Abstract
The present study examines the separate and synergistic effects of macroalgal extract and static magnetic field (SMF) on the germination of soybean seeds (Glycine max (L.) Merrill), cv. Abelina, seedling growth, chlorophyll, and carotenoids content in leaves. Algal extract was produced from freshwater green macroalga (Cladophora glomerata) using ultrasound-assisted extraction. The germination tests were conducted in two stages. Firstly, different concentrations of extracts, 20%, 40%, 60%, 80%, and 100%, were applied to a paper substrate. The best results (taking into account germination percentage, seedlings length and weight, and pigments content in leaves) were observed for 20% and 80% extracts. At the same stage, germination of seeds exposed to SMF (exposure times for 3 and 12 min and magnetic inductions of 250 and 500 mT) was studied. The best developed seedlings were determined for the group treated at 3 min with a magnetic induction of 250 mT. In the final step, the simultaneous effects of 20% and 80% algal extracts and treatment with 3 min at 250 mT SMF were tested. Taking into account all the parameters, the simultaneous use of 20% extract and 3 min of 250 mT magnetic induction is recommended.
1. Introduction
Soybeans are among the most important grain legumes worldwide and their cultivation is gaining popularity, which results from the possibility of its versatile use in different industries [1]. Because of its valuable attributes, soybean accounts for a significant amount of the world’s vegetable oil, animal fodder, and food for human consumption [2,3]. As this plant is very popular, new methods of plant growth-promotion/stimulation are still being sought. Stimulation of seeds using different priming methods as a way to increase their vigor for fast and strong plant development, optimized harvest efficiency, and quality of stimulation has caught the interest of many scientists [4,5,6,7,8,9].
One of these methods is stimulation with algal extracts. Scientist have checked the influence of brown, green, and red macroalgae at different concentrations on soybean seed germination [4,5,7,10,11,12,13,14] and growth parameters [15,16,17], and also checked the impact of biofertilizer based on extract from the brown macroalgae Ascophyllum nodosum [18]. The effects of macroalgal extracts on soybean seeds, obtained by many scientists, are presented in Table 1.

Table 1.
The effects of macroalgal extracts on soybean seeds.
Quite strong global climatic fluctuations are responsible for the decline in crop productivity. Chemicals (fertilizers, plant protection products, growth promoters, etc.) and genetic treatments are commonly used to improve yield. Many scientific studies have found that the influence of magnetic field (MF) on plants is an effective method of reducing diseases and increasing the tolerance of these plants to increasingly unfavorable natural environments [7,9,19,20,21,22,23,24,25,26]. Under unfavorable conditions of abiotic stress, such as drought or soil contamination with heavy metals, MFs mitigate the effects of these stresses by increasing the number of antioxidants and, thus, reducing oxidative stress in plants [6,27,28,29]. On the other hand, MF treatments reduce the plant disease index due to modulation of calcium signaling pathways, as well as prolines and polyamines [30,31].
Studying the effects of magnetic fields on biological structures, including plants, is not entirely new. The first works in this area date back to the second half of the 19th century [32]. The first documented work in this area is the publication of Reinke (1876), which presented the results of experiments on the influence of magnetic fields on plant development [33]. In 1893, Tolomei documented the effect of faster germination in a magnetic field; it was also the first work to prove the effect of magnetotropism [34]. This phenomenon, discovered by Tolomei, was then further investigated by Audus, but only in 1960 [35]. One of the important issues indicating the importance of the undertaken research is that the influence of magnetic fields on seeds does not pose any threat to the environment [27], and, as scientific reports show, it really increases the effectiveness of physiological processes. Pre-treatment of seeds before sowing with magnetic fields is considered eco-friendly technology, safe for the environment and plants, since the field only affects seeds to stimulate their germination, which favors better dynamic emergence and good growth of seedlings [23,27,36]. The results of these treatments are greater plant vigor and a higher yield. Examples of SMF impact on soybean seeds are presented in Table 2.

Table 2.
The effects of SMF on soybean seeds.
There are only a few studies about simultaneous use of macroalgal extracts and SMF on early stages of soybean development. Lewandowska et al., investigated the impact of SMF (time of seed exposurefor 3, 6, and 12 min, magnetic inductionof 400 mT) and C. glomerata 10% extract on the soybean seed variety “Merlin” [14]. The best results (germination percentage and chlorophyll content) were observed for simultaneous application of SMF for 12 min and 10% algal extract. Michalak et al., compared the impact of both SMF (time of seed exposure for 3, 6, and 12 min, magnetic induction of 400 mT) and AMF (alternating magnetic field, time of seed exposure for 1, 2.5, and 5 min, magnetic induction of 30 mT, frequency of 50 Hz) combined with 10% C. glomerata extract on the soybean seed variety “Merlin”[9]. The highest germination percentage and chlorophyll content were obtained for simultaneous use of SMF for 3 min and 10% algal extract and AMF at 2.5 min.
The research hypothesis assumes that soybean seed treatment with a static magnetic field and macroalgae extract will have a synergistic effect, manifested by an increase in seed germination, plant length, biomass weight and chlorophyll content in leaves. In contrast to our previous studies, this time, the impact of different Cladophora glomerata extract concentrations and different magnetic inductions were also checked.
2. Materials and Methods
2.1. Soybean Seeds
Soybean seeds (Glycine max (L.) Merrill), cv. Abelina (not genetically modified) were used in the present study. The seeds came from a harvest in 2020 and were certified. The origin of the seed material was obtained from the SAATBAU Polska plant breeding station. “Abelina”—according to the breeders—belongs to the group of early varieties and is distinguished by its high yield potential and exceptional stability over years. It is a purple flowering variety with a dark stigma that reaches very early maturity. Due to its very intensive early vigor, Abelina obtains a medium to high density of plants and is regarded as the best yielding genotype in relation to the length of the vegetation period. In terms of quality, it is a variety with very high-fat content and a high protein yield.
2.2. Macroalga Biomass
The biomass of the freshwater macroalgae Cladophora glomerata was collected from the surface of a pond in the village of Tomaszówek (Łódź Province, Poland) in October, 2016. The macroalgae was identified on the basis of its morphological characteristics, according to the taxonomic literature for the region [49]. The biomass was air-dried and milled using a grinding mill (Retsch GM300, Haan, Germany). For the production of algal extract, biomass with particle sizes lower than 400 μm was chosen (sieve analysis; Retsch, Haan, Germany).
2.3. Production of the Macroalgal Extract
The algal extract was produced according to the methodology described by Michalak et al. [7]. Dry C. glomerata biomass (4 g) was suspended in an aqueous solution (80 mL). The mixture was subjected to an ultrasound homogenizer for 30 min (parameters: 50 W, ultrasonic frequency 30 kHz, amplitude 100%) (UP 50H; Hielscher Ultrasonics, Teltow, Germany). Then, the mixture was centrifuged for 10 min at a rate of 4000 rpm (Megafuge™ 40 Centrifuge Series, Thermo Scientific, Waltham, MA, USA). The obtained supernatant was treated as 100% algal extract. This concentration was used to prepare 20%, 40%, 60%, and 80% extracts. The pH was checked in 3 replications using a pH-meter (Mettler Toledo SevenMulti). As the extract contained organic matter, a fresh portion was produced before germination tests and stored in a refrigerator.
2.4. Pre-Treatment of Soybean Seeds with SMF
In this research, a permanent magnet was used for pre-sowing stimulation of soybean seeds with a static magnetic field. The inductions of the magnetic field were 250 and 500 mT. The exposure times (3 and 12 min) were chosen according to our previous studies [7,14]. All combinations of magnetic inductions and times of exposure were checked.
