Determination of Eleven Veterinary Drugs in Chicken Meat and Liver
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
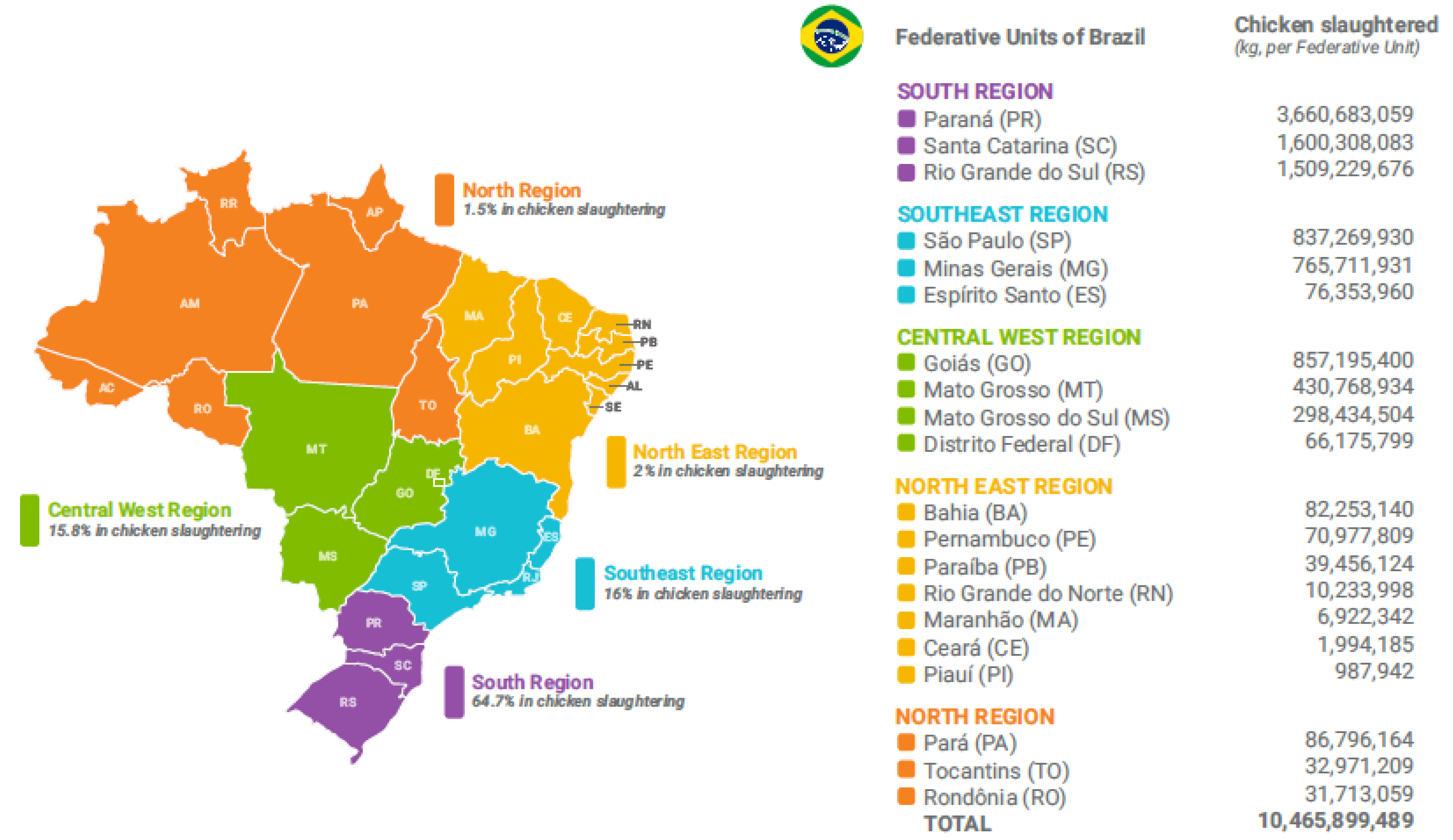
2.1. Sampling Plan
2.2. Chicken Samples Preparation and Chromatographic Analysis
- (a)
- Internal Method POP-QV028/3 (applied to tiamulin, virginiamycin, tylosin, spectinomycin, amoxicillin, trenbolone acetate, halofuginone hydrobromide, dinitolmide + zoalene): The samples were weighed (2.00 ± 0.05 g) and internal standard solution was added. The samples were extracted with acetonitrile:water:acetic acid (70:29:1) v/v then the mixture was manually stirred or vortexed. In case, if further homogenization was needed, ceramic homogenizer or disposable glass sphere were added to the mixture. Afterward, the mixture was placed on a shaking table for 20 min at 200 rpm and then centrifuged at 4000 rpm for 5 min. Then, 500 µL from the extract and 500 µL deionized water were transferred to 15-mL Falcon tubes. These tubes were vortexed, and the solution was filtered through 0.22 µm membrane directly into vial, which were then submitted to LC-MS/MS analysis;
- (b)
- Internal Method POP-QV013/3 (applied to bacitracin and colistin): The samples were weighed (5.00 ± 0.05 g) and internal standard solution was added to these samples, as well as to the calibration curve and blank samples. The samples were extracted with MeOH:H20 (25:75) and 1 mol/L sulfuric acid. Afterward, the mixture was stirred to homogenize it properly. Then, the mixture was placed on a shaking table for 20 min at 200 rpm and then centrifuged at 4000 rpm for 5 min. Then, the samples were frozen (−18 °C) and allowed to rest for 20 min. After that, samples were centrifuged for another 20 min at 4000 rpm and the supernatant was immediately separated to another 50-mL polypropylene tube. The samples were submitted to vacuum-controlled SPE extraction, so that the analytes were allowed to interact with the stationary phase. Afterward, clean-up was performed with deionized water and the cartridges were dried under vacuum in this step. The 15-mL polypropylene tubes were placed under the cartridges and the analytes were eluted with MeOH:Formic acid solution (75:25). The mixture was stirred and filtered through 0.22 µm membrane directly into borosilicate vial, which were then submitted to LC-MS/MS analysis;
- (c)
