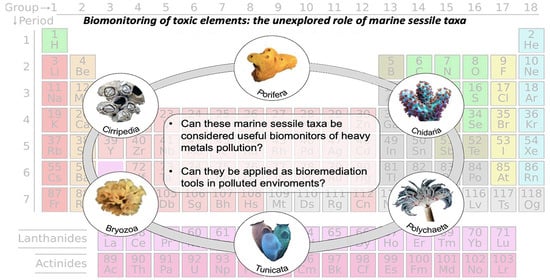
Biomonitoring of Heavy Metals: The Unexplored Role of Marine Sessile Taxa
Abstract
1. Introduction
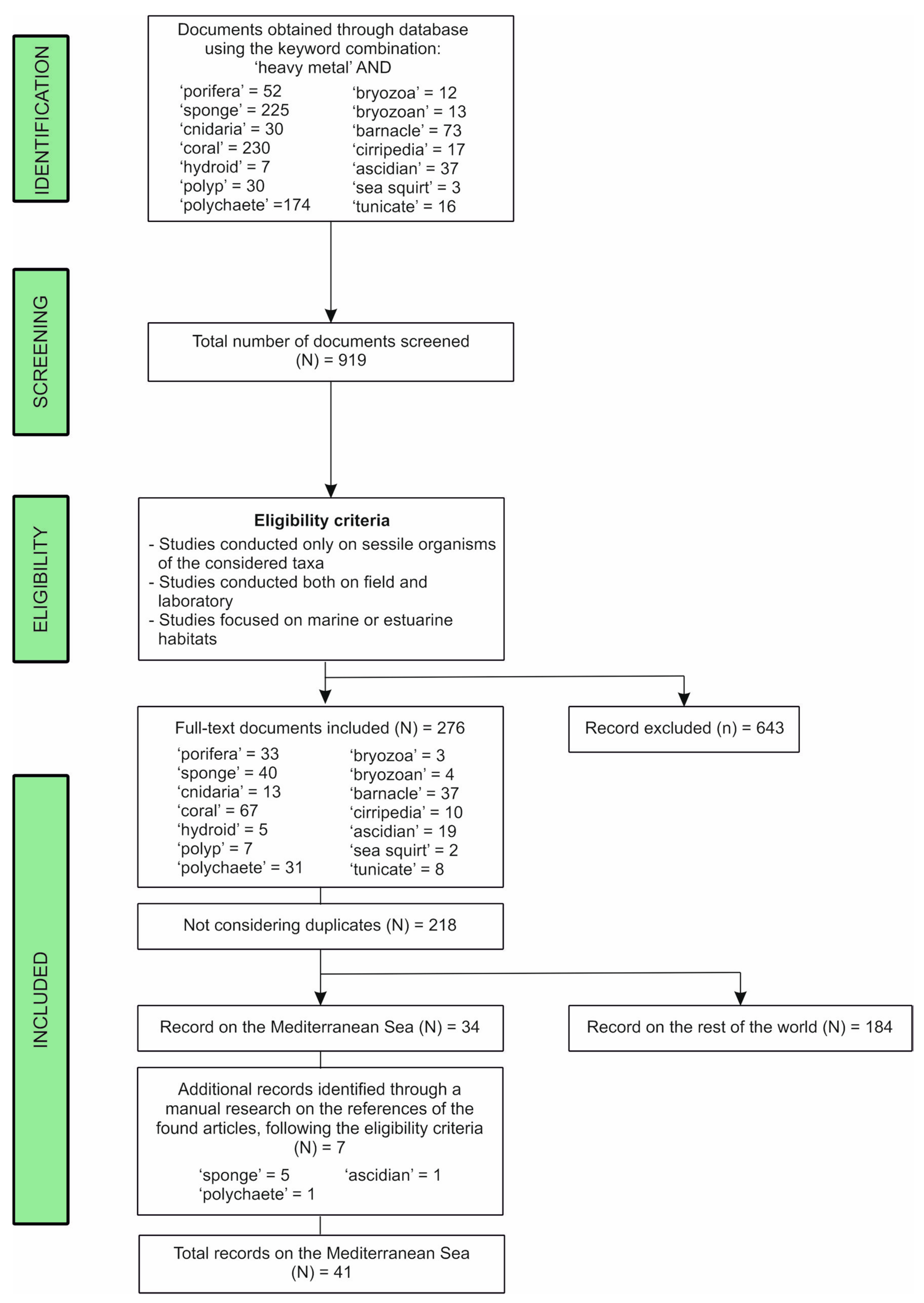
2. Materials and Methods
3. Results
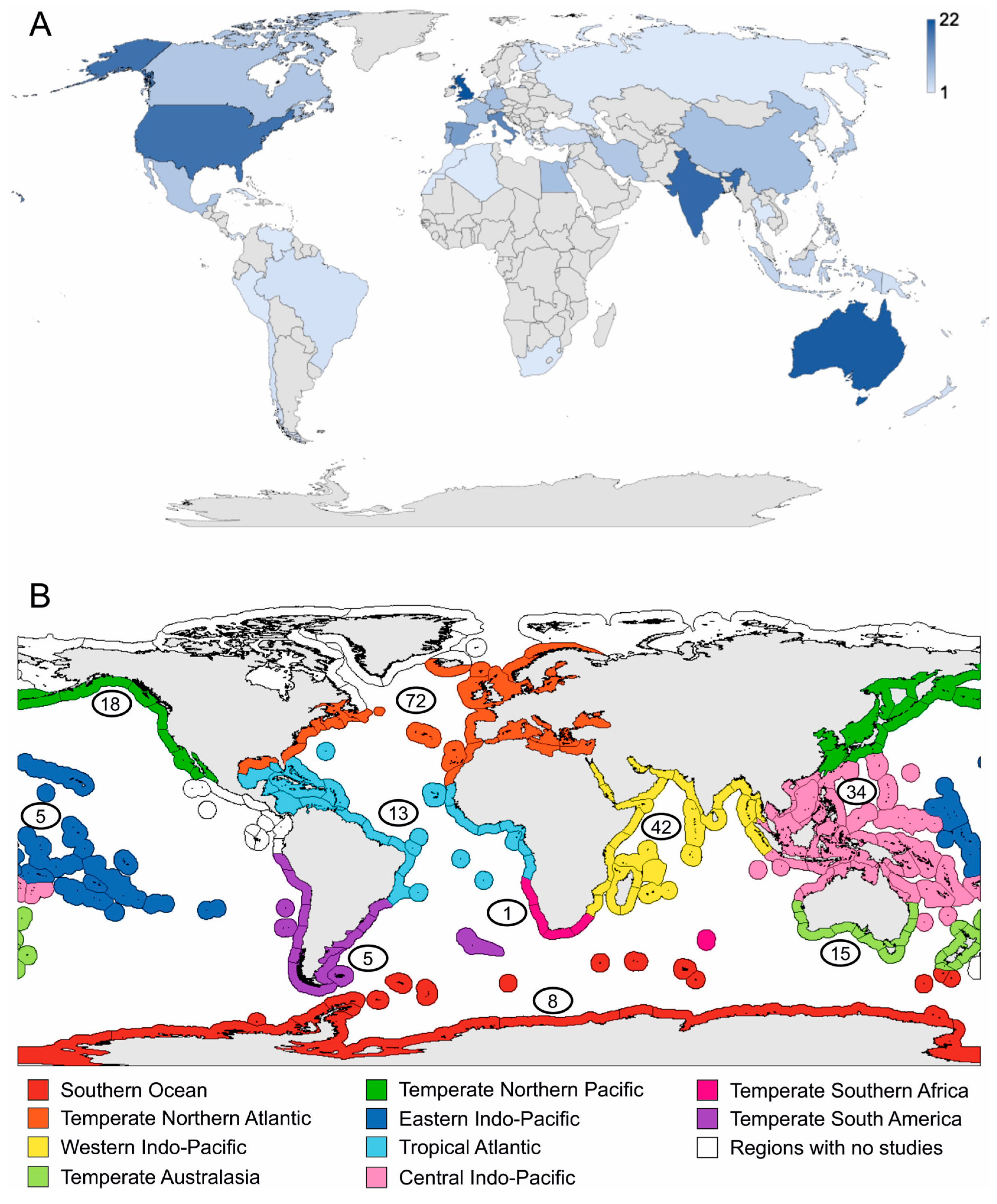
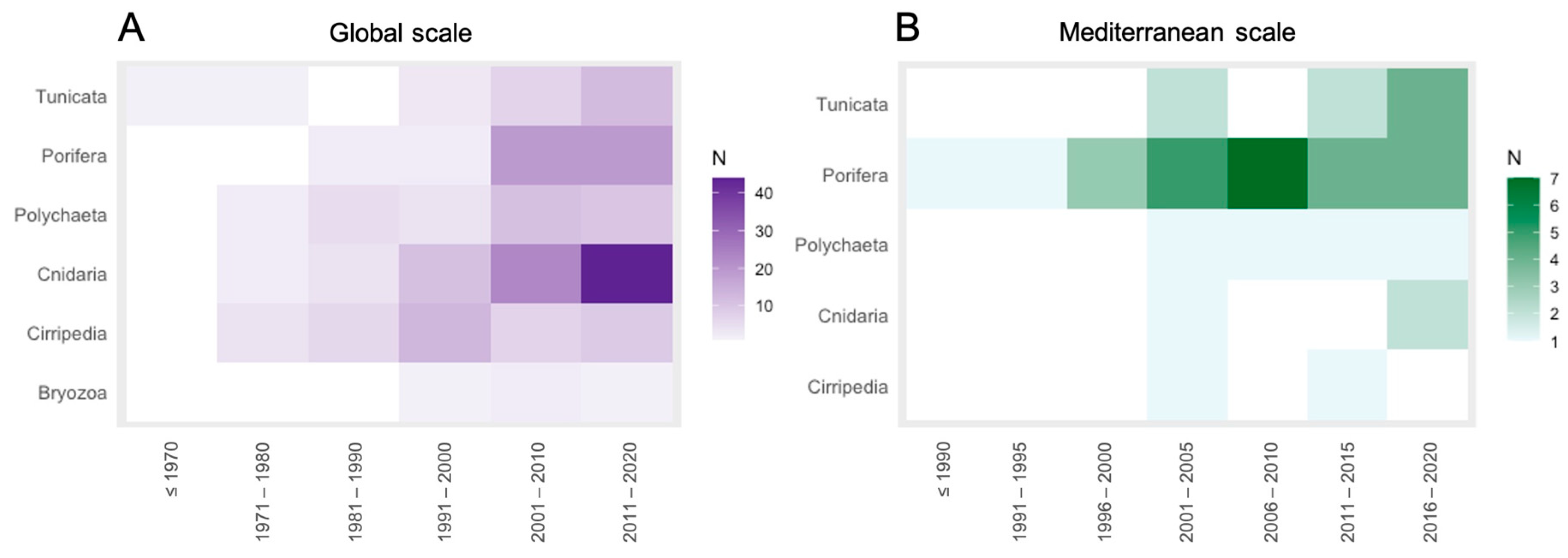
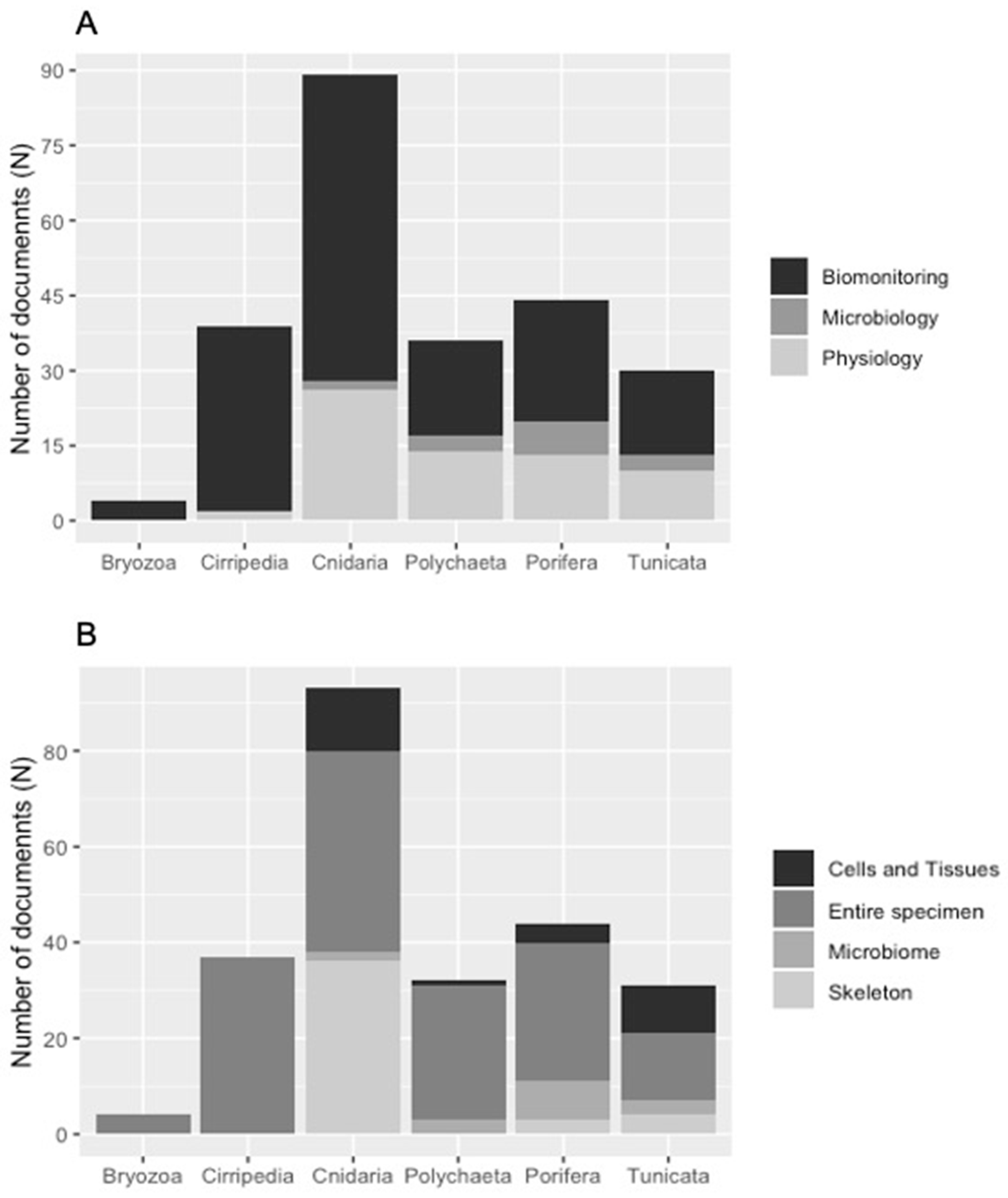
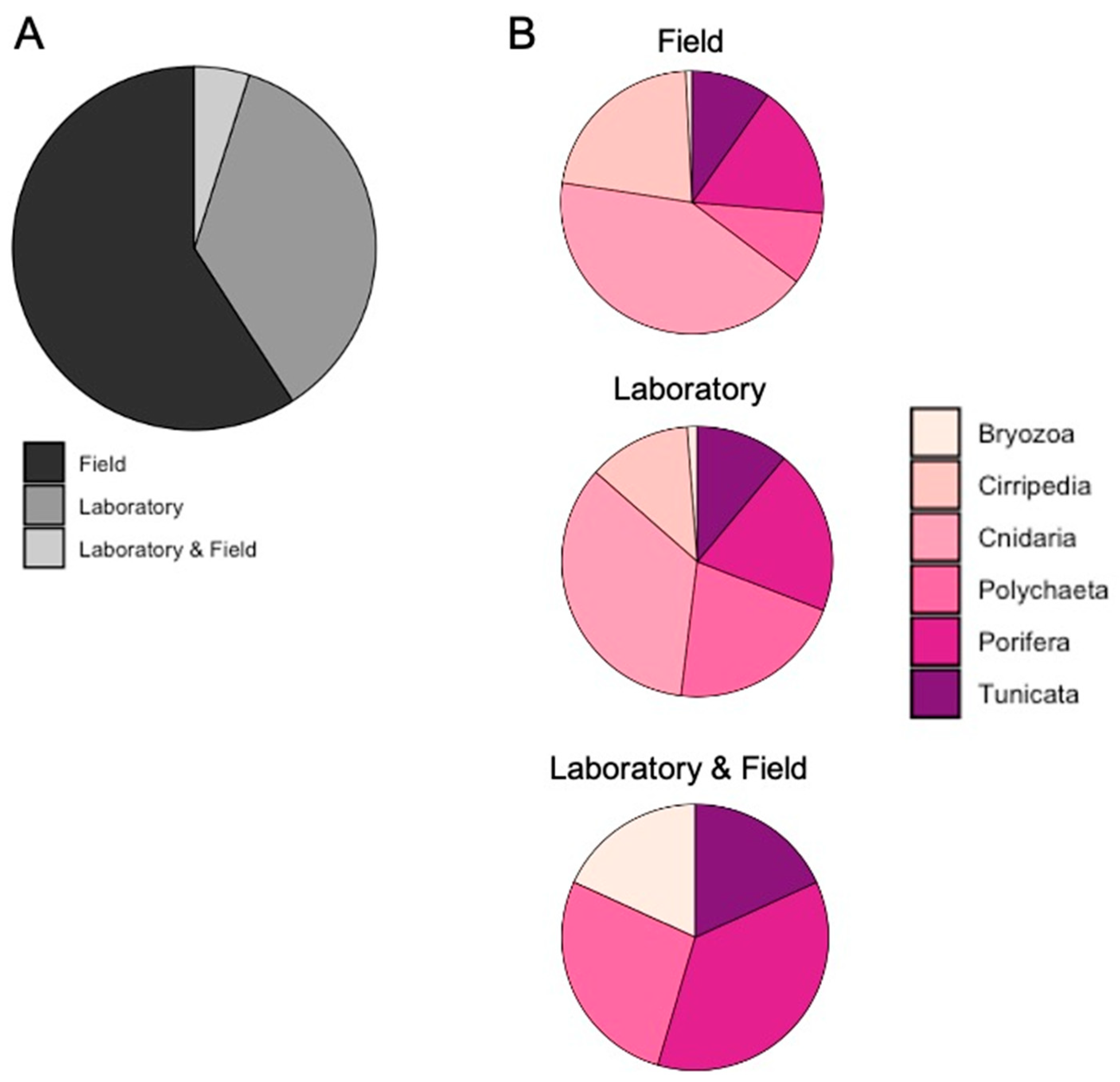
3.1. Literature Analysis at Global Scale
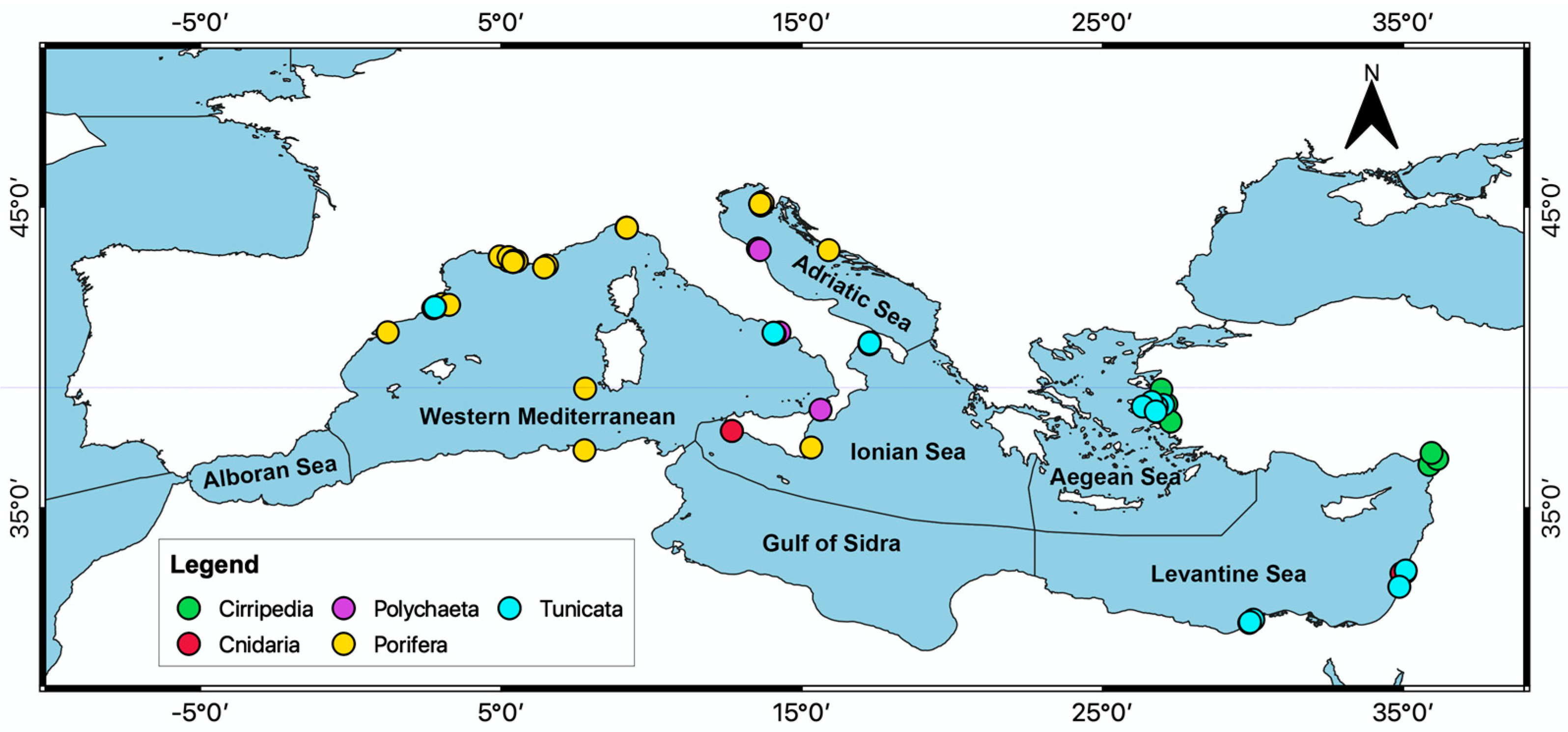
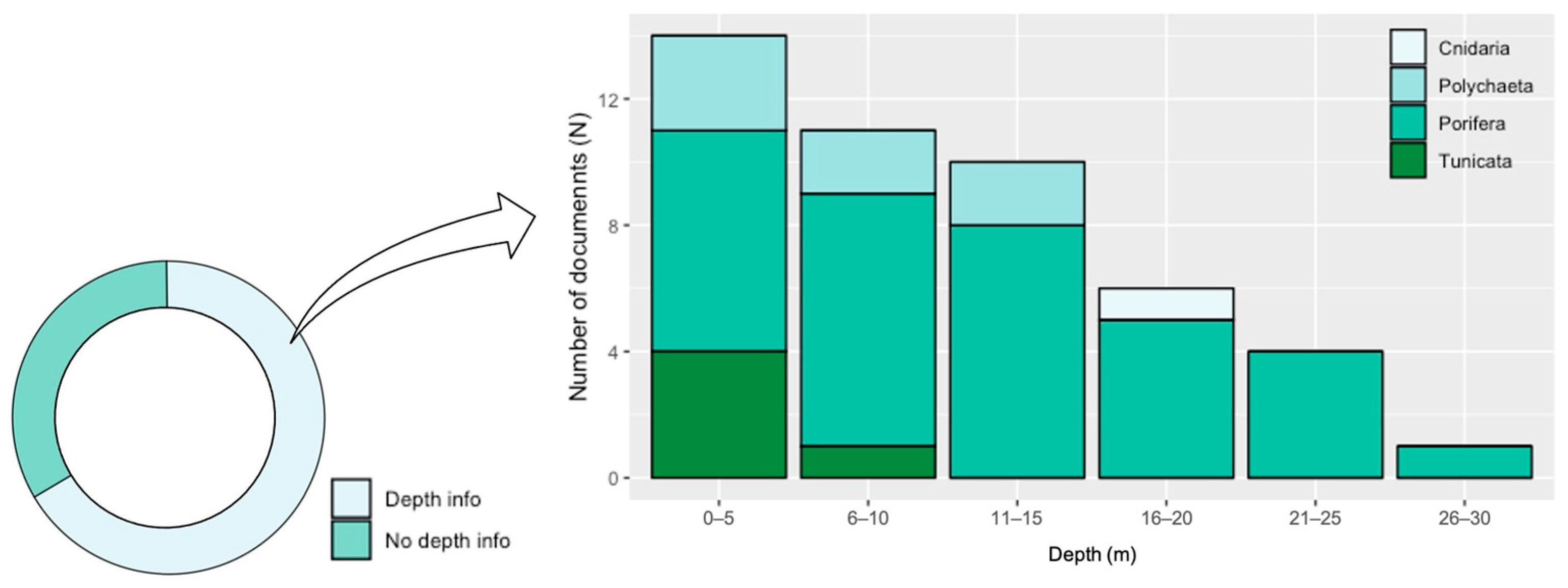
3.2. Literature Analysis Focused on the Mediterranean Sea Province
4. Discussion
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caeiro, S.; Costa, M.H.; Ramos, T.B.; Fernandes, F.; Silveira, N.; Coimbra, A.; Medeiros, G.; Painho, M. Assessing heavy metal contamination in Sado Estuary sediment: An index analysis approach. Ecol. Indic. 2005, 5, 151–169. [Google Scholar] [CrossRef]
- Orlandi, L.; Bentivoglio, F.; Carlino, P.; Calizza, E.; Rossi, D.; Costantini, M.L.; Rossi, L. δ15N variation in Ulva lactuca as a proxy for anthropogenic nitrogen inputs in coastal areas of Gulf of Gaeta (Mediterranean Sea). Mar. Pollut. Bull. 2014, 84, 76–82. [Google Scholar] [CrossRef]
- Tedetti, M.; Guigue, C.; Goutx, M. Utilization of a submersible UV fluorometer for monitoring anthropogenic inputs in the Mediterranean coastal waters. Mar. Pollut. Bull. 2010, 60, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Roveta, C.; Annibaldi, A.; Vagnoni, F.; Mantas, T.P.; Domenichelli, F.; Gridelli, S.; Puce, S. Short-term effects of environmental factors on the asexual reproduction of Aurelia sp. polyps. Chem. Ecol. 2020, 36, 486–492. