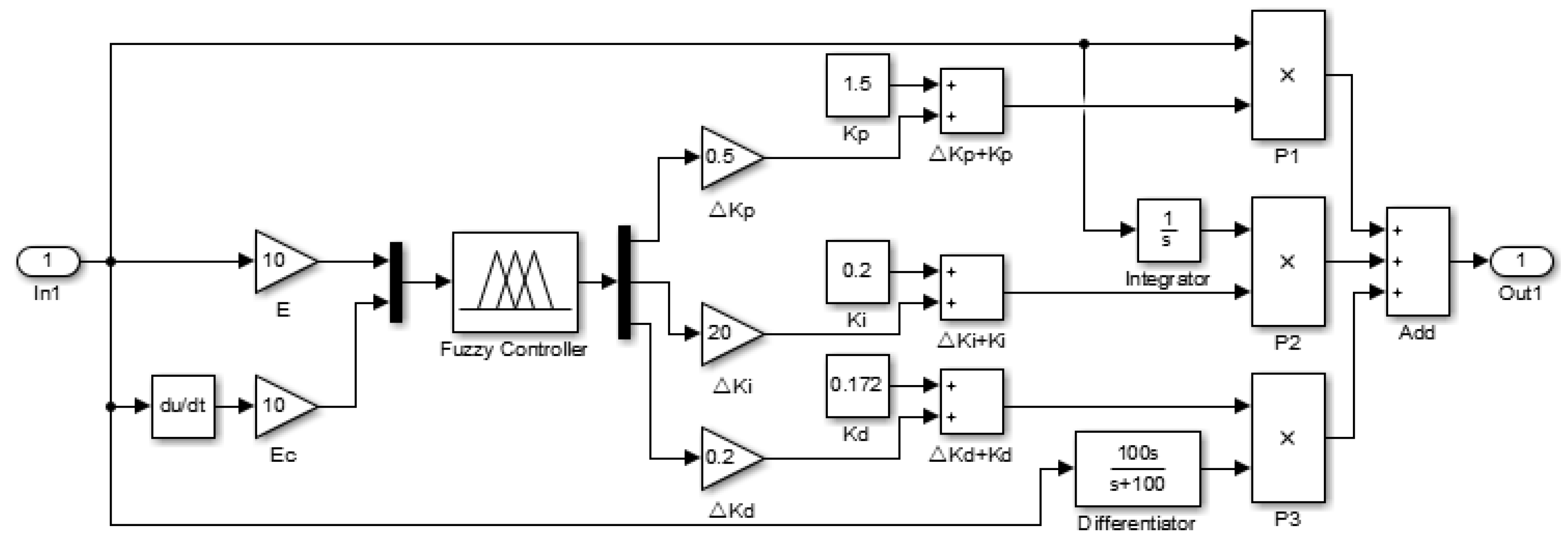
4.2. Design of the Two-Dimensional Fuzzy PID Controller
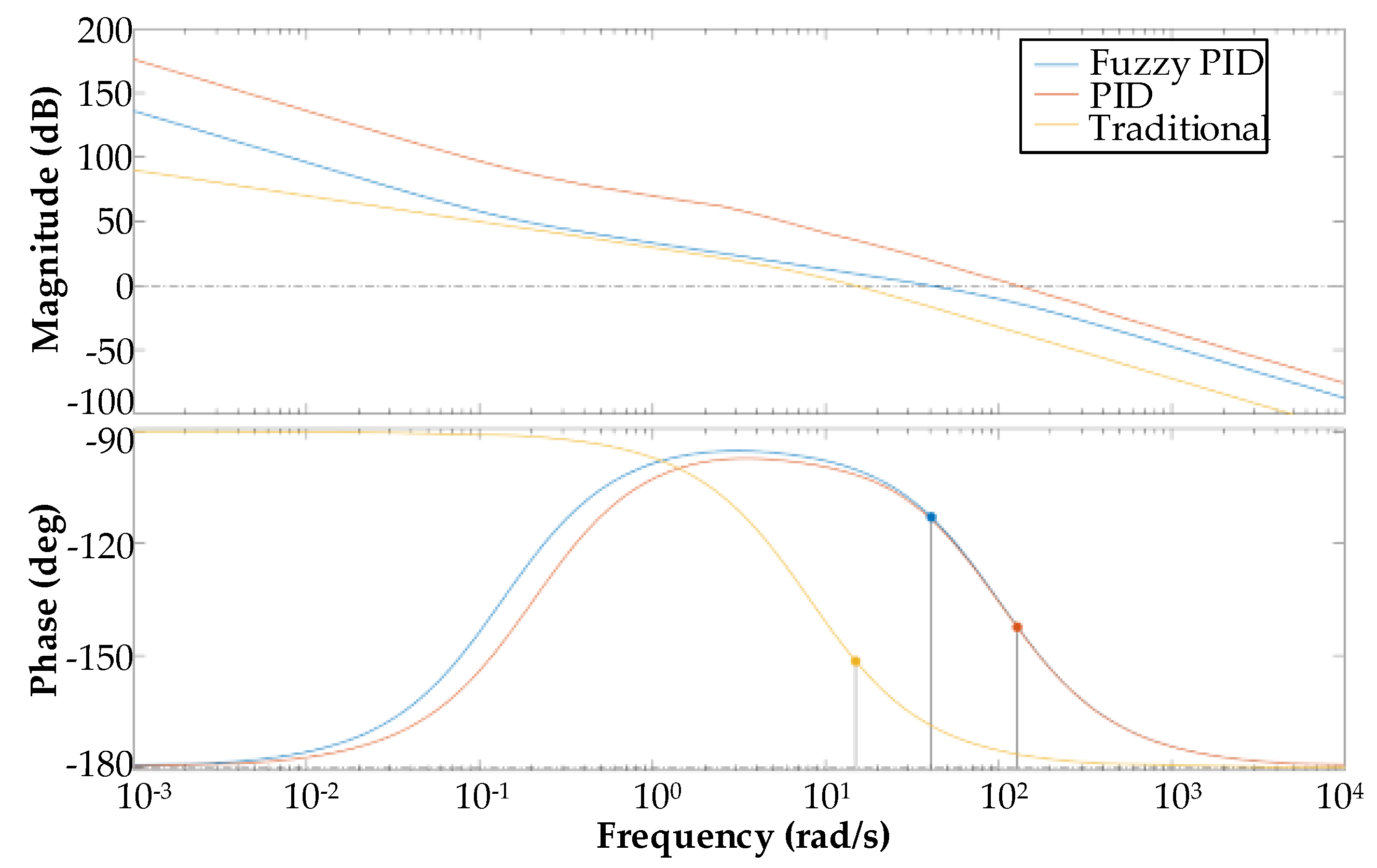
4.3. Control System Simulation
5. Experimental Validation
5.1. Fuzzy PID Controller Anti-Interference Experiment
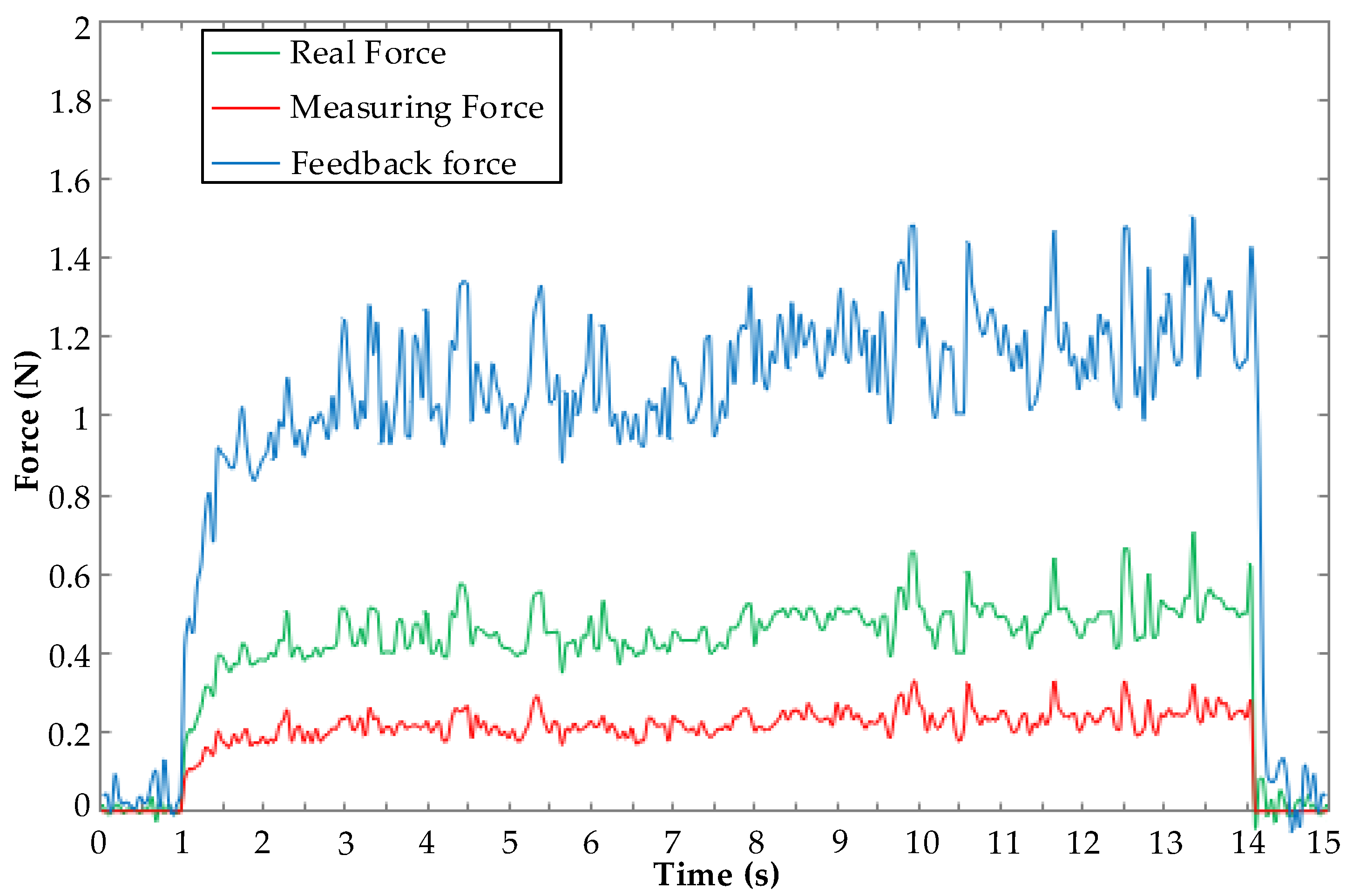
5.2. Force Feedback Experiment
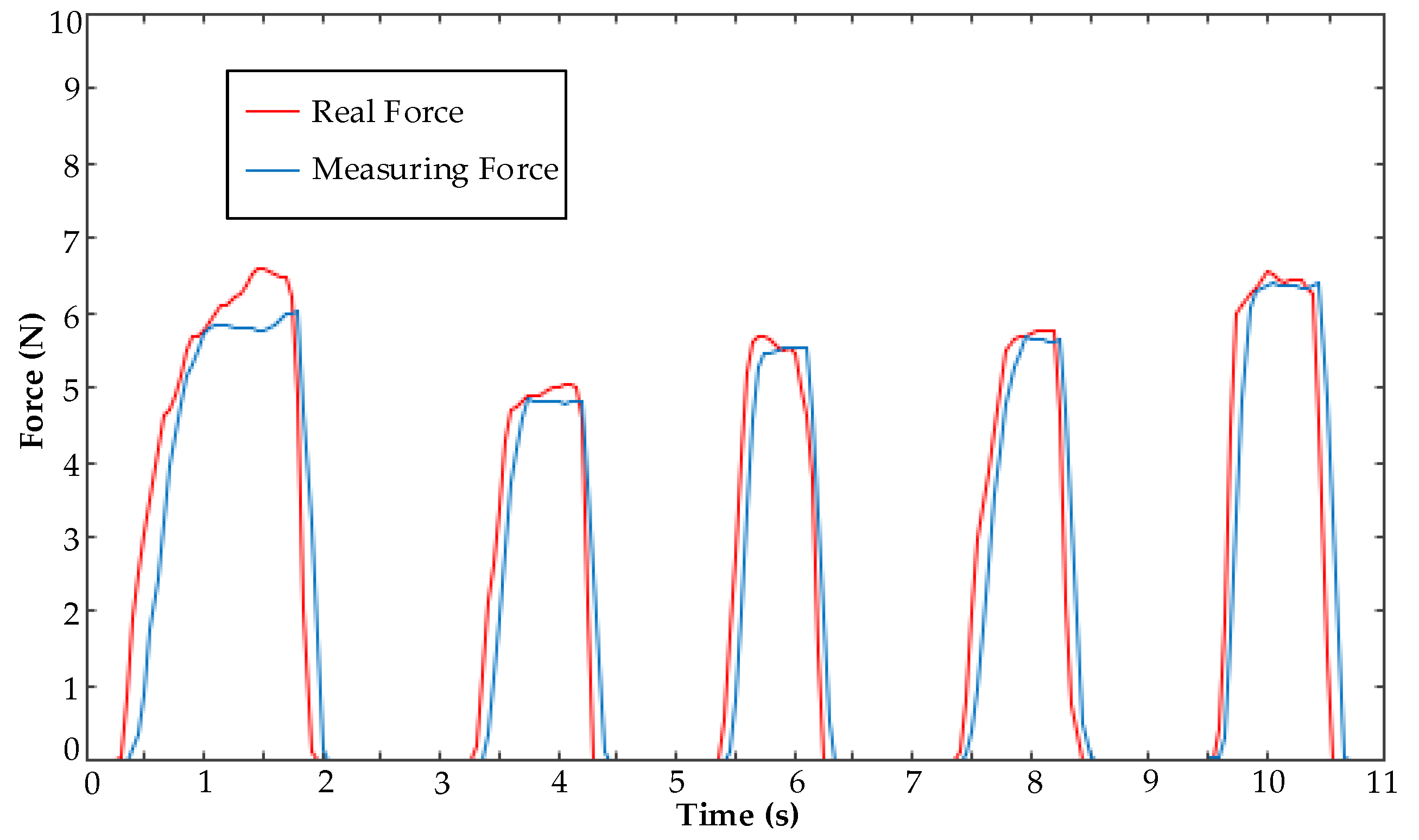
5.3. Clamping Force Experiment
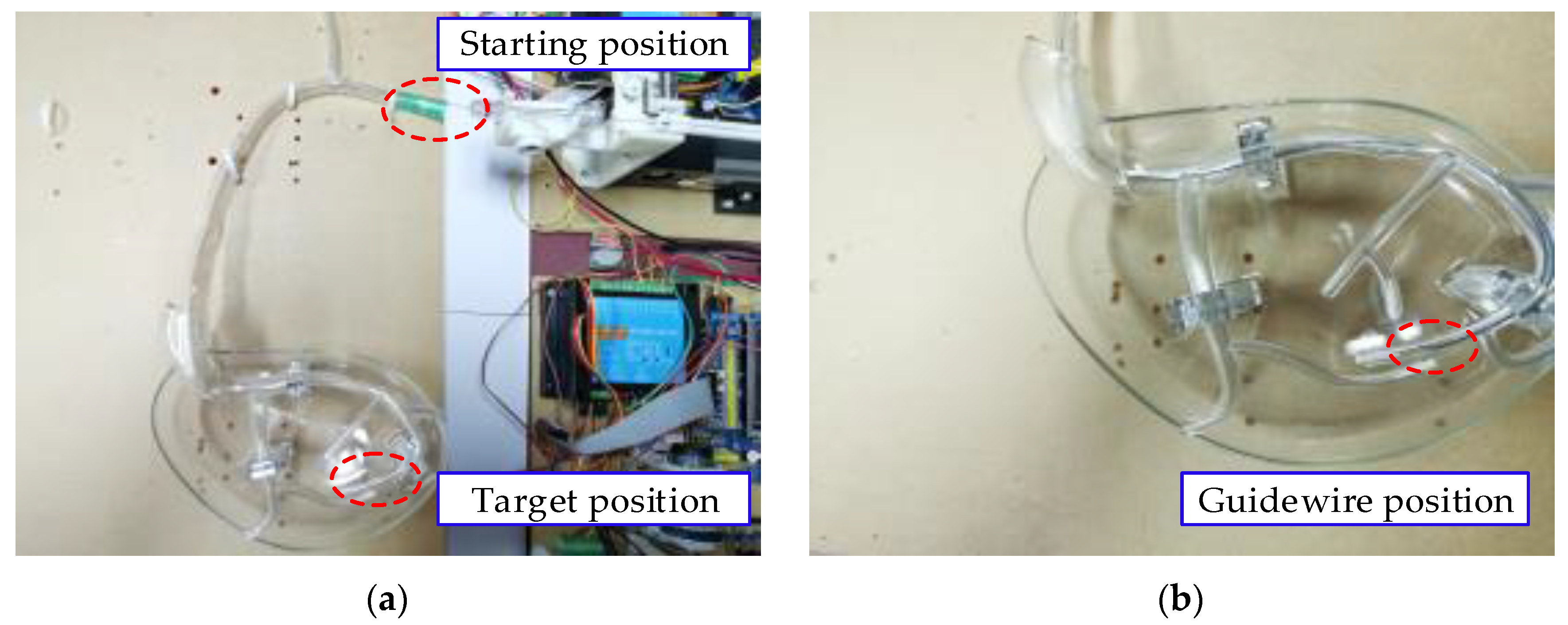
5.4. Vascular Model Experiment
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sachs, D.; Capobianco, R. Minimally invasive sacroiliac joint fusion: One-year outcomes in 40 patients. Adv. Orthop. 2013, 2013, 536128. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, V.; Lee, S.L.; Cundy, T.P.; Yang, G.Z. Emerging robotic platforms for minimally invasive surgery. IEEE Rev. Biomed. Eng. 2013, 6, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, H.; Sun, L.; Yu, H. Operation and force analysis of the guide wire in a minimally invasive vascular interventional surgery robot system. Chin. J. Mech. Eng. 2015, 28, 249–257. [Google Scholar] [CrossRef]
