Biomechanical Analysis of the Spine in Diffuse Idiopathic Skeletal Hyperostosis: Finite Element Analysis
Abstract
:1. Introduction
2. Material and Methods
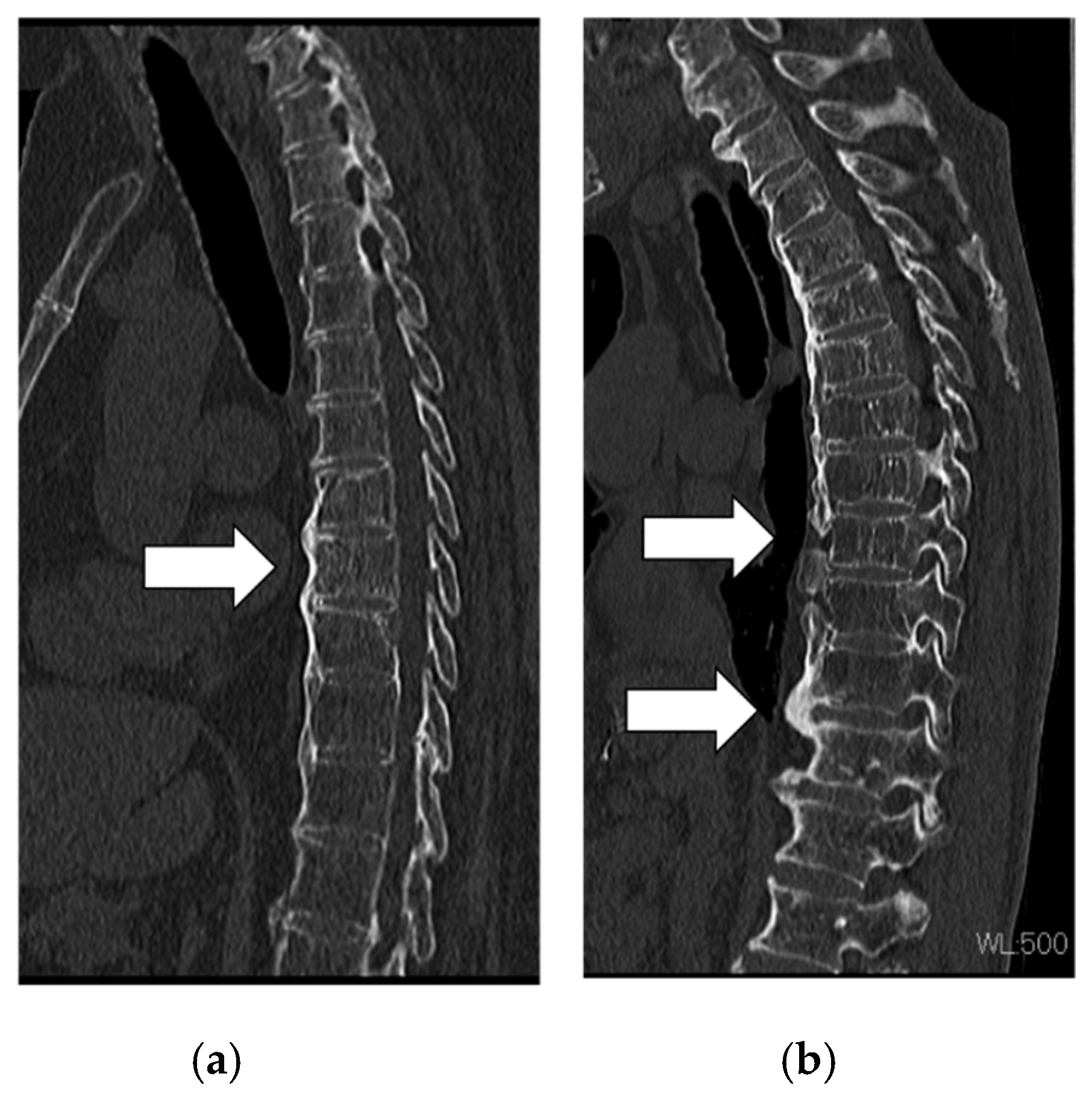
2.1. Patient Images
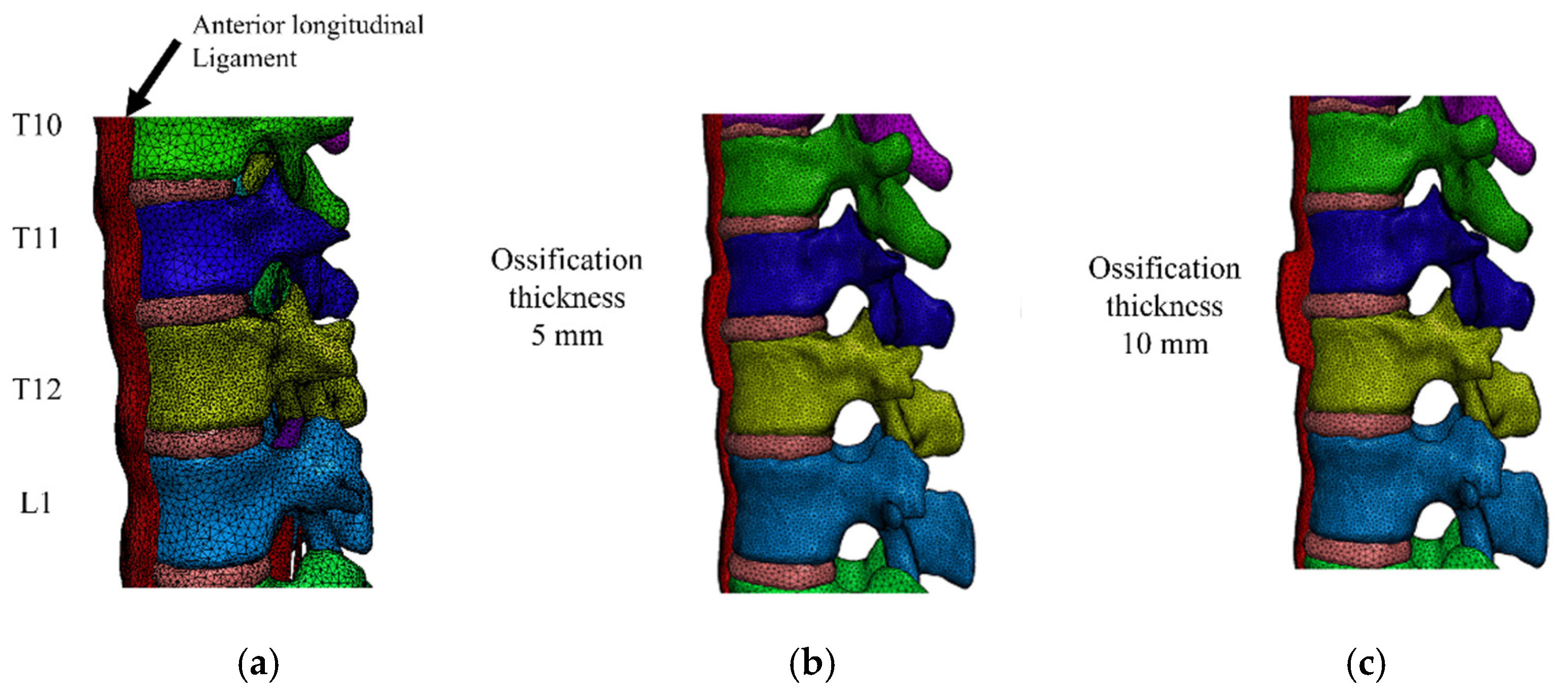
2.2. FEM Model Construction
2.3. Model for DISH
2.4. Load Application
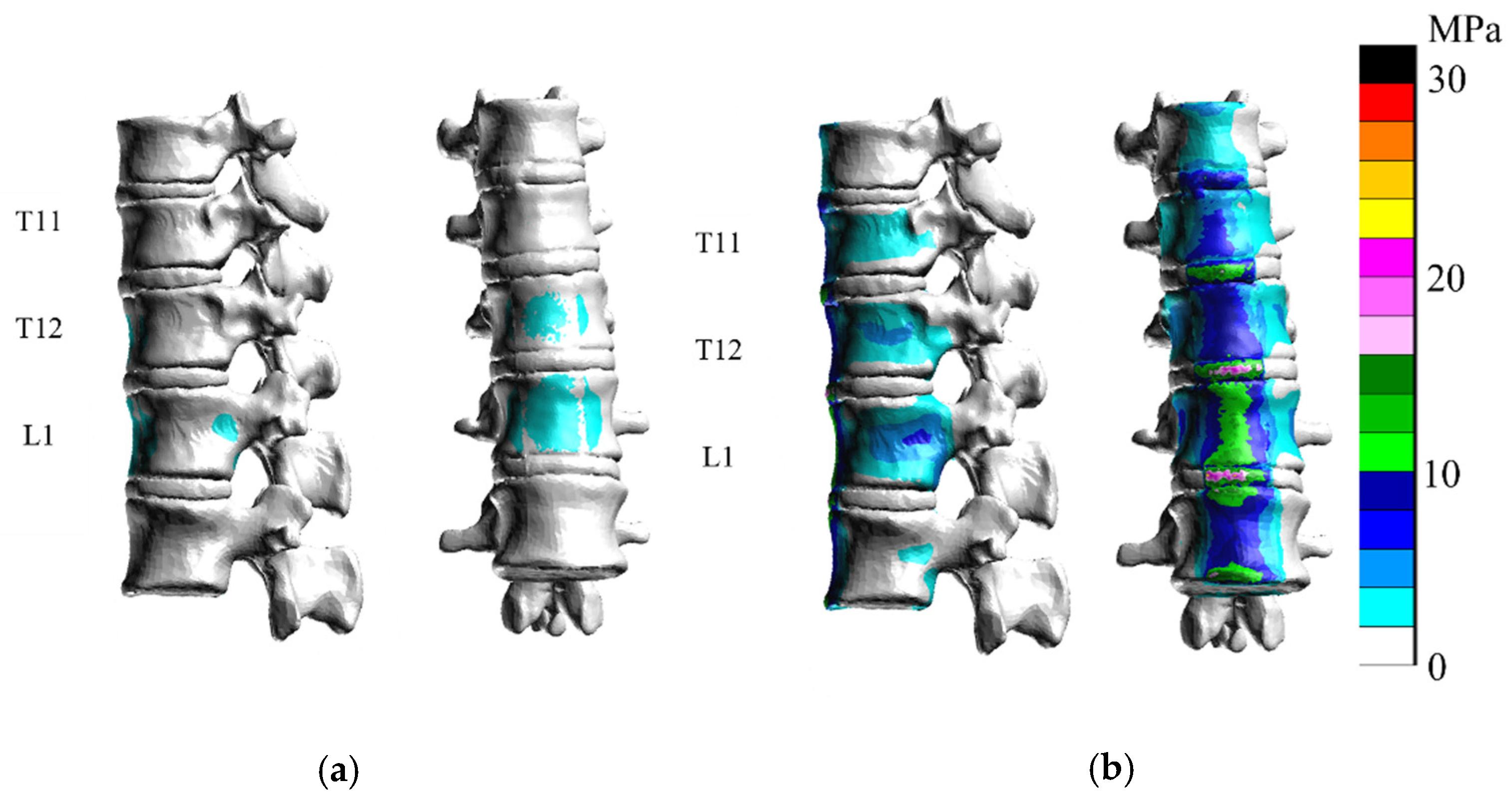
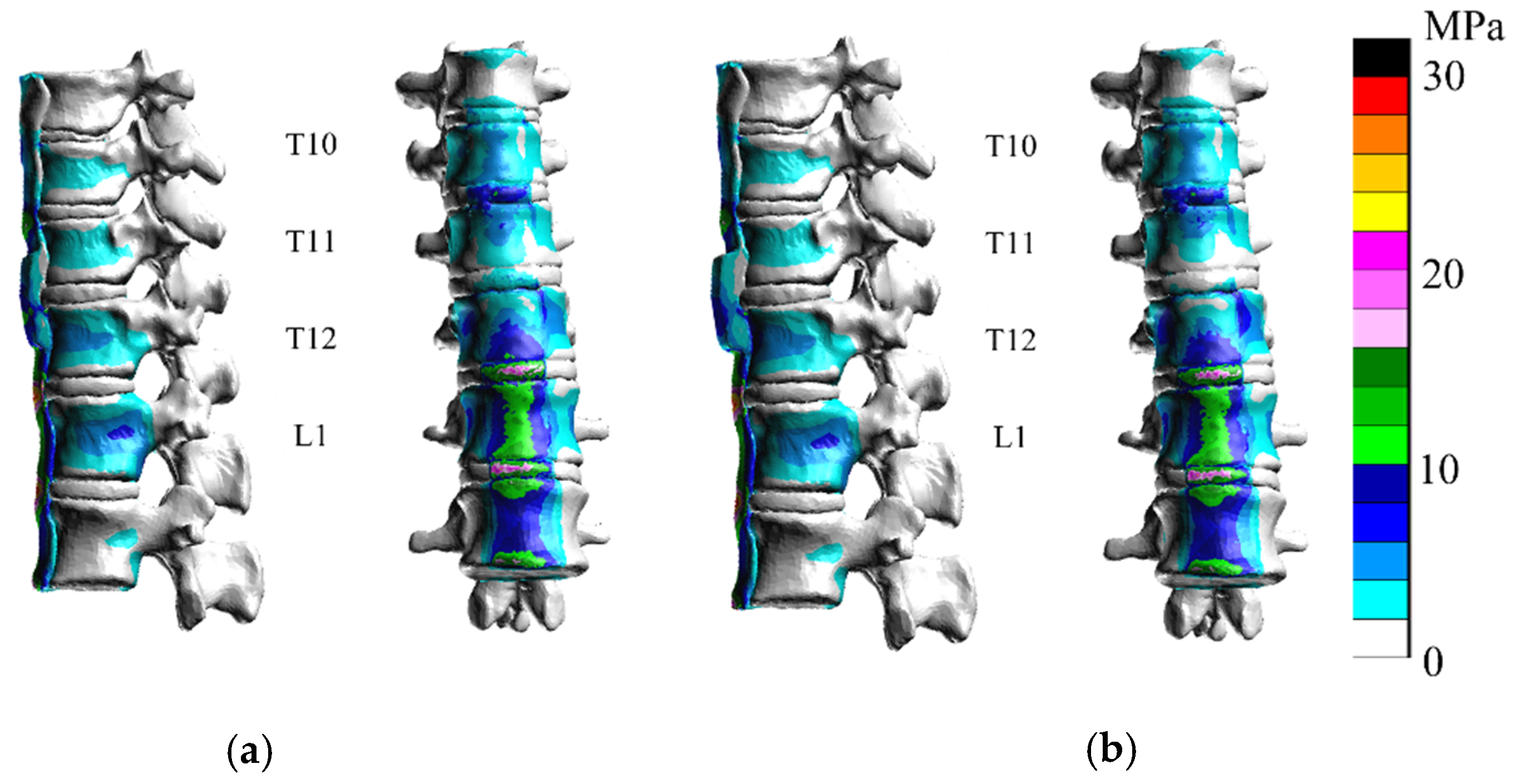
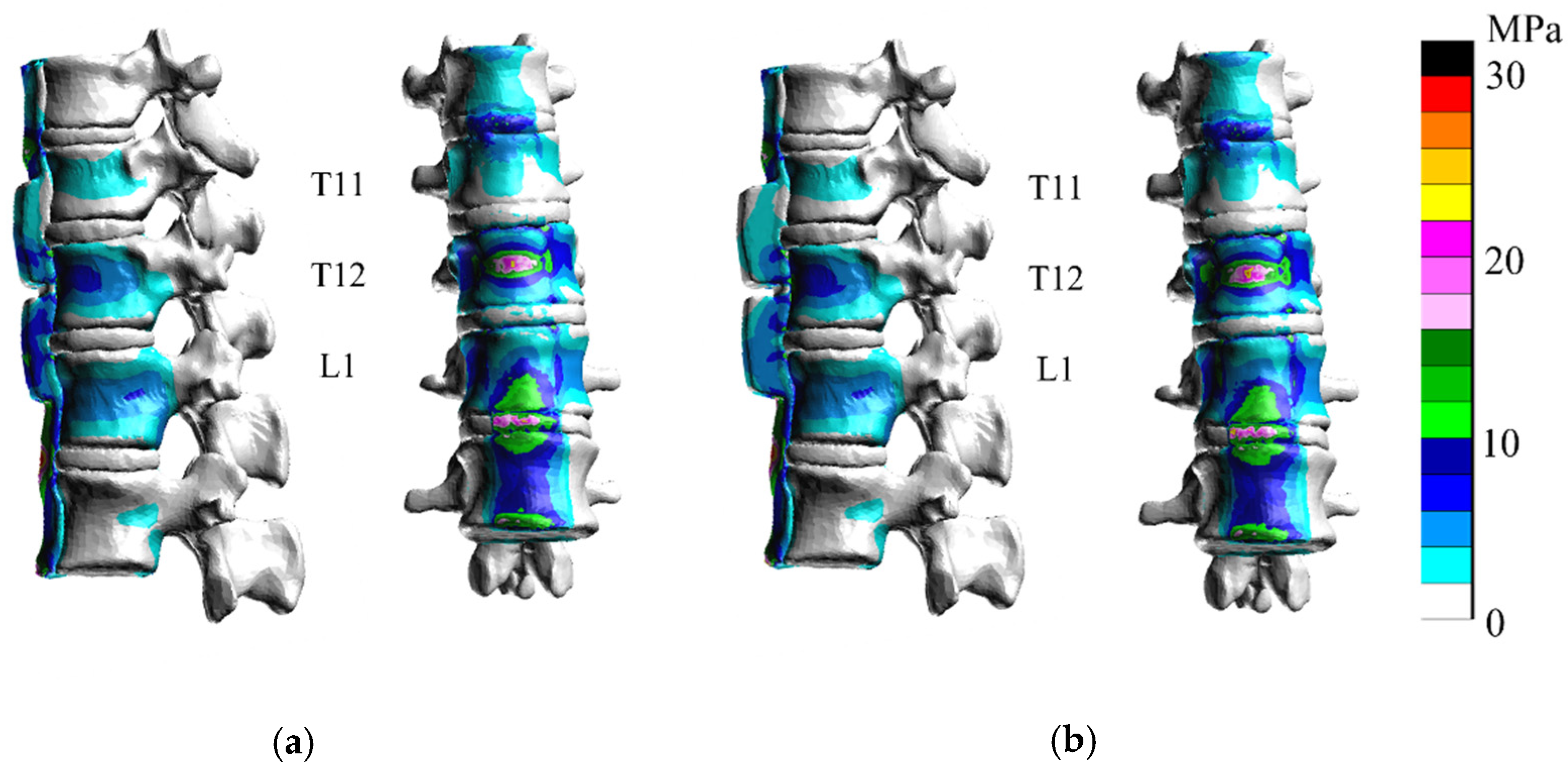
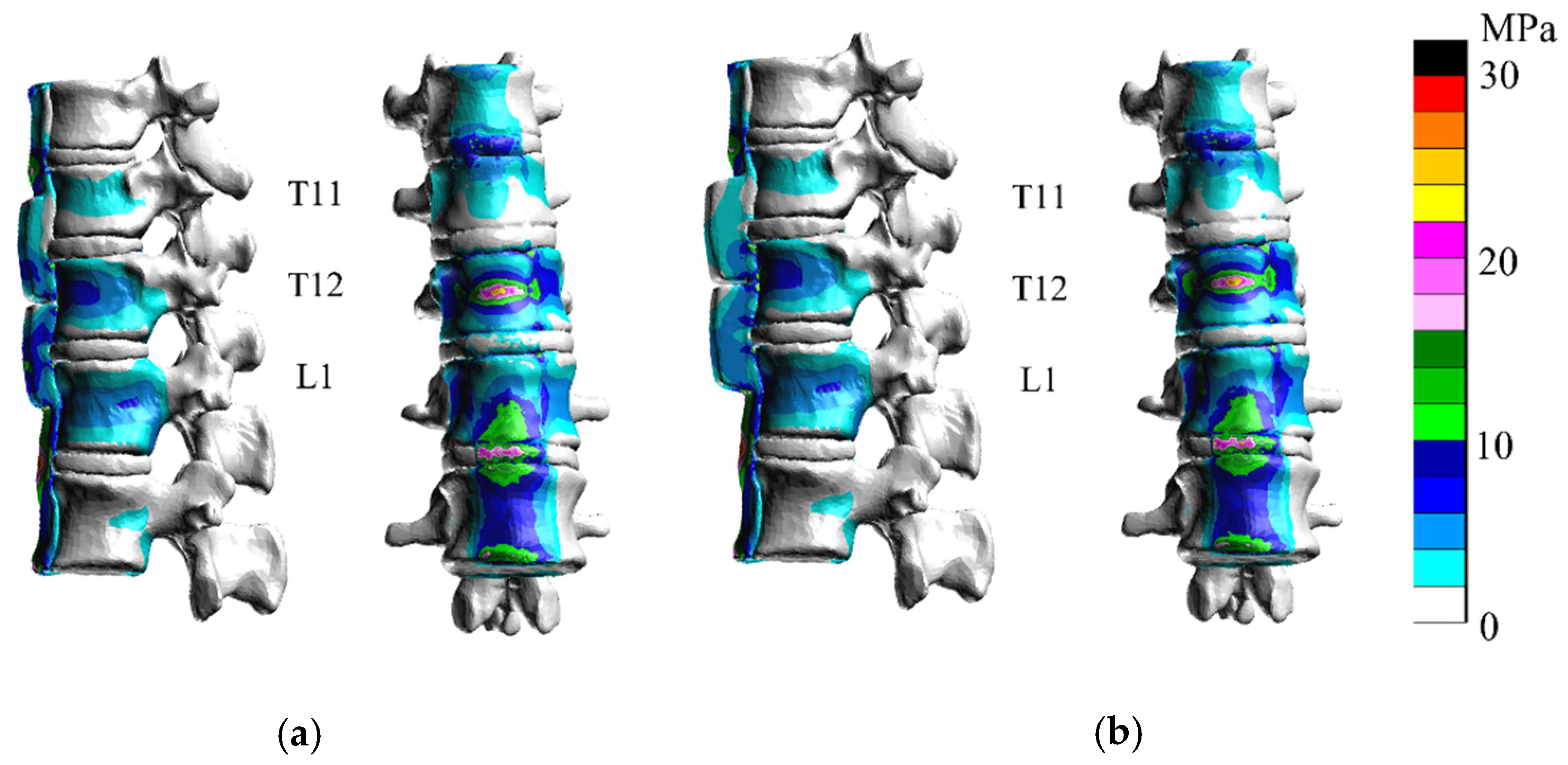
3. Results
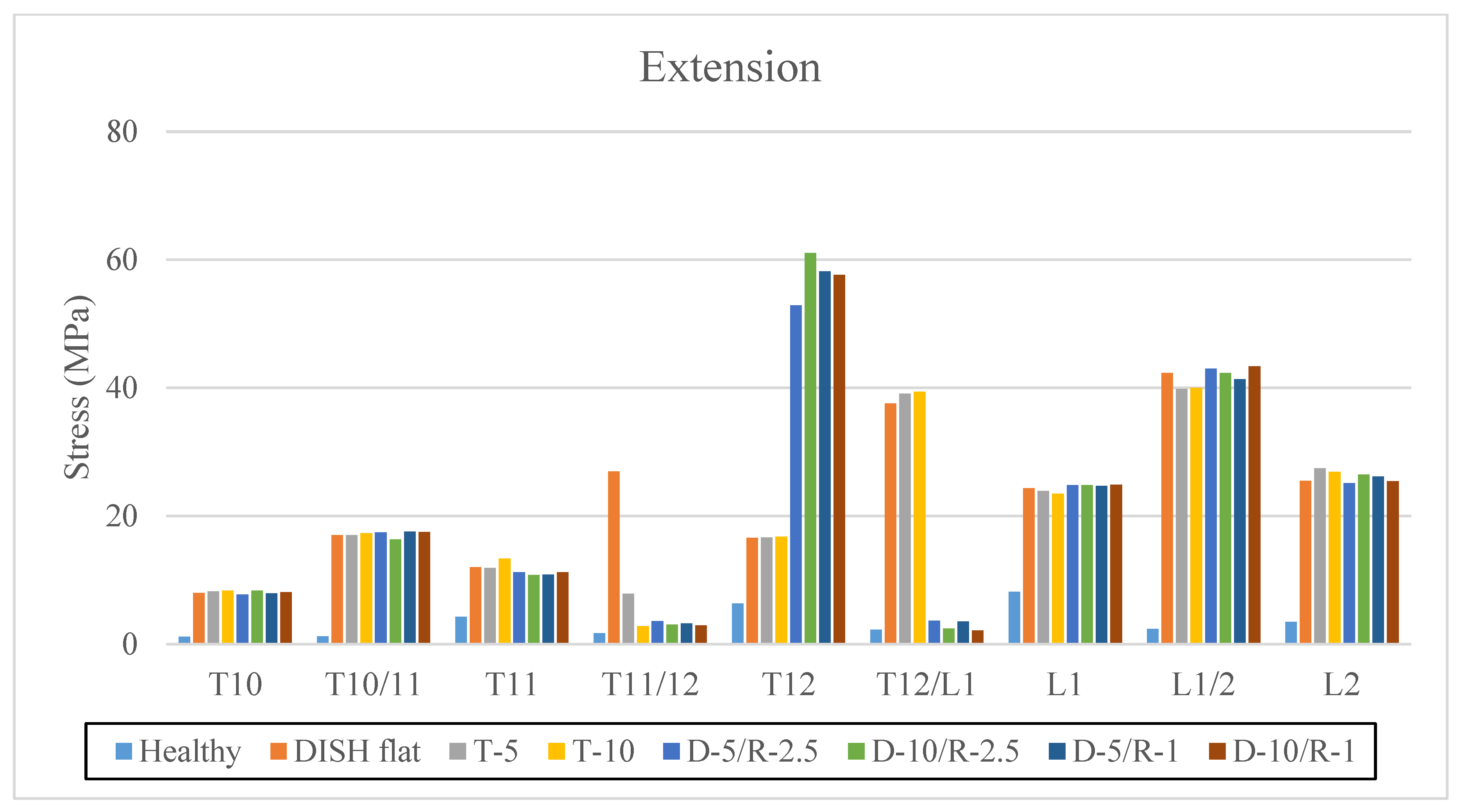
3.1. Extension
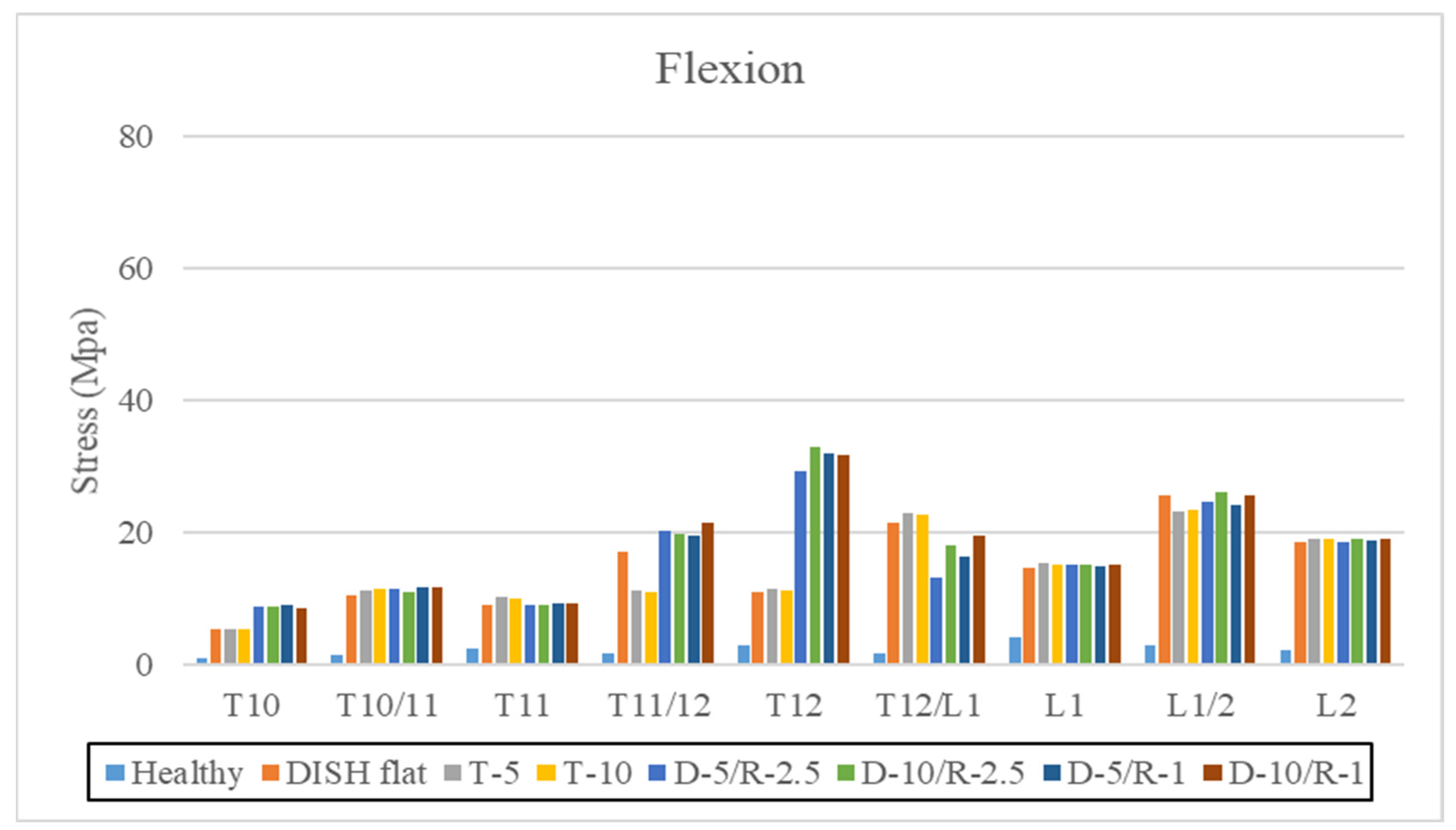
3.2. Flexion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Resnick, D.; Guerra, J., Jr.; Robinson, C.A.; Vint, V.C. Association of diffuse idiopathic skeletal hyperostosis (DISH) and calcification and ossification of the posterior longitudinal ligament. AJR Am. J. Roentgenol. 1978, 131, 1049–1053. [Google Scholar] [CrossRef] [Green Version]
- Okada, E.; Shiono, Y.; Nishida, M.; Mima, Y.; Funao, H.; Shimizu, K.; Kato, M.; Fukuda, K.; Fujita, N.; Yagi, M.; et al. Spinal fractures in diffuse idiopathic skeletal hyperostosis: Advantages of percutaneous pedicle screw fixation. J. Orthop. Surg. (Hong Kong) 2019, 27, 2309499019843407. [Google Scholar] [CrossRef] [Green Version]
- Westerveld, L.A.; van Bemmel, J.C.; Dhert, W.J.; Oner, F.C.; Verlaan, J.J. Clinical outcome after traumatic spinal fractures in patients with ankylosing spinal disorders compared with control patients. Spine J. 2014, 14, 729–740. [Google Scholar] [CrossRef]