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Truzzi, C.; Scarponi, G. Heavy metal distribution in organic and siliceous marine sponge tissues measured by square wave anodic stripping voltammetry. Mar. Pollut. Bull. 2016, 111, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, O.; Benedetti, M.; Beolchini, F.; Dell’Anno, A.; Fattorini, D.; Gorbi, S.; Illuminati, S.; Maradonna, F.; Scarponi, G.; Regoli, F. New Insights for Early Warning and Countermeasures to Aquatic Pollution. In The First Outstanding 50 Years of “Università Politecnica delle Marche”; Longhi, S., Monteriù, A., Freddi, A., Aquilanti, L., Ceravolo, M.G., Carnelavi., O., Giordano, M., Moroncini, G., Eds.; Springer: Cham, Switzerland, 2020; pp. 431–455. [Google Scholar] [CrossRef]
- Duffus, J.H. Heavy metals-a meaningless term? Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Pourret, O.; Hursthouse, A. It’s Time to Replace the Term “Heavy Metals” with “Potentially Toxic Elements” When Reporting Environmental Research. Int. J. Environ. Res. Public. Health 2019, 16, 4446. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, K.-J. What are “heavy metals” in Plant Sciences? Acta Physiol. Plant. 2010, 32, 615–619. [Google Scholar] [CrossRef]
- Chang, L.W.; Magos, L.; Suzuki, T. Toxicology of Metals; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Simkiss, K.; Taylor, M.G. Transport of metals across membranes. In Metal Speciation and Bioavailability in Aquatic Systems; Tessier, A., Turner, D., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 1995; pp. 1–44. [Google Scholar]