2.5. Germination Tests
The germination test of soybean seeds was assessed according to the International Seed Testing Association (ISTA) methodology. A paper substrate was used to perform the germination experiment, which was carried out in 4 replications. One replication consisted of 100 soybean seeds. The temperature in the growth chamber was 25 °C. The germination tests were conducted in two stages. In the first stage, different concentrations of extracts, 20%, 40%, 60%, 80%, and 100%, were applied to a paper substrate to select the best one. Independent of concentration, 240 mL of algal extract per replication was added directly to the paper substrate before soybean seeds were sown. The highest number of normal seedlings was observed at 20% and 80% extract, which was used in further experiments (simultaneous seed stimulation with extract and SMF). At the same time, seeds were exposed to the second factor–static magnetic field. The exposure times were 3 and 12 min and the magnetic inductions were 250 and 500 mT. The best developed seedlings were found after treatment for 3 min and a magnetic induction of 250 mT. In the second step, the simultaneous effects of the best (20% and 80%) algal extracts and seed treatments with 3 min at 250 mT SMF were tested. The experimental groups tested in this study are presented in Figure 1.
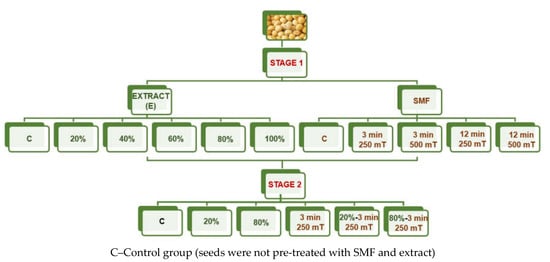
Figure 1.
General scheme of performed experiments.
The counting of soybeans was conducted after 8 days. The seedlings were stored in a growth chamber at a constant temperature of 25 °C. After 8 days, seedlings were carefully extracted from the paper substrate and then divided into normal and abnormal groups, according to ISTA demands. The example of normal and abnormal seedlings is presented in Figure 2. The germination ability was assessed and is given in percentages. The minimum germination requirement was 80% (according to the regulation of The Ministry of Agriculture and Rural Development from 27 May 2020, according to Polish Official Journal from 2 June 2020, item 975). Soybean germination is referred to as epigeal because cotyledons are pulled above the soil surface. Usually, the differences between well-developed and abnormal seedlings are connected with dwarfism, rotten due to primary infection, lack of chlorophyll (albinotic seedling), an irregular hypocotyl shape or its different defects. It should be emphasized that abnormal seedlings simply do not show the ability to develop into a normal, healthy plant (growing in soil under favorable conditions). From all replications, 30 normal seedlings were chosen to measure their root, hypocotyl and epicotyl length, and dried biomass weight.

Figure 2.
Soybean seedlings: normal (3 on the left) and abnormal.
The germination percentage was calculated as number of normal seedlings out of all seedlings. Normal seedlings all have essential structures—the primary root is intact or shows acceptable defects (discolored or necrotic spots, healed cracks and splits, superficial cracks and splits). In addition, a seedling with a defective primary root is classed as normal, if sufficient normal secondary roots have developed. Additionally, the hypocotyl and epicotyl are intact or show acceptable defects: loose twists, superficial cracks and splits, healed cracks and splits. The cotyledons are intact or show acceptable defects—up to 50% of tissue not functioning normally, only one (intact) cotyledons, three cotyledons. Primary leaves are intact or show acceptable defects—only one (intact) primary leaf, three primary leaves, and normal shape but retarded growth. The seedling is abnormal if it is deformed, is fractured, has cotyledons emerging before the primary root, consists of fused twin seedlings, is yellow or white, is spindly or glassy, or is decayed as a result of primary infection.
2.6. Chlorophyll and Carotenoids Measurements
For measurement of the chlorophyll content in soybean leaves, a handheld SPAD chlorophyll meter (Konica, Minolta, Tokyo, Japan) was used, which provided a relative index of chlorophyll content in seedlings. In each experimental group and in each repetition 10 measurements were made from randomly selected seedlings leaves.
For comparison, chlorophyll a, chlorophyll b, and carotenoids contents were me-asured spectrophotometrically (Spectrophotometer Biosens UV 5100, Warsaw, Poland). A total of 100 mg of leaves were grinded in a mortar with 10 mL of 80% acetone (Avantor Performance Materials, Gliwice, Poland). The absorbance was measured at 645, 663, and 470 nm wavelengths, according to the method described by Karthik et al. [50]. The me-asurements were made in duplicate for each replication of the group.
The equations for determining chlorophyll content were described by Arnon [51]:
Chlorophyll a (μg/mL) = 12.7 (A663) − 2.69 (A645)
Chlorophyll b (μg/mL) = 22.9 (A645) − 4.68 (A663)
Total chlorophyll (μg/mL) = 20.2 (A645) + 8.02 (A663)
The equation for determining carotenoids content was described by Lichtenthaler and Welburn [52]:
Carotenoids (μg/mL) = (1000 (A470) − 3.27 [Chl a] − 104 [Chl b])/227
2.7. Statistical Analysis
The measured values were elaborated statistically using Statistica ver. 13.0 (TIBCO Software Inc., Tulsa, OK, USA). Descriptive statistics (average and standard deviations or median and quantiles) for all experimental groups was performed. The normality of the distribution of experimental results was assessed using the Shapiro–Wilk test and the homogeneity of variances using the Brown–Forsythe’s test. Based on these tests, the statistical test, which is used to investigate the significance of differences between the tested groups, was selected. The differences between several groups were investigated with the one-way analysis of variance (ANOVA) using the Tukey multiple comparison test (for normal distribution and the homogeneity of variances) or the Kruskal–Wallis test (for lack of the normal distribution or lack of the homogeneity of variances). The results were considered significantly different when p < 0.05.
3. Results
In the present study, we examined the effect of macroalga C. glomerata extracts (20%, 40%, 60%, 80%, and 100%) and SMF pre-treatment (250 and 500 mT; 3 and 12 min), separately and simultaneously, on the germination on soybean seeds and the quality of germinated seedlings by measuring the number of normal and abnormal seedlings, the relative index of chlorophyll content in their leaves and dried biomass weight.
3.1. Characteristics of Macroalgal Extract
The obtained macroalgal extract has a naturally dark olive-green color. The average pH of 100% extract was 7.30 ± 0.04 in the first stage and 7.55 ± 0.02 in the second one, which is similar to the pH of C. glomerata extract obtained in previous studies, where it was 7.50 [7] and 7.58 [14].
3.2. Germination Tests
In the present research, soybean seeds were subjected to biological (algal extract) and physical (SMF) factors. The results of the germination percentage, chlorophyll and carotenoids content, plant length, and dried biomass weight measurements (mean and standard deviation or median) for soybeans stimulated with algal extracts are presented in Table 3a, for soybean pre-treated with SMF in Table 3b, and for soybean treated simultaneously with algal extract and SMF in Table 3c.

Table 3.
Germination percentage, plant growth parameters and the content of chlorophyll and carotenoids in leaves for treatment of soybean with (a) Cladophora glomerata extract (E), (b) static magnetic field (SMF), and (c) combination of both factors (E + SMF).