- Klein, L.W.; Miller, D.L.; Balter, S.; Laskey, W.; Haines, D.; Norbash, A.; Mauro, M.A.; Goldstein, J.A. Occupational health hazards in the interventional laboratory: Time for a safer environment. Radiology 2009, 250, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Whitby, M.; Martin, C.J. A study of the distribution of dose across the hands of interventional radiologists and cardiologists. Br. J. Radiol. 2005, 78, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Orme, N.M.; Rihal, C.S.; Gulati, R.; Holmes, D.R., Jr.; Lennon, R.J.; Lewis, B.R.; McPhail, I.R.; Thielen, K.R.; Pislaru, S.V.; Sandhu, G.S.; et al. Occupational health hazards of working in the interventional laboratory: A multisite case control study of physicians and allied staff. J. Am. Coll. Cardiol. 2015, 65, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.H.; Stoianovici, D. Medical robotics in computer-integrated surgery. IEEE Trans. Robot. Autom. 2003, 19, 765–781. [Google Scholar] [CrossRef]
- Yu, H.; Wang, H.; Zhang, W.; Liu, H.; Chang, J.; Huang, D. Master-slave system research of a vascular interventional surgical robot. In Proceedings of the 2018 IEEE International Conference on Real-time Computing and Robotics, Kandima, Maldives, 1–5 August 2018; pp. 469–473. [Google Scholar]