- Rainbow, P.S. Trace metal concentrations in aquatic invertebrates: Why and so what? Environ. Pollut. 2002, 120, 497–507. [Google Scholar] [CrossRef]
- Annibaldi, A.; Truzzi, C.; Carnevali, O.; Pignalosa, P.; Api, M.; Scarponi, G.; Illuminati, S. Determination of Hg in farmed and wild atlantic bluefin tuna (Thunnus thynnus L.) muscle. Molecules 2019, 24, 1273. [Google Scholar] [CrossRef]
- Cammilleri, G.; Galluzzo, P.; Pulvirenti, A.; Giangrosso, I.E.; Lo Dico, G.M.; Montana, G.; Lampisi, N.; Mobilia, M.A.; Lastra, A.; Vazzana, M.; et al. Toxic mineral elements in Mytilus galloprovincialis from Sicilian coasts (Southern Italy). Nat. Prod. Res. 2020, 34, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Butler, P.A.; Andren, L.; Bonde, G.J.; Jernelov, A.; Reisch, D.J. Monitoring organisms. In Food and Agricultural Organization Technical Conference on Marine Pollution and its Effects on Living Resources and Fishing, Rome, 1970. Supplement 1: Methods of Detection, Measurement and Monitoring of Pollutants in the Marine Environment; Ruivo, M., Ed.; Fishing News Ltd.: London, UK, 1971; pp. 101–112. [Google Scholar]
- Haug, A.; Melsom, S.; Omang, S. Estimation of heavy metal pollution in two Norwegian fjord areas by analysis of the brown alga Ascophyllum nodosum. Environ. Pollut. 1974, 7, 179–192. [Google Scholar] [CrossRef]