The effects of Cladophora glomerata extract, applied in concentrations of 20%, 40%, 60%, 80%, and 100%, on soybean traits are presented in Table 3a. The germination percentage for all extract concentrations was higher than in the control group. Statistically significant differences were observed between the control group and extract concentrations—20% and 80%—for E 20% it was higher by 26.5% and for E 80% by 29.4% than in the control. Generally, C. glomerata extract positively influenced soybean length–hypocotyl and epicotyl length, as well as root length. Hypocotyl length for all extract concentrations (besides 40%) was higher than in the control group. For this parameter, the best results were obtained for E 20% and E 40% (6.6% higher than in C) and for E 80% (9.4% higher than in C, a statistically significant difference). Epicotyl length greater than in the control group was observed for E 20% (by 19.2%), E 60% (by 7.7%), and E 80% (by 15.4%). Cladophora glomerata extract also stimulated root growth, besides E 80%, when compared with the control group. The longest roots were measured in the E 60% group—longer by 13.1% than C. No significant effect of the extract on the dry weight of plants was observed. Slightly higher dry biomass than in C was found for E 20% and E 100%. All tested concentrations of algae extract increased the chlorophyll content (SPAD measurements) in soybean seedlings when compared to the control group. The highest increase in SPAD value was observed for E 20% (higher by 9.2% than in C) and E 80% (higher by 10.7% than in C). The same concentrations turned out to be the most beneficial in the case of spectrophotometric measurements—for E 20%, the total chlorophyll increased by 7.2% compared to C and for E 80% by 27.0%. The content of carotenoids in soybean seedlings was lower for all groups than in the control group. Comparing the effects of all concentrations of the algae extract on soybean growth and characteristics, two concentrations can be recommended for further research—20 and 80%. However, taking into account the similar values of the soybean parameters for these two concentrations, in further research, 20% extract was used (due to consumption of lower amounts of biomass for extraction, shorter extraction time, etc.).
Table 3b presents the effect of soybean seeds treatment before sowing with the static magnetic field on the plant growth and the pigments content. SMF applied for two different times (3 and 12 min) and doses (250 and 500 mT) did not increase the germination percentage of seeds when compared to the control group. Taking into account the plant length, the stimulatory effects of SMF were observed for the shorter exposure time—3 min. For this time, and for both inductions (250 and 500 mT) root, hypocotyl, and epicotyl lengths were greater than in the control group. For the 3 min and 250 mT group, root length was 18.8% greater than in the control group. For all experimental groups with SMF, the dry soybean biomass was slightly higher than in the control group (max. by 5.4% for 12 min 500 mT). SMF had a positive effect on the pigment contents (chlorophyll and carotenoids) in soybean. Chlorophyll content measured with SPAD was highest for 3 min 500 mT and 12 min 250 mT, greater by 41.9% and 43.5%, respectively, than in the control group. Total chlorophyll was the highest for 3 min 250 mT (83.6% higher than for C). In the case of carotenoids, the highest content was measured for the 3 min 250 mT group and was almost 5 times higher than in the control group. All seedlings pre-treated with SMF had extensive lateral roots. Most of the seedlings in the 12 min 250 mT group had blue discoloration (gray).
Table 3c presents the effects of simultaneous treatment of soybean seeds with algal extract (20% and 80%) and a static magnetic field (3 min 250 mT) on plant growth and the pigment contents. The germination percentage was improved for all the tested groups as compared to C. Plant length was greater than in C only for two groups: E 20% with SMF (by 8.1%) and SMF (by 7.4%). Hypocotyl length was the greatest for the treatment with E 20% and SMF together (9.1% higher than for C) and epicotyl length for treatment with SMF alone (40% higher than for C). Root length was improved only for 2 groups—E 20% with SMF (3.8% higher than for C) and SMF (7.7% higher than for C). Plant weight was not statistically significantly affected by any treatment. Chlorophyll content measured by SPAD was lower for all tested groups than in the control. However, the differences were not statistically significant. Total chlorophyll measured with the spectrophotometric method was improved for all groups. The best results were found for E 80% (1.5 times higher than for C). Carotenoids content was improved for most groups (except E 80% with SMF). The most beneficial results were obtained for E 80% (3 times higher than for C).
4. Discussion
The application of algal extract and pre-sowing treatment of seeds with a static magnetic field can be an alternative choice and supplementation to synthetic chemicals, which can have negative effects on growers, consumers, and the environment. In the literature, the effects of these two factors on soybeans are usually discussed separately. In the present study, their synergistic action was evaluated, which was preceded by the selection of the optimal concentration of algae extract (20%, 40%, 60%, 80%, and 100%), as well as the induction of a static magnetic field (250 and 500 mT) and exposure time (3 and 12 min).
The positive effect of Cladophora glomerata extract on the germination of soybean seeds and seedling development may result from the composition of the algal biomass. As shown in a previous review [53], this macroalga can serve as a source of micro- and macroelements (ash from 2.44% to 39.2%), proteins (from 10.7% to 22.5%), carbohydrates (from 58.4% to 61.7%), vitamins A, B1, B2, C and E, and other antioxidant molecules, such as polyphenols and pigments–carotenoids, chlorophylls. The examined extract from Cladophora glomerata consists of micro- (Cu–0.1726 mg/L, Mn–1.314 mg/L, Zn–0.2614 mg/L, Fe–4.285 mg/L,) and macroelements (Ca–185.6 mg/L, K–829.7 mg/L, Mg–28.92 mg/L, P–19.87 mg/L, S–220.2 mg/L) [7]. Obtained algal extracts, due to their complex biochemical compositions, have broad effects on plants and can protect them from biotic and abiotic stress, enhance nutrient use efficiency, and stimulate beneficial microbiome of plants [10,54]. The effect of algal extracts on cultivated plants depends on many factors, such as type of biostimulant (e.g., seaweed species used for production, harvest season of algal material), biostimulant dose or frequency of application, etc. [4,5,15].
In the preliminary tests, the selection of the appropriate concentration of the tested preparation for stimulating plant growth is essential. The present study indicates that examined concentrations of C. glomerata extract (20%, 40%, 60%, 80%, and 100%) had no phytotoxic effects on soybean germination and growth. Germination percentages in all experimental groups were higher than in the control group, but 20% and 80% concentrations were outstanding. In our previous study, 20% and 80% extracts of C. glomerata also provided the highest germination ability of carrot seedlings [9]. Mathur et al., tested extract obtained from green macroalga—Enteromorpha intestinalis—and applied it at different concentrations—20%, 40%, 60%, and 100%—on soybeans [10]. In this case, the highest germination percentage (100%) was observed for 60% extract, whereas the lowest for concentrations, 20% and 100%, were both 40%. In the case of the control group, the germination percentage was only 20% and for the group treated with chemical fertilizer it was 32%.
In the present study, the highest content of chlorophyll a and b, as well as carotenoids was determined for 80% C. glomerata extract. It aligns with the results presented by Mathur et al., who observed the increase in the content of chlorophyll a and b, as well as carotenoids in soybean with the increase in the concentration of Enteromorpha intestinalis extract (20%, 40%, and 60%) and a decrease for the 100% extract [10]. The application of Biozyme formulation, prepared from Ascophyllum nodosum, at a dose of 500 mL/ha significantly influenced the total chlorophyll content in soybean [55]. However, Joshi-Paneri et al., showed that treatment of soybean seeds with commercial preparation of Stimulagro (liquid extract of Ascophyllum nodosum) at small doses (0.5, 1.0, and 2.0 g/L) had no effect on the chlorophyll content expressed in SPAD units and was comparable to the control group. The enhancement of the leaf chlorophyll level in plants is a well-known property of algal extracts acting as biostimulants for plant growth [56].