- Westerveld, L.A.; Verlaan, J.J.; Oner, F.C. Spinal fractures in patients with ankylosing spinal disorders: A systematic review of the literature on treatment, neurological status and complications. Eur. Spine J. 2009, 18, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Bruges-Armas, J.; Couto, A.R.; Timms, A.; Santos, M.R.; Bettencourt, B.F.; Peixoto, M.J.; Colquhoun, K.; McNally, E.G.; Carneiro, V.; Herrero-Beaumont, G.; et al. Ectopic calcification among families in the Azores: Clinical and radiologic manifestations in families with diffuse idiopathic skeletal hyperostosis and chondrocalcinosis. Arthritis Rheum. 2006, 54, 1340–1349. [Google Scholar] [CrossRef]
- Oudkerk, S.F.; de Jong, P.A.; Attrach, M.; Luijkx, T.; Buckens, C.F.; Mali, W.P.; Oner, F.C.; Resnick, D.L.; Vliegenthart, R.; Verlaan, J.J. Diagnosis of diffuse idiopathic skeletal hyperostosis with chest computed tomography: Inter-observer agreement. Eur Radiol 2017, 27, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Fujimori, T.; Watabe, T.; Iwamoto, Y.; Hamada, S.; Iwasaki, M.; Oda, T. Prevalence, Concomitance, and Distribution of Ossification of the Spinal Ligaments: Results of Whole Spine CT Scans in 1500 Japanese Patients. Spine (Phila Pa 1976) 2016, 41, 1668–1676. [Google Scholar] [CrossRef]
- Galbusera, F.; Bellini, C.M.; Anasetti, F.; Ciavarro, C.; Lovi, A.; Brayda-Bruno, M. Rigid and flexible spinal stabilization devices: A biomechanical comparison. Med. Eng. Phys. 2011, 33, 490–496. [Google Scholar] [CrossRef]