- Haidegger, T. Autonomy for surgical robots: Concepts and paradigms. IEEE Trans. Med. Robot. Bionics 2019, 1, 65–76. [Google Scholar] [CrossRef]
- Weisz, G.; Metzger, D.C.; Caputo, R.P.; Delgado, J.A.; Marshall, J.J.; Vetrovec, G.W.; Reisman, M.; Waksman, R.; Granada, J.F.; Novack, V.; et al. Safety and feasibility of robotic percutaneous coronary intervention: Precise (percutaneous robotically-enhanced coronary intervention) study. J. Am. Coll. Cardiol. 2013, 61, 1596–1600. [Google Scholar] [CrossRef]
- Di Biase, L.; Wang, Y.; Horton, R.; Gallinghouse, G.J.; Mohanty, P.; Sanchez, J.; Patel, D.; Dare, M.; Canby, R.; Price, L.D.; et al. Ablation of atrial fibrillation utilizing robotic catheter navigation in comparison to manual navigation and ablation: Single-center experience. J. Cardiovasc. Electrophysiol. 2009, 20, 1328–1335. [Google Scholar] [CrossRef]
- Kanagaratnam, P.; Koa-Wing, M.; Wallace, D.T.; Goldenberg, A.S.; Peters, N.S.; Davies, D.W. Experience of robotic catheter ablation in humans using a novel remotely steerable catheter sheath. J. Interv. Card. Electrophysiol. 2008, 21, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Gray, W.A. Use of magnetic guidewire navigation in the treatment of lower extremity peripheral vascular disease: Report of the first human clinical experience. Catheter. Cardiovasc. Interv. 2009, 73, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Rafii-Tari, H.; Payne, C.J.; Yang, G.Z. Current and emerging robot-assisted endovascular catheterization technologies: A review. Ann. Biomed. Eng. 2014, 42, 697–715. [Google Scholar] [CrossRef] [PubMed]
- Tercero, C.; Ikeda, S.; Uchiyama, T.; Fukuda, T.; Arai, F.; Okada, Y.; Ono, Y.; Hattori, R.; Yamamoto, T.; Negoro, M.; et al. Autonomous catheter insertion system using magnetic motion capture sensor for endovascular surgery. Int. J. Med. Robot. 2007, 3, 52–58. [Google Scholar] [CrossRef]
- Thakur, Y.; Bax, J.S.; Holdsworth, D.W.; Drangova, M. Design and performance evaluation of a remote catheter navigation system. IEEE. Trans. Biomed. Eng. 2009, 56, 1901–1908. [Google Scholar] [CrossRef]