Examined extracts from Cladophora glomerata had no statistically significant effect on soybean dry mass as compared to the control group, but the highest values were obtained for concentrations 20% (3.52 g) and 100% (3.66 g). Joshi-Paneri et al., who tested an extract from Ascophyllum nodosum at much smaller doses of 0.5, 1.0, and 2.0 g/L on soybean also did not observe an effect on soybean dry mass [56]. Algal-based biostimulants are efficient, not only in germination tests performed under controlled conditions, but also under real field conditions. Rathore et al., showed that among tested concentrations of extracts prepared from red seaweed (Kappaphycus alvarezii at 2.5, 5.0, 7.5, 10, 12.5, and 15%), the last concentration used as a foliar spray provided the highest grain yield (by 57% as compared to the control) of soybean grown under rainfed conditions [4]. Tandon and Dubey also confirmed that the application of extract from Ascophyllum nodosum greatly influenced grain, straw, and total biological yield (grain + stover yield) [55].
Seaweed extracts are used in agriculture as a foliar spray or also as a soil drench to stimulate seedling rooting [15]. In the present study, root length in all experimental groups (except 80%) was higher than in the control group and the best results were obtained for the 60% extract. The response of the soybean to the Cladophora glomerata extract was consistent with that observed in the work of Mathur et al., where root length increased with an increase in Enteromorpha intestinalis extract concentration (20%—4.5 cm, 40%—5.0 cm, 60%—6.6 cm) and decreased for 100% extract—5.2 cm; however, all values were higher than in the control group [10]. Chaikina et al., tested the effects of different concentrations of water-soluble marine algal extracts (from 10−2 g/mL to 10−13 g/mL) including the green algae—Ulva fenestrata and Codium fragile—on the root growth in soybean seedlings and concentrations of 10−2 g/mL exerted an inhibitory effect [16]. For Codium fragile, the highest stimulatory effect on root length was noted for the concentrations of 10−5 g/mL and roots were longer by 18.0% than the control roots [15]. Promotion of soybean root growth is crucial because it enables to obtain seedlings that mature in fewer days after sowing and avoid damage caused by soil pathogens [16].
Algal extracts used as biostimulants of plant growth are known to increase soybean length. Mathur et al., observed that the application of 100% extract from the green algae, Enteromorpha intestinalis (among tested concentrations of 20%, 40%, and 60%), had the greatest effect on shoot length (5.7 cm), which was 63% higher than in the control group. In the present study, comparable results were obtained for 20% (14.4 cm), 60% (14.1 cm), and 80% (14.6 cm) extracts [10]. In the work of Rathore et al., the greatest soybean height was measured for the highest tested concentrations of Kappaphycus alvarezii extract (15% among others 2.5%, 5.0%, 7.5%, 10%, 12.5%) and was 16.8% higher than in the control group [4]. Rathore et al., and Tandon and Dubey also paid attention to another property of algal biostimulants, that they can increase the uptake of nutrients by soybeans, probably due to enhanced root growth (confirmed in this study) and also can enhance the effectiveness of fertilizers [4,55]. In a previous study, we confirmed that carrot seeds treated with an extract from Cladophora glomerata were biofortified with macro- Ca, K, Mg, and S, and microelements Cu, Fe, Mn, and Zn [9]. There are also examples of the influence of much lower concentrations of the tested biostimulants on the growth and development of soybean in the literature. The presented data are inconsistent, which means that for each type of biostimulant, preliminary tests should be carried out to select the optimal concentrations. Kocira et al., showed that commercial extract from Ecklonia maxima (Kelpak) increased soybean height, especially when was applied once at a higher concentration (1% vs. 0.7%), which was 35% greater compared to the control [5]. In the case of the application of extract from brown seaweed—Ascophyllum nodosum—the shoot height decreased slightly with the increase in extract dose (0.5 g/L (25.3 cm), 1.0 g/L (24.2 cm) and 2.0 g/L (24.1 cm)), but was higher than in the control group (22.7 cm) [56].
In the literature, there are some single examples of the simultaneous treatment of soybean with a mixture of extracts. For example, Joshi-Paneri et al., tested the combination of two extracts, from the seaweed Ascophyllum nodosum and from medicinal herb–celandine (Chelidonium majus), both at a dose of 1 g/L [56]. This treatment significantly increased shoot height (13.2%), dry mass (10.7%), and photosynthetic rate (20.3%) compared with the control. This combination also gave better results than when these preparations were used separately.
In the present study, we propose the combination of algal extract and physical factors, which are used for pre-sowing treatment of seeds. Scientific works show that the effects of a magnetic field on biological objects placed in are is manifold. We can distinguish electrodynamic interactions with electric currents occurring in organisms, the formation of magnetomechanical effects inside organisms consisting in the orientation of structures with magnetic anisotropy in homogeneous fields and the displacement of ferromagnetic and paramagnetic substances in fields with non-zero gradients, the effect on uncompensated magnetic spins of elements of paramagnetic radicals and free spins of paramagnetic radicals and free spins, the Dorfman effect, consisting in a reorientation of proteins in a magnetostatic field due to the anisotropy of these molecules, and some components of living organisms exhibit magnetostrictive properties. Therefore, there is a possibility of influencing such components as the magnetic field changes the energy of intra- and inter-atomic interactions in living organisms, the sinusoidal alternating magnetic field causes the induction of currents inside living organisms, and the magnetic field may exert an influence on the depolarization of cells [57,58,59,60]. As a result of the penetration of the magnetic field through a living organisms, resonance phenomena can appear, which can take place, not only in the extracellular space, but also in the cell membrane (i.e., also in ion channels) and inside a cell. Due to the presence of water in living organisms, the interaction with a magnetic field in this context is also important. Water that is subjected to an external magnetic field changes its properties—the rate of crystallization, the concentration of dissolved gases, the rate of coagulation, and settling of suspensions increase. In addition, the pH and the wetting capacity change [29,61,62,63]. In many scientific papers, researchers have shown that a magnetic field changed the characteristics of cell membranes, influenced cell reproduction, and caused certain changes in cell metabolism. At the same time, it was pointed out that a magnetic field influences the growth characteristics and various cell functions, such as the amount of mRNA, gene expression, protein biosynthesis, and enzyme activity, and causes changes in various functions at the organ and tissue levels [29,38,64].
The hypothesis indicating the importance of paramagnetic properties of some atoms in plant cells and pigments, such as chloroplasts, is extremely interesting. In an external magnetic field, the magnetic moments of these atoms change the direction of the field. The magnetic properties of particles determine their ability to absorb and then convert the energy of a magnetic field into different types of energy and then transfer this energy to other structures in plant cells, activating them in this way [62,65,66,67].
Many authors in their scientific publications have shown the positive effects of a stationary magnetic field on plant seeds. Such an influence accelerates plant development [65,68,69], improves seedling germination and growth [70,71,72], and activates protein formation and enzyme activity [27,42,45,72,73,74]. Studies have also shown that stimulating seeds with a magnetic field before sowing increases the germination of difficult-to-germinate seeds and improves quality [75,76,77]. The experiments were and are carried out on a wide range of plants, such as cereals [78,79,80,81,82,83,84,85], legumes [84,86,87], and perennials [88,89,90,91].