- Lu, Y.M.; Hutton, W.C.; Gharpuray, V.M. Can variations in intervertebral disc height affect the mechanical function of the disc? Spine (Phila Pa 1976) 1996, 21, 2208–2216; discussion 2217. [Google Scholar] [CrossRef]
- Cowin, S.C. The mechanical and stress adaptive properties of bone. Ann. Biomed. Eng. 1983, 11, 263–295. [Google Scholar] [CrossRef]
- Cowin, S.C. Mechanical modeling of the stress adaptation process in bone. Calcif. Tissue Int. 1984, 36 (Suppl. 1), S98–S103. [Google Scholar] [CrossRef]
- Périé, D.; Hobatho, M.C.; Baunin, C.; Sales De Gauzy, J. Personalised mechanical properties of scoliotic vertebrae determined in vivo using tomodensitometry. Comput. Methods Biomech. Biomed. Eng. 2002, 5, 161–165. [Google Scholar] [CrossRef]
- Ottardi, C.; Galbusera, F.; Luca, A.; Prosdocimo, L.; Sasso, M.; Brayda-Bruno, M.; Villa, T. Finite element analysis of the lumbar destabilization following pedicle subtraction osteotomy. Med. Eng. Phys. 2016, 38, 506–509. [Google Scholar] [CrossRef]
- Holton, K.F.; Denard, P.J.; Yoo, J.U.; Kado, D.M.; Barrett-Connor, E.; Marshall, L.M. Diffuse idiopathic skeletal hyperostosis and its relation to back pain among older men: The MrOS Study. Semin Arthritis Rheum. 2011, 41, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Okada, E.; Yoshii, T.; Yamada, T.; Watanabe, K.; Katsumi, K.; Hiyama, A.; Watanabe, M.; Nakagawa, Y.; Okada, M.; Endo, T.; et al. Spinal fractures in patients with Diffuse idiopathic skeletal hyperostosis:A nationwide multi-institution survey. J. Orthop. Sci. 2019, 24, 601–606. [Google Scholar] [CrossRef]