- McKenzie, L.A.; Brooks, R.; Johnston, E.L. Heritable pollution tolerance in a marine invader. Environ. Res. 2011, 111, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Batista, D.; Muricy, G.; Rocha, R.C.; Miekeley, N.F. Marine sponges with contrasting life histories can be complementary biomonitors of heavy metal pollution in coastal ecosystems. Environ. Sci. Pollut. Res. 2014, 21, 5785–5794. [Google Scholar] [CrossRef]
- Colozza, N.; Gravina, M.F.; Amendola, L.; Rosati, M.; Akretche, D.E.; Moscone, D.; Arduini, F. A miniaturized bismuth-based sensor to evaluate the marine organism Styela plicata bioremediation capacity toward heavy metal polluted seawater. Sci. Total Environ. 2017, 584, 692–700. [Google Scholar] [CrossRef]
- Giangrande, A.; Licciano, M.; Del Pasqua, M.; Fanizzi, F.P.; Migoni, D.; Stabili, L. Heavy metals in five Sabellidae species (Annelida, Polychaeta): Ecological implications. Environ. Sci. Pollut. R. 2017, 24, 3759–3768. [Google Scholar] [CrossRef]
- Jupp, B.P.; Fowler, S.W.; Dobretsov, S.; van der Wiele, H.; Al-Ghafri, A. Assessment of heavy metal and petroleum hydrocarbon contamination in the Sultanate of Oman with emphasis on harbours, marinas, terminals and ports. Mar. Pollut. Bull. 2017, 121, 260–273. [Google Scholar] [CrossRef]
- Souri, A.; Niyogi, S.; Naji, A. Distribution, source apportionment, bioavailability and ecological risks of metals in reef sediments and corals of the Persian Gulf (Iran): Khark Island, Chirouyeh, and Hendorabi Island. Mar. Pollut. Bull. 2019, 149, 110654. [Google Scholar] [CrossRef]