Recently, more studies have been carried out on the effects of magnetic field biosti-mulation for soybeans. The effects, in terms of seed stimulation before sowing, were compared by measuring the content of sugar, protein, nitrogen, hydrogen peroxide, ascorbic acid, proline, phenol, malondialdehyde, and chlorophyll, as well as enzymatic activities (protease, amylase, catalyst, superoxide dismutase, and peroxides). Most of the publication authors used constant magnetic fields with the induction of several dozen to several hundred mTs [7,14,27,39,40,41,43,44,46]. The time for seed exposure relative to the magnetic field ranged from a few to several dozen minutes in a single or divided dose over several days [47,48]. The stimulated soybean seeds using SMF showed a higher germination capacity and energy compared to the control sample. Moreover, this stimulation had a statistically significant effect on the development of the root system and the content of chlorophyll in the tested seedlings. The dry mass content of plants in general also increased. This was also confirmed by the current research (Table 3); however, an increase in the magnetic field induction over 250 mT slows down this effect, and at higher values, it even works the other way around.
There are also attempts to use magnetic fields with low induction values (a few mT) for seed dressing in a short exposure time (counted in seconds) [38,42]. Some scientists have studied the effect of a magnetic field with very low induction values, which comes directly from the Earth (geomagnetic field, GMF) on plants [92,93,94,95,96]. Such an influence has a positive effect on plant physiology and on development. Scientists have noticed a beneficial effect on shoot formation, root system development, plant dry biomass, and an increase in chlorophyll a and b and total chlorophyll compared to control samples.
Many authors have tried to influence seeds with a magnetic field in combination with another physical field (e.g., electric or electromagnetic field) [77,97,98]. These studies were carried out to evaluate the effect of seed treatment on sugar, protein, nitrogen, hydrogen peroxide, ascorbic acid, proline, phenoldialdehyde, and the content of chlorophyll (Chl a, b and total content of chlorophyll). These works also investigated the specific activity of enzymes, such as protease (PRT), amylase (AMY), catalyst (CAT), superoxide dismutase (SOD), and peroxides (POD). The specific activity of enzymes (during germination and early growth) on biochemical and chlorophyll content were statistically significantly increased as a result of these combined pre-sowing procedures. The effect of stimulating seeds with a magnetic field was usually greater than that of stimulation with another physical field, except for PRT, AMY, and ascorbic acid. However, the effects of both treatments (simultaneously) were much greater compared to the control (seeds that had not been subjected to any pre-sowing treatment). The results of the conducted research have shown that combined pre-sowing seed stimulation treatments enables the potential to enhance soybean biological moieties, chlorophyll contents, and metabolically important enzymes [98,99].
Not only the application of different plant extracts or different physical fields can stimulate plant growth, but also their combinations. In our previous study, we compared the effects of Cladophora glomerata (10%) and two types of magnetic fields, static magnetic field (induction of 400 mT and exposure time 3, 6, and 12 min) and alternating magnetic field (frequency of 50 Hz, an induction of 30 mT and exposure time 1, 2.5, and 5 min), on the germination of soybean seeds and chlorophyll content in seedlings. Synergistic action of algal extract and pre-sowing stimulation of seeds with static magnetic field for 3 min and alternating magnetic field for 2.5 min provided the best results [7,14]. The simultaneous use of 10% algal extract and 3, 6 and 12 min pre-treatment of seeds with 400 mT SMF improved germination percentage as compared to the control and SMF alone. For a 12 min stimulation time, it was also improved as compared to extract alone [14]. According to our research, only simultaneous use of extract and a 3 min SMF stimulation time resulted in higher germination percentage compared to extract alone, SMF, and the control group [7]. In the present study, we found an improvement in the number of germinated seeds for the combination of 20% extract and 3 min 250 mT SMF compared to all the other groups.
In a previous study, we also checked chlorophyll content measured with SPAD chlorophyll meter. Treatment with the combination of 10% extract and 3 min 400 mT SMF improved this parameter compared to all other groups [7]. In the present research, for the use of both stimulating factors together, we did not find any improvement in chlorophyll content measured using a SPAD. However, simultaneous use of 20% extract with SMF and 80% with SMF resulted in higher chlorophyll b and total chlorophyll. The first group also improved carotenoids level.
No prior studies analyzed the influence of algal extract and SMF together on plant and root length, or also plant weight. According to the present research, simultaneous use of 20% extract and 3 min 250 mT SMF improved both root and plant length. In the case of hypocotyl length, the difference was statistically significant. For the 80% extract, there was no difference in root and hypocotyl length, but, for epicotyl length, there was a statistically significant decrease. The applied treatment did not cause any significant changes in plant weight for any of the extract concentrations.
5. Conclusions
In the present paper, C. glomerata, extract tested at five concentrations (20%, 40%, 60%, 80%, and 100%), and SMF seed pre-treatment at two different times (3 and 12 min) and two magnetic induction values (250 and 500 mT) were used to check their impact on soybean (Glycine max (L.) Merrill), cv. Abelina seedlings growth. Both factors (especially 20% extract and 3 min 250 mT SMF used together) improved the analyzed parameters (germination percentage, chlorophyll, and carotenoids content, root length, hypocotyl length, epicotyl length, whole plant length, and dry biomass weight). To date, there are many studies concentrated on the positive impact of both the different types of macroalgal extract and SMF on seeds germination and plant growth. However, there are still very few studies about the results of using these both factors simultaneously. The novelty in our research was also comparing the impact of different concentrations of C. glomerata on soybean seeds germination as well as checking the impact of higher magnetic induction (500 mT) than in studies done by other researchers (up to 400 mT). The effectiveness of the proposed combination of 20% algal extract and seed treatment with SMF (3 min 250 mT) will be examined in field trials.