- Sedney, C.L.; Daffner, S.D.; Obafemi-Afolabi, A.; Gelb, D.; Ludwig, S.; Emery, S.E.; France, J.C. A Comparison of Open and Percutaneous Techniques in the Operative Fixation of Spinal Fractures Associated with Ankylosing Spinal Disorders. Int. J. Spine Surg. 2016, 10, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caron, T.; Bransford, R.; Nguyen, Q.; Agel, J.; Chapman, J.; Bellabarba, C. Spine fractures in patients with ankylosing spinal disorders. Spine (Phila Pa 1976) 2010, 35, E458–E464. [Google Scholar] [CrossRef] [PubMed]
- Krüger, A.; Frink, M.; Oberkircher, L.; El-Zayat, B.F.; Ruchholtz, S.; Lechler, P. Percutaneous dorsal instrumentation for thoracolumbar extension-distraction fractures in patients with ankylosing spinal disorders: A case series. Spine J. 2014, 14, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Shiono, Y.; Funao, H.; Singh, K.; Matsumoto, M. A Novel Groove-Entry Technique for Inserting Thoracic Percutaneous Pedicle Screws. Clin. Spine Surg. 2017, 30, 57–64. [Google Scholar] [CrossRef]
- Hendrix, R.W.; Melany, M.; Miller, F.; Rogers, L.F. Fracture of the spine in patients with ankylosis due to diffuse skeletal hyperostosis: Clinical and imaging findings. AJR Am. J. Roentgenol. 1994, 162, 899–904. [Google Scholar] [CrossRef] [Green Version]
- Okada, E.; Tsuji, T.; Shimizu, K.; Kato, M.; Fukuda, K.; Kaneko, S.; Ogawa, J.; Watanabe, K.; Ishii, K.; Nakamura, M.; et al. CT-based morphological analysis of spinal fractures in patients with diffuse idiopathic skeletal hyperostosis. J. Orthop. Sci. 2017, 22, 3–9. [Google Scholar] [CrossRef]