- AOAC Official Method 2013.06 applied to roxarsone extraction and quantification is well established in the literature [4]. Briefly, the sample was submitted to pressure digestion which occurs using nitric acid in a closed vessel with elevated temperature and pressure by conventional or microwave-assisted heating. Determination occurs using inductively coupled plasma (ICP)/MS [4].
2.3. Statistical Analysis
3. Results and Discussion
3.1. Feed Additives
3.2. Statistical Analyses to Evaluate the Need of Decreasing the National Sampling Plan and Increasing Limit of Non-Compliance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- ABPA Relatório Anual da Associação Brasileira de Proteína Animal. Annual Report from the Brazilian Association of Animal Protein. Available online: http://abpa-br.org/relatorios/ (accessed on 31 August 2021).
- Delatour, T.; Racault, L.; Bessaire, T.; Desmarchelier, A. Screening of veterinary drug residues in food by LC-MS/MS. Background and challenges. Food Addit. Contam. Part A 2018, 35, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Vandenberge, V.; Delezie, E.; Delahaut, P.; Pierret, G.; de Backer, P.; Daeseleire, E.; Croubels, S. Transfer of flubendazole and tylosin from feed at cross-contamination levels to various poultry matrices. Poult. Sci. 2012, 91, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Julshamn, K.; Maage, A.; Norli, H.S.; Grobecker, K.H.; Jorhem, L.; Fecher, P.; Dowell, D. Determination of arsenic, cadmium, mercury, and lead in foods by pressure digestion and inductively coupled plasma/mass spectrometry: First action 2013.06. J. AOAC Int. 2013, 96, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Brazil Secretary of Agricultural Defense. Ordinance No. 87 of 16 August 2018. In Ministry of Agriculture, Livestock and Food Supply (MAPA); Official Gazette of the Federal Government (DOU): Brasilia, Brazil, 16 August 2018. [Google Scholar]
- Brazil Secretary of Agricultural Defense. Sampling Plan for the Meat Chains. Brazilian National Plan for the Control of Residues and Contaminants (PNCRC). Normative Instruction No. 5 of 23 April 2019; Ministry of Agriculture, Livestock and Food Supply (MAPA): Brasilia, Brazil, 2019; Published in the Official Gazette of the Federal Government (DOU). Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/plano-de-nacional-de-controle-de-residuos-e-contaminantes/PNCRC2019SamplingPlan.pdf (accessed on 31 August 2021).
- GSO. Gulf Technical Regulation. Maximum Residues Limits (MRLs) of Veterinary Drugs in Food; GSO: Riyadh, Saudi Arabia, 2015. 2481:2015. Available online: https://www2.sag.gob.cl/pecuaria/establecimientos_habilitados_exportar/normativa/EAU/GSO-2481-2015-en.pdf (accessed on 3 December 2020).