- Swaminathan, R.V.; Rao, S.V. Robotic-assisted transradial diagnostic coronary angiography. Catheter. Cardiovasc. Interv. 2018, 92, 54–57. [Google Scholar] [CrossRef]
- Carrozza, J.P., Jr. Robotic-assisted percutaneous coronary intervention--filling an unmet need. J. Cardiovasc. Transl. Res. 2012, 5, 62–66. [Google Scholar] [CrossRef]
- Maor, E.; Eleid, M.F.; Gulati, R.; Lerman, A.; Sandhu, G.S. Current and future use of robotic devices to perform percutaneous coronary interventions: A review. J. Am. Heart Assoc. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Woo, J.; Song, H.-S.; Cha, H.-J.; Yi, B.-J. Advantage of steerable catheter and haptic feedback for a 5-DOF vascular intervention robot system. Appl. Sci. 2019, 9, 4035. [Google Scholar] [CrossRef]
- Tavallaei, M.A.; Gelman, D.; Lavdas, M.K.; Skanes, A.C.; Jones, D.L.; Bax, J.S.; Drangova, M. Design, development and evaluation of a compact telerobotic catheter navigation system. Int. J. Med. Robot 2016, 12, 442–452. [Google Scholar] [CrossRef]
- Bao, X.; Guo, S.; Xiao, N.; Li, Y.; Yang, C.; Shen, R.; Cui, J.; Jiang, Y.; Liu, X.; Liu, K. Operation evaluation in-human of a novel remote-controlled vascular interventional