- Sairyo, K.; Goel, V.K.; Masuda, A.; Vishnubhotla, S.; Faizan, A.; Biyani, A.; Ebraheim, N.; Yonekura, D.; Murakami, R.; Terai, T. Three-dimensional finite element analysis of the pediatric lumbar spine. Part I: Pathomechanism of apophyseal bony ring fracture. Eur Spine J. 2006, 15, 923–929. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.T.; Qin, D.P.; Zhang, X.G.; Wang, Z.P.; Tong, Z. Biomechanical effects of different vertebral heights after augmentation of osteoporotic vertebral compression fracture: A three-dimensional finite element analysis. J. Orthop. Surg. Res. 2018, 13, 32. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.; Ye, L.Q.; Jiang, X.B.; Yang, P.; Zhou, G.Q.; Yao, Z.S.; Zhang, S.C.; Yang, Z.D. Biomechanical effects of cement distribution in the fractured area on osteoporotic vertebral compression fractures: A three-dimensional finite element analysis. J. Surg. Res. 2015, 195, 246–256. [Google Scholar] [CrossRef]
- Okamoto, Y.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Hayashi, H.; Sakamoto, J.; Kawahara, N.; Tsuchiya, H. The effect of kyphotic deformity because of vertebral fracture: A finite element analysis of a 10° and 20° wedge-shaped vertebral fracture model. Spine J. 2015, 15, 713–720. [Google Scholar] [CrossRef]