- Haynes, T.; Bell, J.; Saunders, G.; Irving, R.; Williams, J.; Bell, G. Marine Strategy Framework Directive Shallow Sublittoral Rock Indicators for Fragile Sponge and Anthozoan Assemblages Part 1: Developing Proposals for Potential Indicators; JNCC Report No. 524; Nature Bureau and Environment Systems Ltd. for JNCC: Peterborough, UK, 2014; p. 85. [Google Scholar]
- Truzzi, C.; Annibaldi, A.; Illuminati, S.; Bassotti, E.; Scarponi, G. Square-wave anodic-stripping voltammetric determination of Cd, Pb, and Cu in a hydrofluoric acid solution of siliceous spicules of marine sponges (from the Ligurian Sea, Italy, and the Ross Sea, Antarctica). Anal. Bioanal. Chem. 2008, 392, 247–262. [Google Scholar] [CrossRef]
- Ledda, F.D.; Ramoino, P.; Ravera, S.; Perino, E.; Bianchini, P.; Diaspro, A.; Gallus, L.; Pronzato, R.; Manconi, R. Tubulin posttranslational modifications induced by cadmium in the sponge Clathrina clathrus. Aquat. Toxicol. 2013, 140, 98–105. [Google Scholar] [CrossRef]
- Vincent, J.B. New evidence against chromium as an essential trace element. J. Nutr. 2017, 147, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, N.; Aksenov, A.; Sorokina, T.; Chashchin, V.; Ellingsen, D.G.; Nieboer, E.; Varakina, Y.; Vaselkina, E.; Kotsur, D.; Thomassen, Y. Essential and non-essential trace elements in fish consumed by indigenous peoples of the European Russian Arctic. Environ. Pollut. 2019, 253, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Directive 2002/32/EC of 7 May 2002 on Undesirable Substances in Animal Feed; Eur-Lex: Brussels, Belgium, 2002; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32002L0032 (accessed on 4 December 2020).