robot. Biomed. Microdevices 2018, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, L.; Liu, J.; Wang, W.; Liu, L.; Xu, Z.; Li, L. Research on motion signal capture accuracy of master manipulator for vascular interventional robot. J. Adv. Mech. Des. Syst. Manuf. 2018, 12, 1–12. [Google Scholar] [CrossRef]
- Yin, X.; Guo, S.; Xiao, N.; Tamiya, T.; Hirata, H.; Ishihara, H. Safety operation consciousness realization of a MR fluids-based novel haptic interface for teleoperated catheter minimally invasive neuro surgery. IEEE ASME Trans. Mechatron. 2016, 21, 1043–1054. [Google Scholar] [CrossRef]
- Zhou, J.; Mei, Z.; Miao, J.; Mao, J.; Wang, L.; Wu, D.; Sun, D.; Zhao, Y. A remote-controlled robotic system with safety protection strategy based on force-sensing and bending feedback for transcatheter arterial chemoembolization. Micromachines 2020, 11, 805. [Google Scholar] [CrossRef]
- Omisore, O.M.; Han, S.; Ren, X.; Wang, S.; Ou, F.; Li, H.; Wang, L. Towards characterization and adaptive compensation of backlash in a novel robotic catheter system for cardiovascular interventions. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 824–838. [Google Scholar] [CrossRef]