- Nishida, N.; Ohgi, J.; Jiang, F.; Ito, S.; Imajo, Y.; Suzuki, H.; Funaba, M.; Nakashima, D.; Sakai, T.; Chen, X. Finite Element Method Analysis of Compression Fractures on Whole-Spine Models Including the Rib Cage. Comput. Math. Methods Med. 2019, 2019, 8348631. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, D.; Kanchiku, T.; Nishida, N.; Ito, S.; Ohgi, J.; Suzuki, H.; Imajo, Y.; Funaba, M.; Chen, X.; Taguchi, T. Finite element analysis of compression fractures at the thoracolumbar junction using models constructed from medical images. Exp. Ther. Med. 2018, 15, 3225–3230. [Google Scholar] [CrossRef]
- Belanger, T.A.; Rowe, D.E. Diffuse idiopathic skeletal hyperostosis: Musculoskeletal manifestations. J. Am. Acad. Orthop. Surg. 2001, 9, 258–267. [Google Scholar] [CrossRef]






| Part | Young’s Modulus E [MPa] | Poisson Ratio |
|---|---|---|
| Cortical bone | 12,000 | 0.3 |
| Cancellous bone | 1500 | 0.3 |
| Annulus fibrosus | 25 | 0.3 |
| Nucleus pulposus | 1 | 0.45 |
| Anterior longitudinal ligament | 68 | 0.3 |
| Posterior longitudinal ligament | 96 | 0.3 |
| Ligamentum flavum | 28.6 | 0.3 |
| Healthy | DISH Flat | Thickness 5 mm (T-5) | Thickness 10 mm (T-10) | Depth of Discontinuity 5.0 mm The Radius of Curvature of the Central Groove 2.5 mm (D-5/R-2.5) | Depth of Discontinuity 10.0 mm The Radius of Curvature of the Central Groove 2.5 mm (D-10/R-2.5) | Depth of Discontinuity 5.0 mm The Radius of Curvature of the Central Groove 1.0 mm (D-5/R-1) | Depth of Discontinuity 10.0 mm The Radius of Curvature of the Central Groove 1.0 mm (D-5/R-1) | |
|---|---|---|---|---|---|---|---|---|