- Pham, C.K.; Murillo, F.J.; Lirette, C.; Maldonado, M.; Colaço, A.; Ottaviani, D.; Kenchington, E. Removal of deep-sea sponges by bottom trawling in the Flemish Cap area: Conservation, ecology and economic assessment. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Dyrynda, P.E.J. Defensive strategies of modular organisms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1986, 313, 227–243. [Google Scholar] [CrossRef]
- Rius, M.; Branch, G.M.; Griffiths, C.L.; Turon, X. Larval settlement behaviour in six gregarious ascidians in relation to adult distribution. Mar. Ecol. Prog. Ser. 2010, 418, 151–163. [Google Scholar] [CrossRef]
- Keen, S.L. Recruitment of Aurelia aurita (Cnidaria: Scyphozoa) larvae is position-dependent, and independent of conspecific density, within a settling surface. Mar. Ecol. Prog. Ser. 1987, 38, 151–160. [Google Scholar] [CrossRef]
- Miranda, L.S.; Collins, A.G.; Marques, A.C. Molecules Clarify a Cnidarian Life Cycle—The “Hydrozoan” Microhydrula limopsicola Is an Early Life Stage of the Staurozoan Haliclystus antarcticus. PLoS ONE 2010, 5, e10182. [Google Scholar] [CrossRef]
- Crisp, D.J.; Meadows, P.S. The chemical basis of gregariousness in cirripedes. Proc. R. Soc. Lond. B Biol. Sci. 1962, 156, 500–520. [Google Scholar] [CrossRef]
- Burke, R.D. Pheromones and gregarious settlement of marine invertebrate larvae. Bull. Mar. Sci. 1986, 39, 323–331. [Google Scholar]
- Millot, C.; Taupier-Letage, I. Circulation in the Mediterranean Sea. In The Mediterranean Sea; Springer: Berlin/Heidelberg, Germany, 2005; Volume 5K, pp. 29–66. [Google Scholar] [CrossRef]
- Bethoux, J.P.; Courau, P.; Nicolas, E.; Ruizpino, D. Trace-metal pollution in the Mediterranean Sea. Oceanol. Acta 1990, 13, 481–488. [Google Scholar]
- Danovaro, R. Pollution threats in the Mediterranean Sea: An overview. Chem. Ecol. 2003, 19, 15–32. [Google Scholar] [CrossRef]
- Tovar-Sánchez, A.; Rodríguez-Romero, A.; Engel, A.; Zäncker, B.; Fu, F.; Marañón, E.; Pérez-Lorenzo, M.; Bressac, M.; Wagener, T.; Triquet, S.; et al. Characterizing the surface microlayer in the Mediterranean Sea: Trace metal concentrations and microbial plankton abundance. Biogeosciences 2020, 17, 2349–2364. [Google Scholar] [CrossRef]
- Adkins, J.F.; Henderson, G.M.; Wang, S.L.; O’Shea, S.; Mokadem, F. Growth rates of the deep-sea scleractinia Desmophyllum cristagalli and Enallopsammia rostrata. Earth Planet. Sci. Lett. 2004, 227, 481–490. [Google Scholar] [CrossRef]
- Lough, J.M. Coral calcification from skeletal records revisited. Mar. Ecol. Prog. Ser. 2008, 373, 257–264. [Google Scholar] [CrossRef]
- DeLong, K.L.; Quinn, T.M.; Taylor, F.W. Reconstructing twentieth-century sea surface temperature variability in the southwest Pacific: A replication study using multiple coral Sr/Ca records from New Caledonia. Paleoceanography 2007, 22, PA4212. [Google Scholar] [CrossRef]
- Carilli, J.E.; Norris, R.D.; Black, B.; Walsh, S.M.; McField, M. Century-scale records of coral growth rates indicate that local stressors reduce coral thermal tolerance threshold. Glob. Change Biol. 2010, 16, 1247–1257. [Google Scholar] [CrossRef]
- David, C.P. Heavy metal concentrations in growth bands of corals: A record of mine tailings input through time (Marinduque Island, Philippines). Mar. Pollut. Bull. 2003, 46, 187–196. [Google Scholar] [CrossRef]
- Nour, H.E.S.; Nouh, E.S. Using coral skeletons for monitoring of heavy metals pollution in the Red Sea Coast, Egypt. Arab. J. Geosci. 2020, 13, 341. [Google Scholar] [CrossRef]
- Readman, J.W.; Tolosa, I.; Law, A.T.; Bartocci, J.; Azemard, S.; Hamilton, T.; Mee, L.D.; Wagener, A.; Le Tissier, M.; Roberts, C.; et al. Discrete bands of petroleum hydrocarbons and molecular organic markers identified within massive coral skeletons. Mar. Pollut. Bull. 1996, 32, 437–443. [Google Scholar] [CrossRef]
- Yang, T.; Diao, X.; Cheng, H.; Wang, H.; Zhou, H.; Zhao, H.; Chen, C.M. Comparative study of polycyclic aromatic hydrocarbons (PAHs) and heavy metals (HMs) in corals, sediments and seawater from coral reefs of Hainan, China. Environ. Pollut. 2020, 264, 114719. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, O.A.; Edinger, E.N. Ages and growth rates of some deep-sea gorgonian and antipatharian corals of Newfoundland and Labrador. Can. J. Fish. Aquat. Sci. 2009, 66, 142–152. [Google Scholar] [CrossRef]
- Samperiz, A.; Robinson, L.F.; Stewart, J.A.; Strawson, I.; Leng, M.J.; Rosenheim, B.E.; Ciscato, E.R.; Hendry, K.R.; Santodomingo, N. Stylasterid corals: A new paleotemperature archive. Earth Planet. Sci. Lett. 2020, 545, 116407. [Google Scholar] [CrossRef]
- Webster, N.S.; Webb, R.I.; Ridd, M.J.; Hill, R.T.; Negri, A.P. The effects of copper on the microbial community of a coral reef sponge. Environ. Microbiol. 2001, 3, 19–31. [Google Scholar] [CrossRef]
- Mohanty, S.; Bapuji, M.; Mishra, R.K.; Sree, A.; Ray, P.; Mohapatra, S.B.; Rath, C.C. Studies on metal tolerance of bacterial associates of marine sedentary organisms. Asian J. Microbiol. Biotechnol. Environ. Sci. 2004, 6, 291–296. [Google Scholar]
- Selvin, J.; Priya, S.S.; Kiran, G.S.; Bhosle, S. Biomonitoring of heavy metal pollution in the marine environment using indicator organisms. In Causes and Effects of Heavy Metal Pollution; Sánchez, M.L., Ed.; Nova Publishers: New York, NY, USA, 2008; Volume 4, pp. 251–264. [Google Scholar]