- Commission Regulation of the European Union (EU). No. 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union 2010, 1, 1–72. Available online: https://ec.europa.eu/health/sites/default/files/files/eudralex/vol-5/reg_2010_37/reg_2010_37_en.pdf (accessed on 3 December 2020).
- Ministry of Agriculture (MOA). National Standard No. 235; Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2002. Available online: http://english.agri.gov.cn (accessed on 3 December 2020).
- Health Canada (HC). List of Maximum Residue Limits (MRLs) for Veterinary Drugs in Foods. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/maximum-residue-limits-mrls/list-maximum-residue-limits-mrls-veterinary-drugs-foods.html (accessed on 3 December 2020).
- USA Government Publishing Office. USA Code of Federal Regulation. In Title 21—Food and Drugs. Chapter I—Food and Drug Administration, Department of Health and Human Services. Subchapter E—Animal Drugs, Feeds, and Related Products Part 556—Tolerances for Residues of New Animal Drugs in Food; 2020. Available online: https://www.govinfo.gov/content/pkg/CFR-2020-title21-vol6/pdf/CFR-2020-title21-vol6-chapI-subchapE.pdf (accessed on 3 December 2020).
- Codex Alimentarius. Index of Veterinary Drugs. Maximum Residue Limits (MRLs) and Risk Management Recommendations (RMRs) for Residues of Veterinary Drugs in Food CX/MRL 2-2018. 2018. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/vetdrugs/veterinary-drugs/en/ (accessed on 3 December 2020).
- Ledesma, C.; Rosario, C.; Gracia-Mora, J.; Tapia, G.; Gutiérrez, L.; Sumano, H. Antibacterial activity of amoxicillin in vitro and its oral bioavailability in broiler chickens under the influence of 3 water sanitizers. Poult. Sci. 2018, 97, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Yadav, A.S.; Tripathi, V.; Singh, R.P. Antimicrobial resistance profile of Salmonella present in poultry and poultry environment in north India. Food Control 2013, 33, 545–548. [Google Scholar] [CrossRef]
- Plumb, D.C. Plumb’s Veterinary Drug Handbook, 9th ed.; PharmaVet Inc.: New York, NY, USA; Wiley-Blackwell: Hoboken, NJ, USA, 2018; ISBN 978-1-119-34445-2. [Google Scholar]
- Krueger, L.A.; Spangler, D.A.; Vandermyde, D.R.; Sims, M.D.; Ayangbile, G.A. Avi-Lution ® supplemented at 1.0 or 2.0 g/kg in feed improves the growth performance of broiler chickens during challenge with bacitracin-resistant Clostridium perfringens. Poult. Sci. 2017, 96, 2595–2600. [Google Scholar] [CrossRef]
- Aronson, J.K. The International Encyclopedia of Adverse Drug Reactions and Interactions, 2016. Spectinomycin. In Meyler’s Side Effects of Drugs, 16th ed.; Elsevier: Amsterdam, The Netherlands; Wolters Kluwer Medknow: Mumbai, India, 2016; p. 469. [Google Scholar] [CrossRef]
- Haagsna, N.; Scherpenisse, P.; Simmonds, R.J.; Wood, S.A.; Rees, S.A. High-performance liquid chromatographic determination of spectinomycin in swine, calf and chicken plasma using post-column derivatization. J. Chromatogr. B Biomed. Sci. Appl. 1995, 672, 165–171. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Arsenic-Based Animal Drugs and Poultry. Product Safety Information. 2019. Available online: https://www.fda.gov/animal-veterinary/product-safety-information/arsenic-based-animal-drugs-and-poultry (accessed on 2 December 2020).
- Kawalek, J.C.; Carson, M.; Conklin, S.; Lancaster, V.; Howard, K.; Ward, J.; Farrell, D.; Myers, M.; Swain, H.; Jeanettes, P.; et al. Final Report on Study 275.30: Provide Data on Various Arsenic Species Present in Broilers Treated with Roxarsone: Comparison with Untreated Birds; FDA: Silver Spring, MD, USA, 2011; 39p. Available online: https://www.fda.gov/media/80665/download (accessed on 2 December 2020).