| elements | 903,621 | 903,621 | 916,543 | 920,383 | 928,076 | 941,138 | 933,367 | 939,741 |
| nodes | 203,312 | 203,312 | 206,149 | 206,992 | 208,975 | 211,550 | 209,951 | 211,200 |
| Extension | ||||||||
|---|---|---|---|---|---|---|---|---|
| Healthy | DISH Flat | T-5 | T-10 | D-5/R-2.5 | D-10/R-2.5 | D-5/R-1 | D-10/R-1 | |
| T10 | 1.17 | 7.99 | 8.25 | 8.4 | 7.79 | 8.35 | 7.93 | 8.11 |
| T10/11 | 1.22 | 17.03 | 17.04 | 17.32 | 17.49 | 16.38 | 17.61 | 17.53 |
| T11 | 4.27 | 12.05 | 11.94 | 13.37 | 11.23 | 10.81 | 10.85 | 11.26 |
| T11/12 | 1.7 | 26.94 | 7.88 | 2.85 | 3.64 | 3.08 | 3.26 | 2.92 |
| T12 | 6.37 | 16.64 | 16.67 | 16.81 | 52.89 | 61.04 | 58.2 | 57.62 |
| T12/L1 | 2.27 | 37.55 | 39.11 | 39.43 | 3.69 | 2.44 | 3.53 | 2.12 |
| L1 | 8.2 | 24.36 | 23.88 | 23.49 | 24.79 | 24.81 | 24.7 | 24.89 |
| L1/2 | 2.38 | 42.33 | 39.81 | 40.03 | 43.02 | 42.33 | 41.36 | 43.36 |
| L2 | 3.47 | 25.47 | 27.42 | 26.87 | 25.15 | 26.45 | 26.19 | 25.44 |
| Flexions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Healthy | DISH Flat | T-5 | T-10 | D-5/R-2.5 | D-10/R-2.5 | D-5/R-1 | D-10/R-1 | |
| T10 | 0.96 | 5.44 | 5.47 | 5.5 | 8.79 | 8.89 | 8.95 | 8.65 |
| T10/11 | 1.39 | 10.55 | 11.28 | 11.384 | 11.52 | 11.03 | 11.66 | 11.69 |
| T11 | 2.4 | 9.15 | 10.23 | 9.99 | 9.01 | 8.97 | 9.23 | 9.23 |
| T11/12 | 1.77 | 16.99 | 11.18 | 11.01 | 20.31 | 19.88 | 19.5 | 21.43 |
| T12 | 3.02 | 11.09 | 11.51 | 11.33 | 29.27 | 32.91 | 32.05 | 31.74 |
| T12/L1 | 1.67 | 21.49 | 22.97 | 22.79 | 13.28 | 18.01 | 16.28 | 19.61 |