- Bao, X.; Guo, S.; Xiao, N.; Li, Y.; Shi, L. Compensatory force measurement and multimodal force feedback for remote-controlled vascular interventional robot. Biomed. Microdevices 2018, 20, 74. [Google Scholar] [CrossRef]
- Cha, H.J.; Yi, B.J.; Won, J.Y. An assembly-type master-slave catheter and guidewire driving system for vascular intervention. Proc. IMechE Part H J. Eng. Med. 2017, 231, 69–79. [Google Scholar] [CrossRef]
- Shen, H.; Wang, C.; Xie, L.; Zhou, S.; Gu, L.; Xie, H. A novel remote-controlled robotic system for cerebrovascular intervention. Int. J. Med. Robot. Comput. Assist. Surg. 2018, 14, e1943. [Google Scholar] [CrossRef]
- Bao, X.; Guo, S.; Xiao, N.; Li, Y.; Yang, C.; Jiang, Y. A cooperation of catheters and guidewires-based novel remote-controlled vascular interventional robot. Biomed. Microdevices 2018, 20, 20. [Google Scholar] [CrossRef]

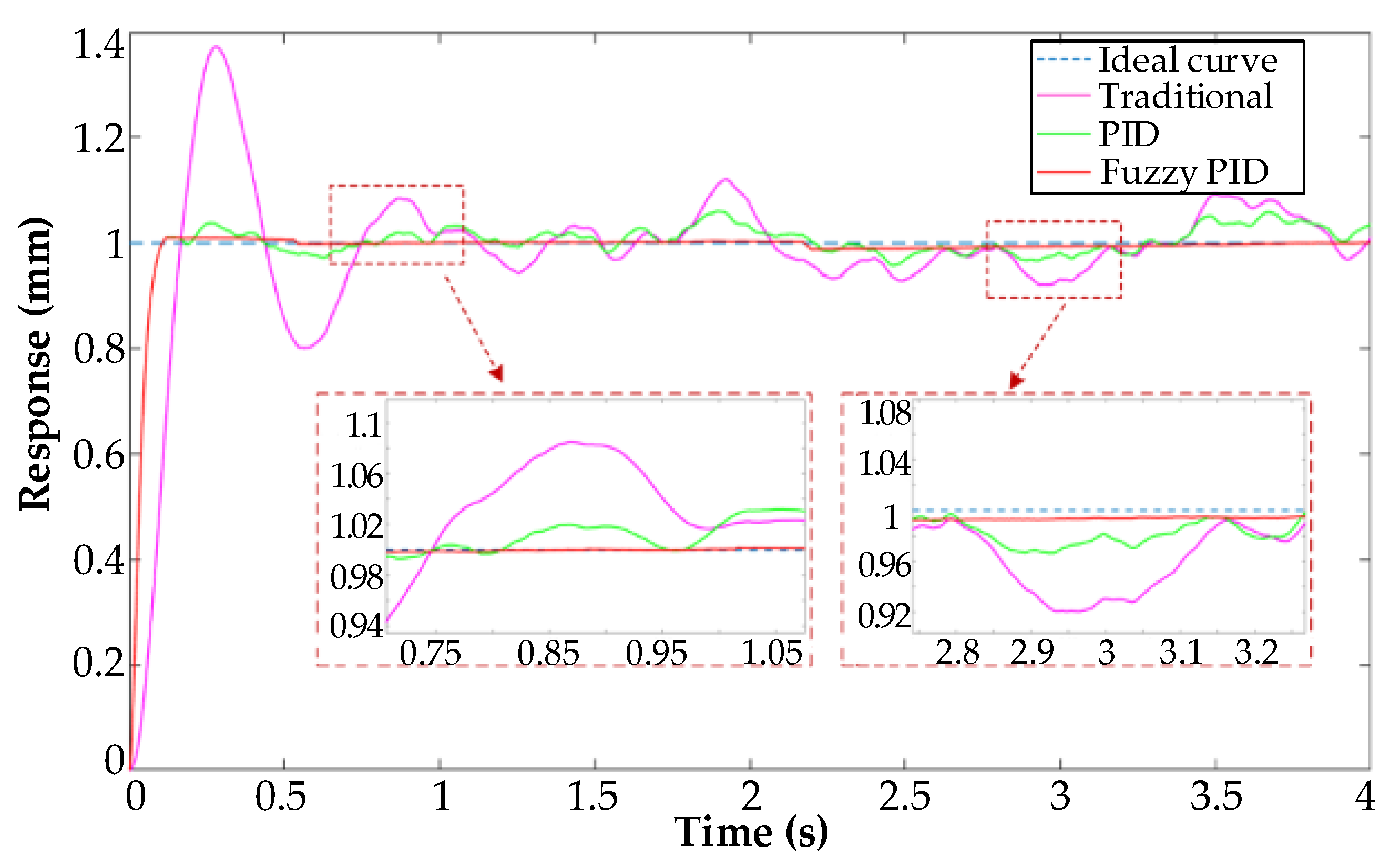



| Controller | Rise Time (s) | Maximum Deviation (mm) | Mean Deviation (mm) |
|---|---|---|---|
| Traditional controller | 0.44 | 0.3778 | 0.1153 |
| PID controller | 0.13 | 0.1222 | 0.0724 |