Author Contributions
Conceptualization, K.D., S.L., J.D. and I.M.; methodology, K.D., S.L., R.M., M.P., J.D. and I.M.; software, K.D., I.M.; validation, S.L. and R.M.; formal analysis, K.D. and I.M.; investigation, K.D., S.L., R.M., M.P., J.D. and I.M.; resources, S.L., J.D. and I.M.; data curation, K.D.; writing—original draft preparation, K.D.; writing—review and editing, S.L., J.D. and I.M.; visualization, K.D., I.M., S.L.; supervision, S.L., J.D. and I.M.; project administration, S.L. and I.M.; funding acquisition, S.L. and I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre in Poland (grant entitled: “Eco-friendly technologies for the management of seaweed biomass for products useful for sustainable agriculture and biosorbents used for the removal of heavy metal ions from the environment”, No 2019/33/B/NZ9/01844) and the National Centre for Research and Development in Poland (grant entitled: “Increasing productivity and sustainability of European plant protein production by closing the grain legume yield gap”, No SUSCROP/I/LegumeGap/01/2019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Griebsch, A.; Matschiavelli, N.; Lewandowska, S.; Schmidtke, K. Presence of Bradyrhizobium sp. under continental conditions in Central Europe. Agriculture 2020, 10, 446. [Google Scholar] [CrossRef]
- Young, V.R. Soy protein in relation to human protein and amino acid nutrition. J. Am. Diet. Assoc. 1991, 91, 828–835. [Google Scholar] [CrossRef]
- Henley, E.C.; Kuster, J.M. Protein quality evaluation by protein digestibility corrected amino acid scoring. Food Technol. 1994, 48, 74–77. [Google Scholar]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zodape, S.T.; Patolia, J.S. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kuboń, M.; Czerwińska, E.; Piskier, T. Morphological and biochemical responses of Glycine max (L.) Merr. to the use of seaweed extract. Agronomy 2019, 9, 93. [Google Scholar] [CrossRef]
- Kataria, S.; Rastogi, A.; Bele, A.; Jain, M. Role of nitric oxide and reactive oxygen species in static magnetic field pre-treatment induced tolerance to ambient UV-B stress in soybean. Physiol. Mol. Biol. Plants 2020, 26, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Lewandowska, S.; Niemczyk, K.; Detyna, J.; Bujak, H.; Arik, P.; Bartniczak, A. Germination of soybean seeds exposed to the static/alternating magnetic field and algal extract. Eng. Life Sci. 2019, 19, 986–999. [Google Scholar] [CrossRef]
- Lewandowska, S.; Łoziński, M.; Marczewski, K.; Kozak, M.; Schmidtke, K. Influence of priming on germination, development, and yield of soybean varieties. Open Agric. 2020, 5, 930–935. [Google Scholar] [CrossRef]
- Michalak, I.; Bartniczak, A.; Baśladyńska, S.; Lewandowska, S.; Detyna, J.; Łoziński, M.; Niemczyk, K.; Bujak, H. Cladophora glomerata extract and static magnetic field influences the germination of seeds and multielemental composition of carrot. Ecol. Chem. Eng. 2020, 27, 629–641. [Google Scholar] [CrossRef]
- Mathur, C.; Rai, S.; Sase, N.; Krish, S.; Jayasri, M.A. Enteromorpha intestinalis derived seaweed liquid fertilizers as prospective biostimulant for Glycine max. Braz. Arch. Biol. Technol. 2015, 58, 813–820. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Hara, P.; Treder, K.; Findura, P.; Bartoš, P.; Filip, M. Biochemical and economical effect of application biostimulants containing seaweed extracts and amino acids as an element of agroecological management of bean cultivation. Sci. Rep. 2020, 10, 17759. [Google Scholar] [CrossRef]
- Kocira, S.; Kocira, A.; Kornas, R.; Koszel, M.; Szmigielski, M.; Krajewska, M.; Szparaga, A.; Krzysiak, Z. Effects of seaweed extract on yield and protein content of two common bean (Phaseolus vulgaris L.) cultivars. Legum. Res. Int. J. 2018, 41, 589–593. [Google Scholar] [CrossRef]
- Michalak, I.; Lewandowska, S.; Detyna, J.; Olsztyńska-Janus, S.; Bujak, H.; Pacholska, P. The effect of macroalgal extracts and near infrared radiation on germination of soybean seedlings: Preliminary research results. Open Chem. 2018, 16, 1066–1076. [Google Scholar] [CrossRef]
- Lewandowska, S.; Michalak, I.; Niemczyk, K.; Detyna, J.; Bujak, H.; Arik, P. Influence of the static magnetic field and algal extract on the germination of soybean seeds. Open Chem. 2019, 17, 516–525. [Google Scholar] [CrossRef]
- Anisimov, M.M.; Chaikina, E.L. Effect of seaweed extracts on the growth of seedling roots of soybean (Glycine max ( L.) Merr.) seasonal changes in the activity. Int. J. Curr. Res. Acad. Rev. 2014, 2, 19–23. [Google Scholar]
- Chaikina, E.L.; Dega, L.A.; Pislyagin, E.A.; Anisimov, M.M. Effects of water soluble extracts from marine algae on root growth in soybean seedlings. Russ. Agric. Sci. 2015, 41, 11–13. [Google Scholar] [CrossRef]
- da Costa, M.A.; Alves, H.J.; Alab, J.C.; Albrecht, L.P.; Albrecht, A.J.P.; Marra, B.M. Kappaphycus alvarezii extract used for the seed treatment of soybean culture. Afr. J. Agric. Res. 2017, 12, 1054–1058. [Google Scholar] [CrossRef]
- Grigore, A.E.; Sîrbu, C.E.; Cioroianu, T.M.; Dumitru, M.; Nicu, E. Fertilizers with natural organic substances—Development and effect on soybean yield. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, Albena, Bulgaria, 28 June–7 July 2019; Volume 19, pp. 217–223. [Google Scholar]
- Radhakrishnan, R.; Kumari, B.D.R. Influence of pulsed magnetic field on soybean (Glycine max L.) seed germination, seedling growth and soil microbial population. Indian J. Biochem. Biophys. 2013, 50, 312–317. [Google Scholar]
- Radhakrishnan, R.; Kumari, B.D.R. Pulsed magnetic field: A contemporary approach offers to enhance plant growth and yield of soybean. Plant Physiol. Biochem. 2012, 51, 139–144. [Google Scholar] [CrossRef]
- Poinapen, D.; Brown, D.C.W.; Beeharry, G.K. Seed orientation and magnetic field strength have more influence on tomato seed performance than relative humidity and duration of exposure to non-uniform static magnetic fields. J. Plant Physiol. 2013, 170, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Pietruszewski, S.; Szecówka, P.S.; Kania, K. Effect of pre-sowing magnetic stimulation on germination of kernels of various spring wheat varieties. Acta Agrophys. 2013, 20, 415–425. [Google Scholar]
- Podleśny, J.; Pietruszewski, S. The effect of magnetic stimulation of seeds on growth and cropping of seed pea grown at varying soil moisture content. Agric. Eng. 2007, 11, 207–212. (In Polish) [Google Scholar]
- Matwijczuk, A.; Kornarzyński, K.; Pietruszewski, S. Effect of magnetic field on seed germination and seedling growth of sunflower. Int. Agrophys. 2012, 26, 271–278. [Google Scholar] [CrossRef]
- Barnes, F.S. Mechanisms for electric and magnetic fields effects on biological cells. IEEE Trans. Magn. 2005, 41, 4219–4224. [Google Scholar] [CrossRef]
- Blanchard, J.P. Modeling biological effects from magnetic fields. IEEE Aerosp. Electron. Syst. Mag. 1996, 11, 6–10. [Google Scholar] [CrossRef]
- Kataria, S.; Baghel, L.; Jain, M.; Guruprasad, K.N. Magnetopriming regulates antioxidant defense system in soybean against salt stress. Biocatal. Agric. Biotechnol. 2019, 18, 101090. [Google Scholar] [CrossRef]
