Low Temperature Resistance Increase for Bitumen by Compounding with Tar
Abstract
1. Introduction
2. Materials and Methods
2.1. Bitumen
2.2. Tar
2.3. Compounding
- A sample of the virgin bitumen with penetration grade 100–130 has been selected in the required volume and prepared for compounding;
- The virgin bitumen was gradually heated up to the temperature of 120 °C;
- A previously prepared portion of the tar has been added at the mixing of the sample of the virgin bitumen with the rate of 450–500 revolutions per minute;
- The virgin bitumen and the tar were mixed at a steady pace with the rate of 450–500 rotations per minute at the constant temperature of 120 °C for 30–40 min;
- The prepared portion of the compounded bitumen cooled down to room temperature.
2.4. Bitumen Aging
2.5. Bending Beam Rheometer
2.6. Group Chemical Composition of Bitumen
2.7. NMR-Spectroscopy
3. Results and Discussion
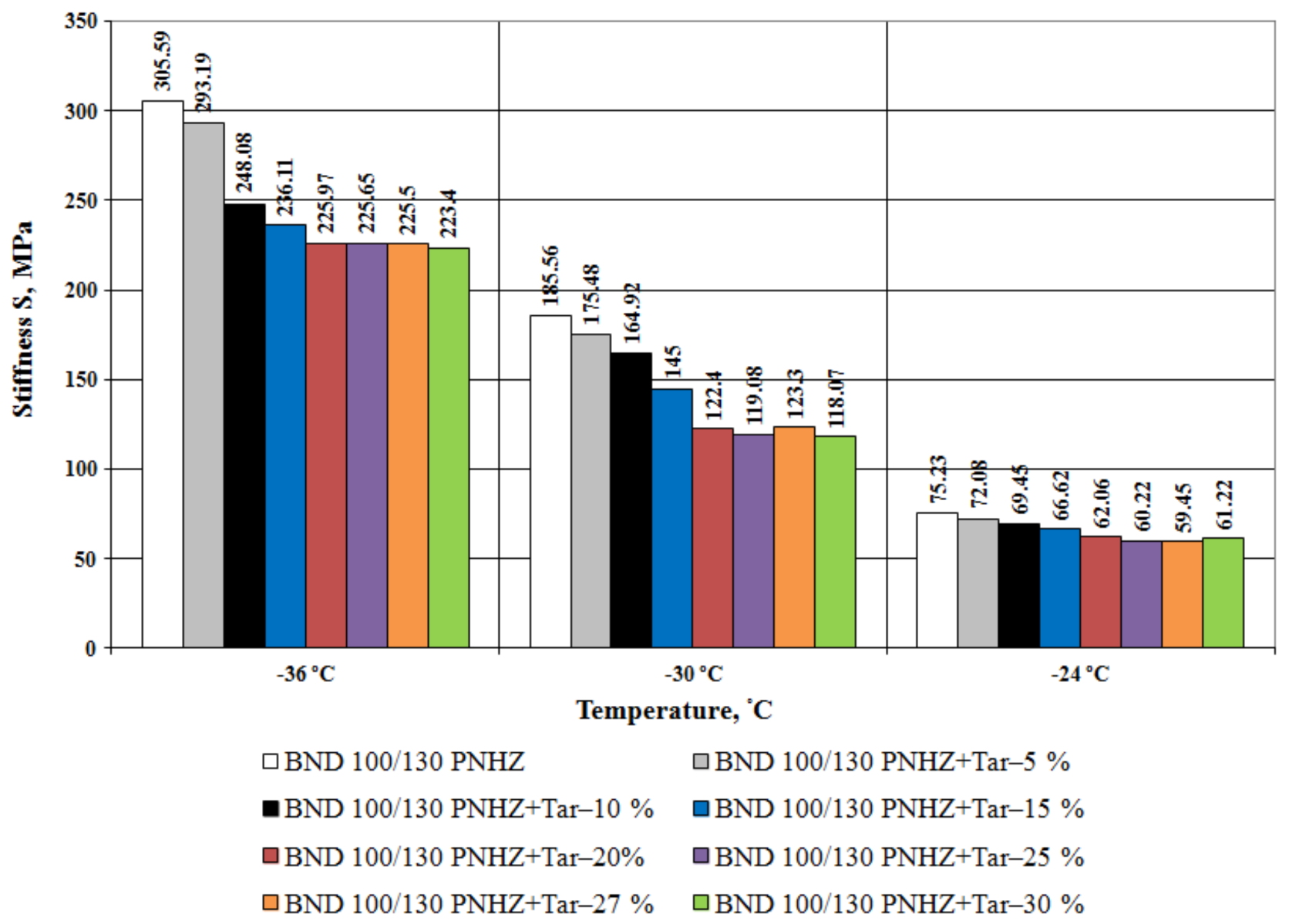