| Fuzzy PID controller | 0.12 | 0.0400 | 0.0147 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Wang, H.; Chang, J.; Niu, J.; Wang, F.; Yan, Y.; Tian, H.; Fang, J.; Lu, H. A Novel Vascular Intervention Surgical Robot Based on Force Feedback and Flexible Clamping. Appl. Sci. 2021, 11, 611. https://doi.org/10.3390/app11020611
Yu H, Wang H, Chang J, Niu J, Wang F, Yan Y, Tian H, Fang J, Lu H. A Novel Vascular Intervention Surgical Robot Based on Force Feedback and Flexible Clamping. Applied Sciences. 2021; 11(2):611. https://doi.org/10.3390/app11020611
Chicago/Turabian StyleYu, Haoyang, Hongbo Wang, Jingyuan Chang, Jianye Niu, Fuhao Wang, Yonggan Yan, Hesuo Tian, Junyu Fang, and Haixia Lu. 2021. "A Novel Vascular Intervention Surgical Robot Based on Force Feedback and Flexible Clamping" Applied Sciences 11, no. 2: 611. https://doi.org/10.3390/app11020611
APA StyleYu, H., Wang, H., Chang, J., Niu, J., Wang, F., Yan, Y., Tian, H., Fang, J., & Lu, H. (2021). A Novel Vascular Intervention Surgical Robot Based on Force Feedback and Flexible Clamping. Applied Sciences, 11(2), 611. https://doi.org/10.3390/app11020611