- Selvin, J.; Priya, S.S.; Kiran, G.S.; Thangavelu, T.; Bai, N.S. Sponge-associated marine bacteria as indicators of heavy metal pollution. Microbiol. Res. 2009, 164, 352–363. [Google Scholar] [CrossRef]
- Mangano, S.; Michaud, L.; Caruso, C.; Giudice, A.L. Metal and antibiotic resistance in psychrotrophic bacteria associated with the Antarctic sponge Hemigellius pilosus (Kirkpatrick, 1907). Polar Biol. 2014, 37, 227–235. [Google Scholar] [CrossRef]
- Bauvais, C.; Zirah, S.; Piette, L.; Chaspoul, F.; Domart-Coulon, I.; Chapon, V.; Gallice, P.; Rebuffat, S.; Pérez, T.; Bourguet-Kondracki, M.L. Sponging up metals: Bacteria associated with the marine sponge Spongia officinalis. Mar. Environ. Res. 2015, 104, 20–30. [Google Scholar] [CrossRef]
- Karimi, E.; Gonçalves, J.M.; Reis, M.; Costa, R. Draft Genome Sequence of Microbacterium sp. Strain Alg239_V18, an Actinobacterium Retrieved from the Marine Sponge Spongia sp. Genome Announc. 2017, 5, e01457-16. [Google Scholar] [CrossRef]
- Schmitt, S.; Wehrl, M.; Siegl, A.; Hentschel, U. Review article Marine sponges as models for commensal microbe-host interactions. Symbiosis 2007, 44, 43–50. [Google Scholar]
- Santos-Gandelman, J.F.; Giambiagi-deMarval, M.; Oelemann, W.M.R.; Laport, M.S. Biotechnological potential of sponge-associated bacteria. In Current Pharmaceutical Biotechnology; Bentham Science Publishers: Valencia, Spain, 2014; Volume 15, pp. 143–155. [Google Scholar]
- Thomas, T.; Moitinho-Silva, L.; Lurgi, M.; Björk, J.R.; Easson, C.; Astudillo-García, C.; Olson, J.B.; Erwin, P.M.; Lopez-Legentil, S.; Luter, H.; et al. Diversity, structure and convergent evolution of the global sponge microbiome. Nat. Commun. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jeanthon, C.; Prieur, D. Resistance to heavy metals of heterotrophic bacteria isolated from the deep-sea hydrothermal vent polychaete, Alvinella pompejana. Prog. Oceanogr. 1990, 24, 81–88. [Google Scholar] [CrossRef]
- Prieur, D.; Chamroux, S.; Durand, P.; Erauso, G.; Fera, P.; Jeanthon, C.; Le Borgne, G.; Mével, G.; Vincent, P. Metabolic diversity in epibiotic microflora associated with the Pompeii worms Alvinella pompejana and A. caudata (Polychaetae: Annelida) from deep-sea hydrothermal vents. Mar. Biol. 1990, 106, 361–367. [Google Scholar] [CrossRef]
- Rizzo, C.; Michaud, L.; Graziano, M.; De Domenico, E.; Syldatk, C.; Hausmann, R.; Giudice, A.L. Biosurfactant activity, heavy metal tolerance and characterization of Joostella strain A8 from the Mediterranean polychaete Megalomma claparedei (Gravier, 1906). Ecotoxicology 2015, 24, 1294–1304. [Google Scholar] [CrossRef]
- Ueki, T. Bioaccumulation of vanadium by vanadium-resistant bacteria isolated from the intestine of Ascidia sydneiensis samea. Mar. Biotechnol. 2016, 18, 359–371. [Google Scholar] [CrossRef]
- Ueki, T.; Fujie, M.; Satoh, N. Symbiotic bacteria associated with ascidian vanadium accumulation identified by 16S rRNA amplicon sequencing. Mar. Genom. 2019, 43, 33–42. [Google Scholar] [CrossRef]
- Liberti, A.; Bertocci, I.; Pollet, A.; Musco, L.; Locascio, A.; Ristoratore, F.; Spagnuolo, A.; Sordino, P. An indoor study of the combined effect of industrial pollution and turbulence events on the gut environment in a marine invertebrate. Mar. Environ. Res. 2020, 158, 104950. [Google Scholar] [CrossRef]
- Batel, R.; Bihari, N.; Rinkevich, B.; Dapper, J.; Schäcke, H.; Schröder, H.; Müller, W. Modulation of organotin-induced apoptosis by the water pollutant methyl mercury in a human lymphoblastoid tumor cell line and a marine sponge. Mar. Ecol. Prog. Ser. 1993, 93, 245–251. [Google Scholar] [CrossRef]
- Jones, R.J. Testing the ‘photoinhibition’ model of coral bleaching using chemical inhibitors. Mar. Ecol. Prog. Ser. 2004, 284, 133–145. [Google Scholar] [CrossRef]
- Lockyer, A.; Binet, M.T.; Styan, C.A. Importance of sperm density in assessing the toxicity of metals to the fertilization of broadcast spawners. Ecotoxicol. Environ. Saf. 2019, 172, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.H.; Horton, A.A. Short-term effects of the heavy metals, Silver and copper, on polyps of the common jellyfish, Aurelia aurita. J. Exp. Mar. Biol. Ecol. 2014, 461, 154–161. [Google Scholar] [CrossRef]
- Duarte, C.M.; Pitt, K.A.; Lucas, C.H.; Purcell, J.E.; Uye, S.I.; Robinson, K.; Brotz, L.; Decker, M.B.; Sutherland, K.R.; Malej, A.; et al. Is global ocean sprawl a cause of jellyfish blooms? Front. Ecol. Environ. 2013, 11, 91–97. [Google Scholar] [CrossRef]
- Purcell, J.E. Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Ann. Rev. Mar. Sci. 2012, 4, 209–235. [Google Scholar] [CrossRef]
- Purcell, J.E.; Uye, S.I.; Lo, W.T. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Viarengo, A.; Burlando, B.; Giordana, A.; Bolognesi, C.; Gabrielides, G.P. Networking and expert-system analysis: Next frontier in biomonitoring. Mar. Environ. Res. 2000, 49, 483–486. [Google Scholar] [CrossRef]
- Agell, G.; Uriz, M.J.; Cebrian, E.; Martí, R. Does stress protein induction by copper modify natural toxicity in sponges? Environ. Toxicol. Chem. Int. J. 2001, 20, 2588–2593. [Google Scholar] [CrossRef]