- Binhi, V.N. Theoretical concepts in magnetobiology. Electro. Magnetobiol. 2001, 20, 43–58. [Google Scholar] [CrossRef]
- Ghodbane, S.; Lahbib, A.; Sakly, M.; Abdelmelek, H. Bioeffects of static magnetic fields: Oxidative stress, genotoxic effects, and cancer studies. Biomed Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Plant responses to electromagnetic fields. In Biological and Medical Aspects of Electromagnetic Fields; Greenebaum, B., Barnes, F., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 89–109. ISBN 9780849395383. [Google Scholar]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Pietruszewski, S. The application of magnetic field as ecological method of quality culture improvement. Pol. J. Food Eng. 2013, 5, 25–33. (In Polish) [Google Scholar]
- Reinke, J. Untersuchungen der Wachstrum. Botan. Ztg. 1876, 34, 183–195. [Google Scholar]
- Tolomei, G. Anzione del magnetismo sullagerminazione. Malpighia 1893, 7, 183–195. [Google Scholar]
- Audus, L.J. Magnetotropism: A New Plant GrowthRespense. Nature 1960, 185, 132–134. [Google Scholar] [CrossRef]
- Podleśny, J.; Podleśna, A.; Gładyszewska, B.; Bojarszczuk, J. Effect of pre-sowing magnetic field treatment on enzymes and phytohormones in pea (Pisum sativum L.) seeds and seedlings. Agronomy 2021, 11, 494. [Google Scholar] [CrossRef]
- Phirke, P.S.; Patil, M.N.; Umbarkar, S.P.; Dudhe, Y.H. The application of magnetic treatment to seeds: Methods and responses. Seed Sci. Technol. 1996, 24, 365–373. [Google Scholar]
- Atak, Ç.; Emiroğlu, Ö.; Alikamenğlu, S.; Razkoulieva, A. Stimulation of regeneration by magnetic field in soybean (Glycine max L. Merrill) tissue cultures. J. Cell Mol. Biol. 2003, 2, 113–119. [Google Scholar]
- Shine, M.B.; Guruprasad, K.N.; Anand, A. Enhancement of germination, growth, and photosynthesis in soybean by pre-treatment of seeds with magnetic field. Bioelectromagnetics 2011, 32, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Shine, M.B.; Guruprasad, K.; Anand, A. Effect of stationary magnetic field strengths of 150 and 200 mT on reactive oxygen species production in soybean. Bioelectromagnetics 2012, 33, 428–437. [Google Scholar] [CrossRef] [PubMed]
- García, A.S.; Reina, F.G.; Franco, Y.P.; Páez, D.D. Stimulation of germination and growth in soybean seeds by stationary magnetic field treatment. Asian J. Agric. Biol. 2013, 1, 85–90. [Google Scholar]
- Atak, Ç.; Çelik, Ö.; Olgun, A.; Alikamanoğlu, S.; Rzakoulieva, A. Effect of magnetic field on peroxidase activities of soybean tissue culture. Biotechnol. Biotechnol. Equip. 2007, 21, 166–171. [Google Scholar] [CrossRef][Green Version]
- Baghel, L.; Kataria, S.; Guruprasad, K.N. Static magnetic field treatment of seeds improves carbon and nitrogen metabolism under salinity stress in soybean. Bioelectromagnetics 2016, 37, 455–470. [Google Scholar] [CrossRef]
- Kataria, S.; Baghel, L.; Guruprasad, K.N. Pre-treatment of seeds with static magnetic field improves germination and early growth characteristics under salt stress in maize and soybean. Biocatal. Agric. Biotechnol. 2017, 10, 83–90. [Google Scholar] [CrossRef]
- Kataria, S.; Baghel, L.; Guruprasad, K.N. Alleviation of adverse effects of ambient UV stress on growth and some potential physiological attributes in soybean (Glycine max) by seed pre-treatment with static magnetic field. J. Plant Growth Regul. 2017, 36, 550–565. [Google Scholar] [CrossRef]
- Baghel, L.; Kataria, S.; Guruprasad, K.N. Effect of static magnetic field pretreatment on growth, photosynthetic performance and yield of soybean under water stress. Photosynthetica 2018, 56, 718–730. [Google Scholar] [CrossRef]
- Payez, A.; Ghanati, F. Comparison of static and electromagnetic field effects on redox system of soybean (Glycine max L. Merrill) seedlings. J. Plant Process. Funct. 2018, 6, 1–6. [Google Scholar]
- Shokrollahi, S.; Ghanati, F.; Sajedi, R.H.; Sharifi, M. Possible role of iron containing proteins in physiological responses of soybean to static magnetic field. J. Plant Physiol. 2018, 226, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Starmach, K. Family: Cladophora Kutzing 1843. Identification Key. [Translation from: Flora Słodkowodna Polski 10, 227–263, 1972.]; Freshwater Biological Association: Windermere, FL, UK, 1975; Volume 83. [Google Scholar]
- Karthik, T.; Sarkar, G.; Babu, S.; Amalraj, L.D.; Jayasri, M.A. Preparation and evaluation of liquid fertilizer from Turbinaria ornata and Ulva reticulata. Biocatal. Agric. Biotechnol. 2020, 28, 101712. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Michalak, I.; Messyasz, B. Concise review of Cladophora spp.: Macroalgae of commercial interest. J. Appl. Phycol. 2021, 33, 133–166. [Google Scholar] [CrossRef]
- EL Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- Tandon, S.; Dubey, A. Effects of biozyme (Ascophyllum nodosum) biostimulant on growth and development of soybean [Glycine Max (L.) Merill]. Commun. Soil Sci. Plant Anal. 2015, 46, 845–858. [Google Scholar] [CrossRef]
- Joshi-Paneri, J.; Chamberland, G.; Donnelly, D. Effects of Chelidonium majus and Ascophyllum nodosum extracts on growth and photosynthesis of soybean. Acta Agrobot. 2020, 73, 1–6. [Google Scholar] [CrossRef]
- Cieśla, A.; Kraszewski, W.; Skowron, M.; Syrek, P. The effects of magnetic fields on seed germination. Prz. Elektrotechniczny 2015, 91, 125–128. [Google Scholar] [CrossRef]
- Cieśla, A.; Kraszewski, W.; Skowron, M.; Syrek, P. Determination of safety zones in the context of the magnetic field impact on the surrounding during magnetic therapy. Prz. Elektrotechniczny 2011, 87, 79–83. [Google Scholar]
- Rosen, A.D. Studies on the efect of static magnetic fields on biological systems. PIERS Online 2010, 6, 133–136. [Google Scholar] [CrossRef]
- Tenforde, T.S. Mechanisms for biological effects of magnetic fields. In Biological Effects and Dosimetry of Static and ELF Electromagnetic Fields; Springer US: Boston, MA, USA, 1985; pp. 71–92. [Google Scholar]
- Zhang, X.; Yarema, K.; Xu, A. Biological Effects of Static Magnetic Fields; Springer: Singapore, 2017; ISBN 978-981-10-3577-7. [Google Scholar]
- Ueno, S. Biological effects of magnetic fields. IEEE Transl. J. Magn. Jpn. 1992, 7, 580–585. [Google Scholar] [CrossRef]
- Kovacs, P.E.; Valentine, R.L.; Alvarez, P.J.J. The effect of static magnetic fields on biological systems: Implications for enhanced biodegradation. Crit. Rev. Environ. Sci. Technol. 1997, 27, 319–382. [Google Scholar] [CrossRef]
- Frankel, R.B.; Liburdy, R.P. Biological effects of static magnetic fields. In Handbook of Biological Effects of Electromagnetic Fields; Polk, C., Postow, E., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 149–183. ISBN 9781315891910. [Google Scholar]
- Aladjadjiyan, A. Influence of stationary magnetic field on lentil seeds. Int. Agrophys. 2010, 24, 321–324. [Google Scholar]
- Ueno, S.; Okano, H. Static, low-frequency, and pulsed magnetic fields in biological systems. In Electromagnetic Fields in Biological Systems; CRC Press: Boca Raton, FL, USA, 2016; pp. 115–196. [Google Scholar]