| L1 | 4.2 | 14.6 | 15.38 | 15.13 | 15.09 | 15.18 | 14.84 | 15.24 |
| L1/2 | 2.89 | 25.63 | 23.28 | 23.32 | 24.52 | 26.19 | 24.04 | 25.68 |
| L2 | 2.23 | 18.62 | 19.1 | 19.12 | 18.51 | 18.96 | 18.73 | 18.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, N.; Jiang, F.; Ohgi, J.; Fuchida, M.; Kitazumi, R.; Yamamura, Y.; Tome, R.; Imajo, Y.; Suzuki, H.; Funaba, M.; et al. Biomechanical Analysis of the Spine in Diffuse Idiopathic Skeletal Hyperostosis: Finite Element Analysis. Appl. Sci. 2021, 11, 8944. https://doi.org/10.3390/app11198944
Nishida N, Jiang F, Ohgi J, Fuchida M, Kitazumi R, Yamamura Y, Tome R, Imajo Y, Suzuki H, Funaba M, et al. Biomechanical Analysis of the Spine in Diffuse Idiopathic Skeletal Hyperostosis: Finite Element Analysis. Applied Sciences. 2021; 11(19):8944. https://doi.org/10.3390/app11198944
Chicago/Turabian StyleNishida, Norihiro, Fei Jiang, Junji Ohgi, Masahiro Fuchida, Rei Kitazumi, Yuto Yamamura, Rui Tome, Yasuaki Imajo, Hidenori Suzuki, Masahiro Funaba, and et al. 2021. "Biomechanical Analysis of the Spine in Diffuse Idiopathic Skeletal Hyperostosis: Finite Element Analysis" Applied Sciences 11, no. 19: 8944. https://doi.org/10.3390/app11198944
APA StyleNishida, N., Jiang, F., Ohgi, J., Fuchida, M., Kitazumi, R., Yamamura, Y., Tome, R., Imajo, Y., Suzuki, H., Funaba, M., Chen, X., & Sakai, T. (2021). Biomechanical Analysis of the Spine in Diffuse Idiopathic Skeletal Hyperostosis: Finite Element Analysis. Applied Sciences, 11(19), 8944. https://doi.org/10.3390/app11198944