- Berthet, B.; Mouneyrac, C.; Pérez, T.; Amiard-Triquet, C. Metallothionein concentration in sponges (Spongia officinalis) as a biomarker of metal contamination. Comp. Biochem. Phys. C Toxicol. Pharmacol. 2005, 141, 306–313. [Google Scholar] [CrossRef]
- Amiard, J.C.; Amiard-Triquet, C.; Barka, S.; Pellerin, J.; Rainbow, P.S. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef]
- Franchi, N.; Boldrin, F.; Ballarin, L.; Piccinni, E. CiMT-1, an unusual chordate metallothionein gene in Ciona intestinalis genome: Structure and expression studies. J. Exp. Zool. A Ecol. Genet. Physiol. 2011, 315, 90–100. [Google Scholar] [CrossRef]
- Zanette, J.; Monserrat, J.M.; Bianchini, A. Biochemical biomarkers in barnacles Balanus improvisus: Pollution and seasonal effects. Mar. Environ. Res. 2015, 103, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Karntanut, W.; Pascoe, D. The toxicity of copper, cadmium and zinc to four different Hydra (Cnidaria: Hydrozoa). Chemosphere 2002, 47, 1059–1064. [Google Scholar] [CrossRef]
- Piola, R.F.; Johnston, E.L. Differential tolerance to metals among populations of the introduced bryozoan Bugula neritina. Mar. Biol. 2006, 148, 997–1010. [Google Scholar] [CrossRef]
- Capdevila, M.; Atrian, S. Metallothionein protein evolution: A miniassay. J. Biol. Inorg. Chem. 2011, 16, 977–989. [Google Scholar] [CrossRef]
- Casado-Martinez, M.C.; Smith, B.D.; Luoma, S.N.; Rainbow, P.S. Metal toxicity in a sediment-dwelling polychaete: Threshold body concentrations or overwhelming accumulation rates? Environ. Pollut. 2010, 158, 3071–3076. [Google Scholar] [CrossRef]
- Domingos, R.F.; Gélabert, A.; Carreira, S.; Cordeiro, A.; Sivry, Y.; Benedetti, M.F. Metals in the aquatic environment—Interactions and implications for the speciation and bioavailability: A critical overview. Aquat. Geochem. 2015, 21, 231–257. [Google Scholar] [CrossRef]
- Goldberg, E.D.; Bowen, V.T.; Farrington, J.W.; Harvey, G.; Martin, J.H.; Parker, P.L.; Risebrough, R.W.; Robertson, W.; Schneider, E.; Gamble, E. The Mussel Watch. In Environmental Conservation; The Foundation of Environmental Conservation: La Jolla, CA, USA, 1978; Volume 5, pp. 101–105. [Google Scholar]
- Roose, P.; Albaigés, J.; Bebianno, M.J.; Camphuysen, C.; Cronin, M.; de Leeuw, J.; Gabrielsen, G.; Hutchinson, T.; Hylland, K.; Jansson, B.; et al. Chemical Pollution in Europe’s Seas: Programmes, Practices and Priorities for Research; Marine Board Position Paper, 16; Calewaert, J.B., McDonough, N., Eds.; Marine Board-ESF: Ostend, Belgium, 2011. [Google Scholar]
- Gabrielides, G. MED POL Biomonitoring Programme Concerning the Effects of Pollutants on Marine Organisms along the Mediterranean Coasts; UNEP(OCA)/MED WG 132/3; Athens: Athens, Greece, 1997. [Google Scholar]
- Da Silva, M.; Passarini, M.R.Z.; Bonugli, R.C.; Sette, L.D. Cnidarian-derived filamentous fungi from Brazil: Isolation, characterisation and RBBR decolourisation screening. Environ. Technol. 2008, 29, 1331–1339. [Google Scholar] [CrossRef]
- Rosa, I.C.; Costa, R.; Gonçalves, F.; Pereira, J.L. Bioremediation of Metal-Rich Effluents: Could the Invasive Bivalve Corbicula fluminea Work as a Biofilter? J. Environ. Qual. 2014, 43, 1536–1545. [Google Scholar] [CrossRef]
- Tamilselvi, M.; Akram, A.S.; Arshan, M.K.; Sivakumar, V. Comparative study on bioremediation of heavy metals by solitary ascidian, Phallusia nigra, between Thoothukudi and Vizhinjam ports of India. Ecotoxicol. Environ. Saf. 2015, 121, 93–99. [Google Scholar] [CrossRef]
- Sicuro, B.; Castelar, B.; Mugetti, D.; Pastorino, P.; Chiarandon, A.; Menconi, V.; Galloni, M.; Prearo, M. Bioremediation with freshwater bivalves: A sustainable approach to reducing the environmental impact of inland trout farms. J. Environ. Manag. 2020, 276, 111327. [Google Scholar] [CrossRef]
- Relini, G.; Giaccone, G. Priority habitats according to the SPA/BIO protocol (Barcelona Convention) present in Italy. Identification sheets. Biol. Mar. Mediterr. 2009, 16 (Suppl. 2). [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roveta, C.; Annibaldi, A.; Afghan, A.; Calcinai, B.; Di Camillo, C.G.; Gregorin, C.; Illuminati, S.; Pulido Mantas, T.; Truzzi, C.; Puce, S. Biomonitoring of Heavy Metals: The Unexplored Role of Marine Sessile Taxa. Appl. Sci. 2021, 11, 580. https://doi.org/10.3390/app11020580
Roveta C, Annibaldi A, Afghan A, Calcinai B, Di Camillo CG, Gregorin C, Illuminati S, Pulido Mantas T, Truzzi C, Puce S. Biomonitoring of Heavy Metals: The Unexplored Role of Marine Sessile Taxa. Applied Sciences. 2021; 11(2):580. https://doi.org/10.3390/app11020580
Chicago/Turabian StyleRoveta, Camilla, Anna Annibaldi, Afghan Afghan, Barbara Calcinai, Cristina Gioia Di Camillo, Chiara Gregorin, Silvia Illuminati, Torcuato Pulido Mantas, Cristina Truzzi, and Stefania Puce. 2021. "Biomonitoring of Heavy Metals: The Unexplored Role of Marine Sessile Taxa" Applied Sciences 11, no. 2: 580. https://doi.org/10.3390/app11020580
APA StyleRoveta, C., Annibaldi, A., Afghan, A., Calcinai, B., Di Camillo, C. G., Gregorin, C., Illuminati, S., Pulido Mantas, T., Truzzi, C., & Puce, S. (2021). Biomonitoring of Heavy Metals: The Unexplored Role of Marine Sessile Taxa. Applied Sciences, 11(2), 580. https://doi.org/10.3390/app11020580