- Wang, X.; Wang, M.; Zhang, K.; Hou, T.; Zhang, L.; Fei, C.; Xue, F.; Hang, T. Determination of virginiamycin M1 residue in tissues of swine and chicken by ultra-performance liquid chromatography tandem mass spectrometry. Food Chem. 2018, 250, 127–133. [Google Scholar] [CrossRef]
- Ribeiro, G.O.; May, M.L.; Parr, S.L.; Schunicht, O.C.; Burciaga-Robles, L.O.; Hannon, S.J.; Grimson, T.M.; Booker, C.W.; McAllister, T.A. Effects of conventional and nonconventional growth-enhancing technologies for finishing feedlot beef steers. Appl. Anim. Sci. 2020, 36, 524–536. [Google Scholar] [CrossRef]
- Liu, J.; Song, S.; Wu, A.; Wu, X.; Xiao, J.; Xu, C. Development of a gold nanoparticle-based lateral-flow strip for the detection of dinitolmide in chicken tissue. Anal. Methods 2020, 12, 3210–3217. [Google Scholar] [CrossRef]
- Alhaji, N.B.; Haruna, A.E.; Muhammad, B.; Lawan, M.K.; Isola, T.O. Antimicrobials usage assessments in commercial poultry and local birds in North-central Nigeria: Associated pathways and factors for resistance emergence and spread. Prev. Vet. Med. 2018, 154, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius. CAC/GL 71-2009: Guidelines for the design and implementation of national regulatory food safety assurance programmes associated with the use of veterinary drugs in food producing animals. Codex Texts 2009. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/guidelines/en/ (accessed on 15 October 2020).
- Brazil Secretary of Agricultural Defense. Normative Instruction No. 42 of 20 December 1999. Brazilian National Plan for the Control of Residues and Contaminants (PNCRC); Ministry of Agriculture, Livestock and Food Supply (MAPA): Brasilia, Brazil, 1999; Published in the Official Gazette of the Federal Government (DOU). Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/plano-de-nacional-de-controle-de-residuos-e-contaminantes/documentos-da-pncrc/instrucao-normativa-sda-n-o-42-de-20-de-dezembro-de-1999.pdf/@@download/file/instrucao-normativa-sda-n-o-4 (accessed on 15 October 2020).

| This Study | MRL in Meat (µg/kg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Residues (µg/kg) * | Number of Samples ** | Brazil a | Middle East b | EU c | China d | Canada e | USA f | CODEX g | ||
| Matrix | Number of Samples | MRL | |||||||||
| Amoxicillin | <10 | 300 | Chicken meat | 600 | 50 | 10 | 50 | 50 | 10 | 10 | 50 |
| Bacitracin | <75 | 300 | Egg | 300 | 500 | 500 | 150 | 500 | 500 | 500 | - |
| Colistin | <75 | 300 | Egg | 300 | 300 | 150 | 150 | 150 | - | - | 150 |
| Dinitolmide and zoalene | <5 | 60 | - | - | - | 3000 | - | 3000 | 3000 | 3000 | - |
| Spectinomycin | <50 | 300 | Kidney | 600 | 5000 | 100 | 300 | 500 | 100 | 100 | 500 |
| Halofuginone hydrobromide | <3 | 60 | - | - | - | 100 | 10 | 10 | 10 | - | - |
| Roxarsone | <70 | 300 | - | - | - | 500 | - | 500 | - | - | - |
| Tiamulin | <25 | 300 | Egg | 300 | 1000 | 100 | 100 | 100 | 100 | - | - |
| Tylosin | <25 | 300 | *** | 600 | 100 | 200 | 100 | 200 | 200 | 200 | 100 |
| 17-α-Trenbolone | <2 | 60 | Swine urine | 60 | 2 | 2000 | - | - | 2 | - | 2 |
| Virginiamycin | <20 | 300 | - | - | - | 200 | 10 | 100 | 100 | 100 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, A.; Novo, C.S.; Feddern, V.; Coldebella, A.; Scheuermann, G.N. Determination of Eleven Veterinary Drugs in Chicken Meat and Liver. Appl. Sci. 2021, 11, 8731. https://doi.org/10.3390/app11188731
Barros A, Novo CS, Feddern V, Coldebella A, Scheuermann GN. Determination of Eleven Veterinary Drugs in Chicken Meat and Liver. Applied Sciences. 2021; 11(18):8731. https://doi.org/10.3390/app11188731
Chicago/Turabian StyleBarros, Amanda, Cauê S. Novo, Vivian Feddern, Arlei Coldebella, and Gerson N. Scheuermann. 2021. "Determination of Eleven Veterinary Drugs in Chicken Meat and Liver" Applied Sciences 11, no. 18: 8731. https://doi.org/10.3390/app11188731
APA StyleBarros, A., Novo, C. S., Feddern, V., Coldebella, A., & Scheuermann, G. N. (2021). Determination of Eleven Veterinary Drugs in Chicken Meat and Liver. Applied Sciences, 11(18), 8731. https://doi.org/10.3390/app11188731