- Zannella, S. Biological effects of magnetic fields. In Proceedings of the CAS—CERN Accelerator School: Measurement and Alignment of Accelerator and Detector Magnets, Anacapri, Italy, 11–17 April 1997; pp. 375–386. [Google Scholar]
- Florez, M.; Carbonell, M.; Martinez, E. Exposure of maize seeds to stationary magnetic fields: Effects on germination and early growth. Environ. Exp. Bot. 2007, 59, 68–75. [Google Scholar] [CrossRef]
- Gouda, O.E.; Amer, G.M. Performance of crops growth under low frequency electric and magnetic fields. In Proceedings of the 2009 6th International Multi-Conference on Systems, Signals and Devices, Djerba, Tunisia, 23–26 March 2009; pp. 1–7. [Google Scholar]
- Carbonnel, M.V.; Martínez, E.; Florez, M.; Maqueda, R.; Pintor-López, A.; Amaya, J.M. Magnetic field treatments improve germination and seedling growth in Festuca arundinacea Schreb. and Lolium perenne L. Seed Sci. Technol. 2008, 36, 31–37. [Google Scholar] [CrossRef]
- Iqbal, M.; ul Haq, Z.; Malik, A.; Ayoub, C.M.; Jamil, Y.; Nisar, J. Pre-sowing seed magnetic field stimulation: A good option to enhance bitter gourd germination, seedling growth and yield characteristics. Biocatal. Agric. Biotechnol. 2016, 5, 30–37. [Google Scholar] [CrossRef]
- Martínez, E.; Florez, M.; Maqueda, R.; Carbonell, M.V.; Amaya, J.M. Pea (Pisum sativum, L.) and lentil (Lens culinaris, Medik) growth stimulation due to exposure to 125 and 250 mT stationary fields. Pol. J. Environ. Stud. 2009, 16, 657–663. [Google Scholar]
- Rǎcuciu, M.; Creangǎ, D.; Horga, I. Plant growth under static magnetic field influence. Rom. Rep. Phys. 2008, 53, 331–336. [Google Scholar]
- Çelik, Ö.; Büyükuslu, N.; Atak, Ç.; Rzakoulieva, A. Effects of magnetic field on activity of superoxide dismutase and catalase in Glycine max (L.) Merr. roots. Pol. J. Environ. Stud. 2009, 18, 175–183. [Google Scholar]
- Pietruszewski, S.; Muszyński, S.; Dziwulska, A. Electromagnetic fields and electromagnetic radiation as non-invasive external stimulants for seeds (selected methods and responses). Int. Agrophys. 2007, 21, 95–100. [Google Scholar]
- Aladjadjiyan, A. The use of physical methods for plant growing stimulation in Bulgaria. J. Cent. Eur. Agric. 2007, 8, 369–380. [Google Scholar]
- Pietruszewski, S. Influence of magnetic and electric fields on seeds germination of selected cultivated plants. Acta Sci. Pol. Tech. Agrar. 2002, 1, 75–81. (In Polish) [Google Scholar]
- Torres, C.; Díaz, J.; Cabal, P. Magnetic fields effect over seeds germination of rice (Oryza sativa L.) and tomato (Solanum lycopersicum L.). Agron. Colomb. 2008, 26, 177–185. [Google Scholar]
- Florez, M.; Carbonell, M.V.; Martínez, E. Early sprouting and first stages of growth of rice seeds exposed to a magnetic field. Electromagn. Biol. Med. 2004, 23, 157–166. [Google Scholar] [CrossRef]
- Vashisth, A.; Nagarajan, S. Exposure of seeds to static magnetic field enhances germination and early growth characteristics in chickpea (Cicer arietinum L.). Bioelectromagnetics 2008, 29, 571–578. [Google Scholar] [CrossRef]
- Vashisth, A.; Nagarajan, S. Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. J. Plant Physiol. 2010, 167, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.; Florez, M.; Carbonell, M.V. Stimulatory effect of the magnetic treatment on the germination of cereal seeds. Int. J. Environ. Agric. Biotechnol. 2017, 2, 375–381. [Google Scholar] [CrossRef]
- Minucci, S.; Calabro, G.; Astolfi, S.; Campiglia, E. Effects of static magnetic fields on the germination and early growth of durum wheat seeds. In Proceedings of the 2019 19th International Symposium on Electromagnetic Fields in Mechatronics, Electrical and Electronic Engineering (ISEF), Nancy, France, 29–31 August 2019; pp. 1–2. [Google Scholar]
- Cakmak, T.; Dumlupinar, R.; Erdal, S. Acceleration of germination and early growth of wheat and bean seedlings grown under various magnetic field and osmotic conditions. Bioelectromagnetics 2009, 31, 120–129. [Google Scholar] [CrossRef]
- Florez, M.; Martinez, E.; Carbonell, V. Germination and initial growth of triticale seeds under stationary magnetic fields. J. Adv. Agric. 2014, 2, 72–79. [Google Scholar] [CrossRef]
- Podleśny, J.; Pietruszewski, S.; Podleśna, A. Efficiency of the magnetic treatment of broad bean seeds cultivated under experimental plot conditions. Int. Agrophys. 2004, 18, 65–71. [Google Scholar]
- Podleśny, J.; Pietruszewski, S.; Podleśna, A. Influence of magnetic stimulation of seeds on the formation of morphological features and yielding of the pea. Int. Agrophys. 2005, 19, 61–68. [Google Scholar]
- Çelik, Ö.; Atak, Ç.; Rzakulieva, A. Stimulation of rapid regeneration by a magnetic field in Paulownia node cultures. J. Cent. Eur. Agric. 2008, 9, 297–304. [Google Scholar] [CrossRef]
- Dardeniz, A.; Tayyar, S.; Yalcin, S. Influence of lowfrequency electromagnetic field on the vegetative growth of grape cv. Uslu. J. Cent. Eur. Agric. 2007, 7, 389–395. [Google Scholar]
- Dhawi, F.; Al-Khayri, J.M. The effect of magnetic resonance imaging on date palm (Phoenix dactylifera L.) elemental composition. Commun. Biometry Crop. Sci. 2009, 4, 14–20. [Google Scholar]
- Ulgen, C.; Birinci Yıldırım, A.; Uçar Turker, A. Effect of magnetic field treatments on seed germination of Melissa officinalis L. Int. J. Second Metab. 2017, 4, 63–69. [Google Scholar] [CrossRef]
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445. [Google Scholar] [CrossRef] [PubMed]
- Polk, C. Biological effects of low-level low-frequency electric and magnetic fields. IEEE Trans. Educ. 1991, 34, 243–249. [Google Scholar] [CrossRef]
- Florez, M.; Martínez, E.; Carbonell, M.V. Effect of magnetic field treatment on germination of medicinal plants Salvia officinalis L. and Calendula officinalis L. Pol. J. Environ. Stud. 2012, 21, 57–63. [Google Scholar]
- Bertea, C.M.; Narayana, R.; Agliassa, C.; Rodgers, C.T.; Maffei, M.E. Geomagnetic field (Gmf) and plant evolution: Investigating the effects of Gmf reversal on Arabidopsis thaliana development and gene expression. J. Vis. Exp. 2015, 105. [Google Scholar] [CrossRef] [PubMed]
- Jan, L.; Fefer, D.; Košmelj, K.; Gaberščik, A.; Jerman, I. Geomagnetic and strong static magnetic field effects on growth and chlorophyll a fluorescence in Lemna minor. Bioelectromagnetics 2015, 36, 190–203. [Google Scholar] [CrossRef]
- Lazim, S.K.; Nasur, A.F. The effect of magnetic field and ultraviolet-C radiation on germination and growth seedling of sorghum (Sorghum bicolor L. Moench). J. Agric. Vet. Sci. 2017, 10, 30–36. [Google Scholar] [CrossRef]
- Asghar, T.; Jamil, Y.; Iqbal, M.; Zia-ul-Haq; Abbas, M. Laser light and magnetic field stimulation effect on biochemical, enzymes activities and chlorophyll contents in soybean seeds and seedlings during early growth stages. J. Photochem. Photobiol. B Biol. 2016, 165, 283–290. [Google Scholar] [CrossRef]
- Asghar, T.; Iqbal, M.; Jamil, Y.; Zia-ul-Haq; Nisar, J.; Shahid, M. Comparison of He-Ne laser and sinusoidal non-uniform magnetic field seed pre-sowing treatment effect on Glycine max (Var 90-I) germination, growth and yield. J. Photochem. Photobiol. B Biol. 2017, 166, 212–219. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).