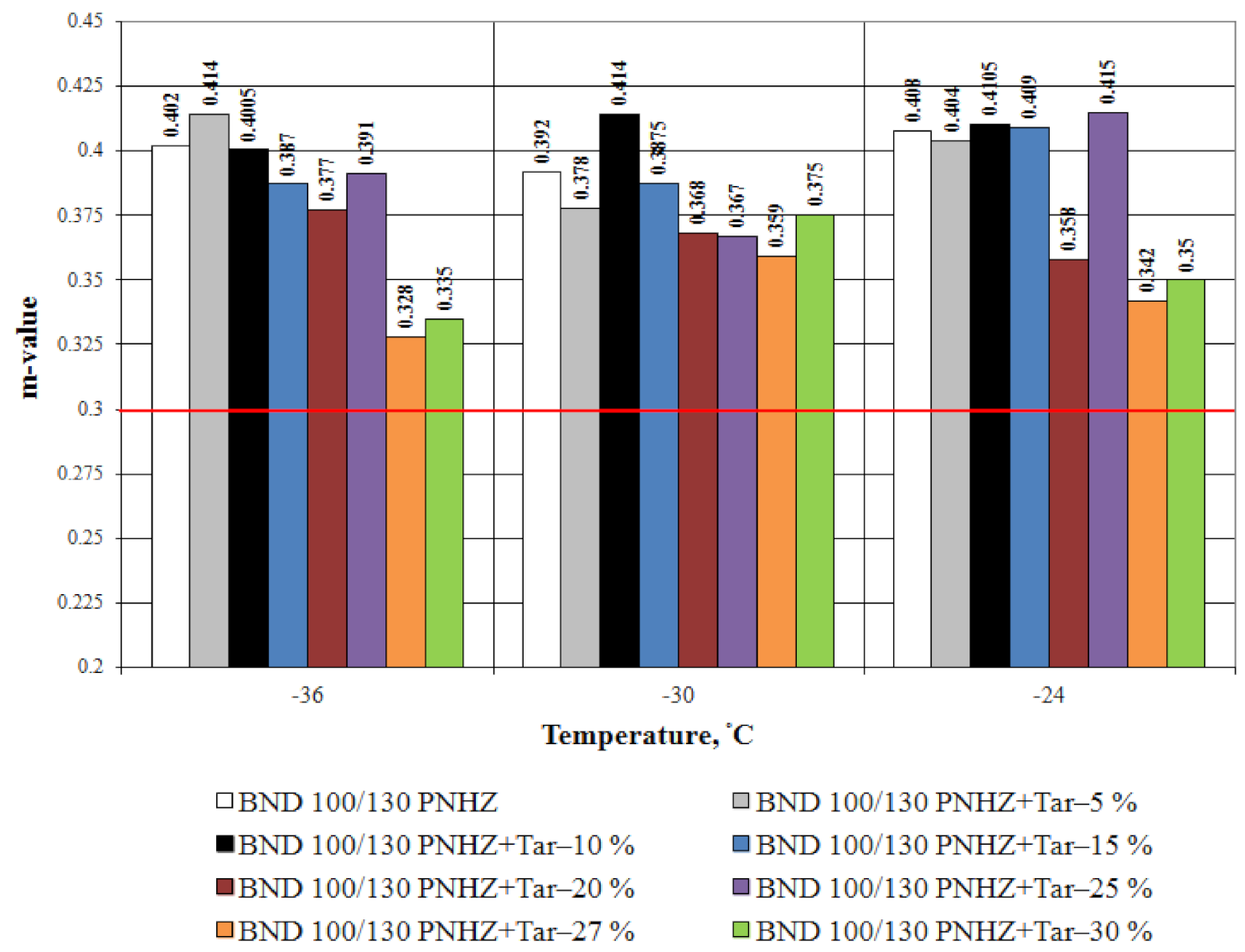
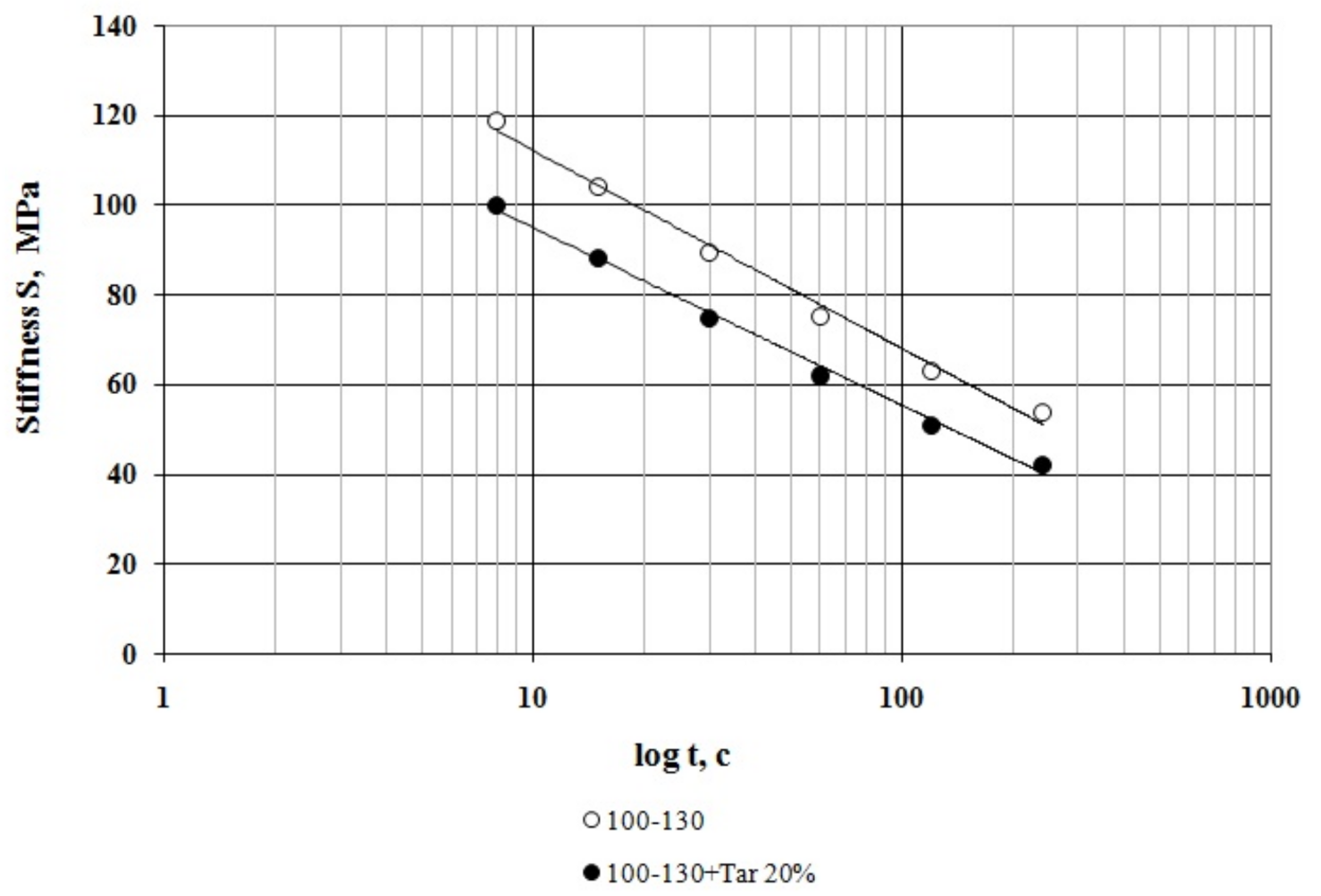
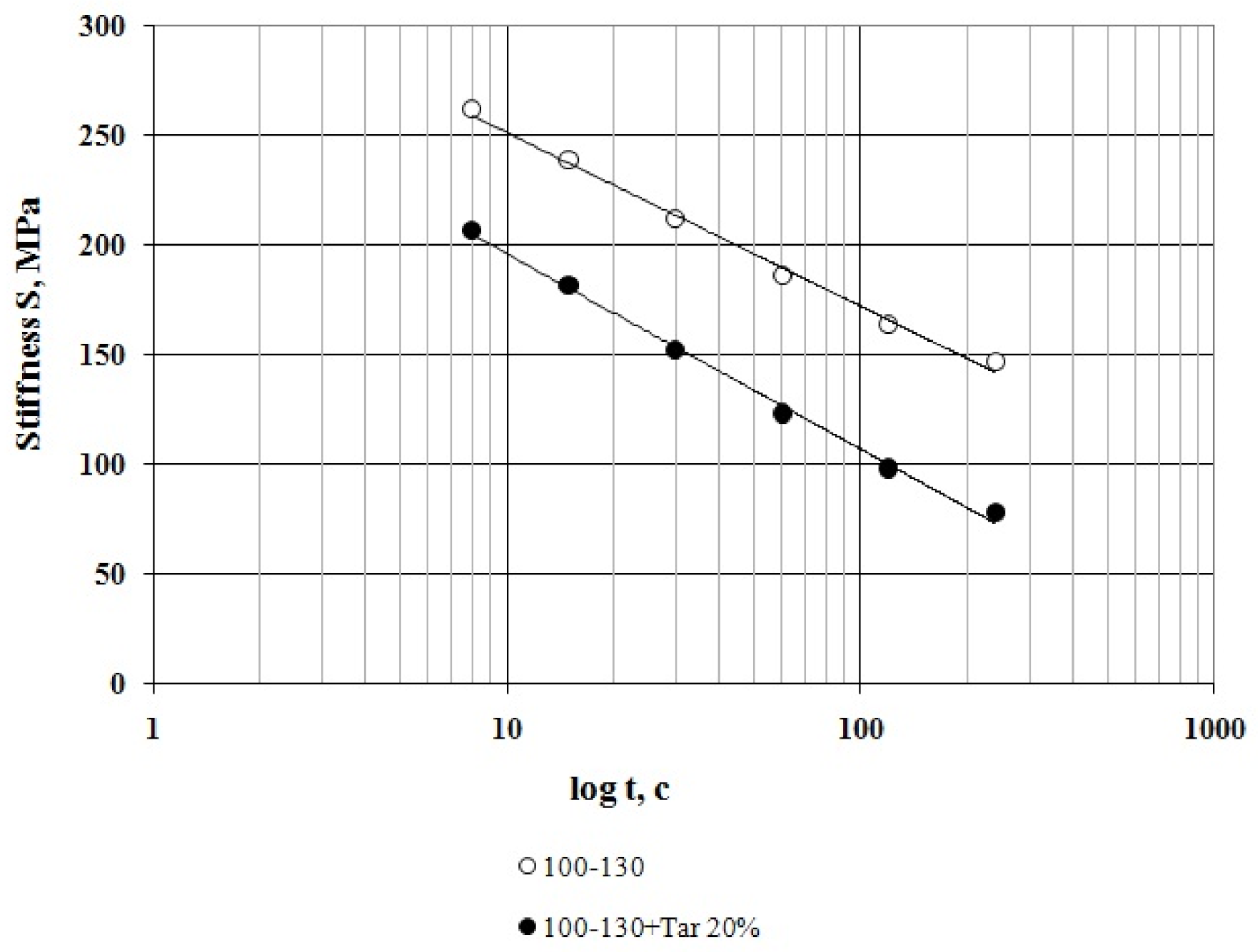
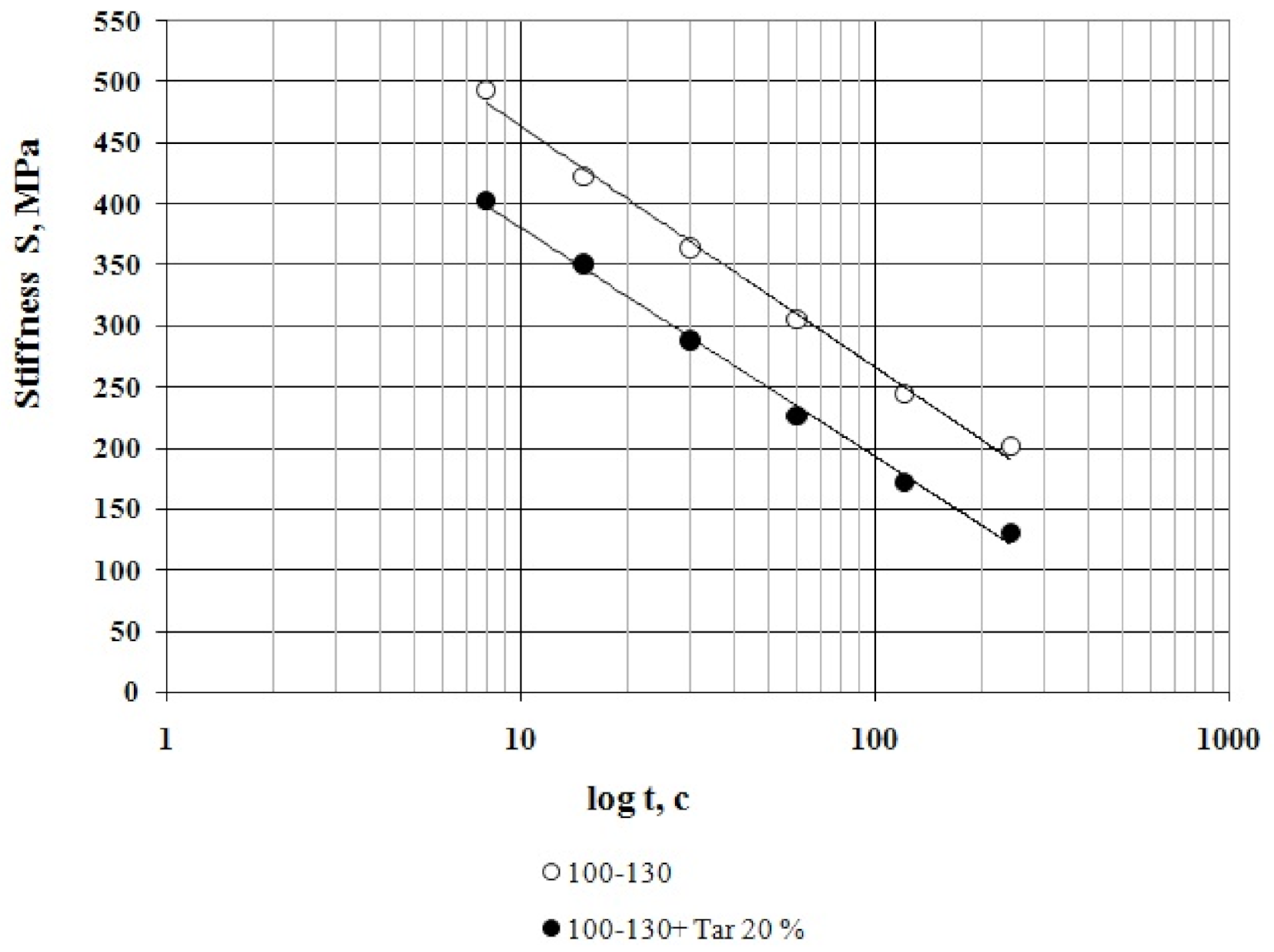
3.1. Bitumen Stiffness
3.2. Standard Indicators of Bitumens
3.3. Chemical Structure of Bitumens
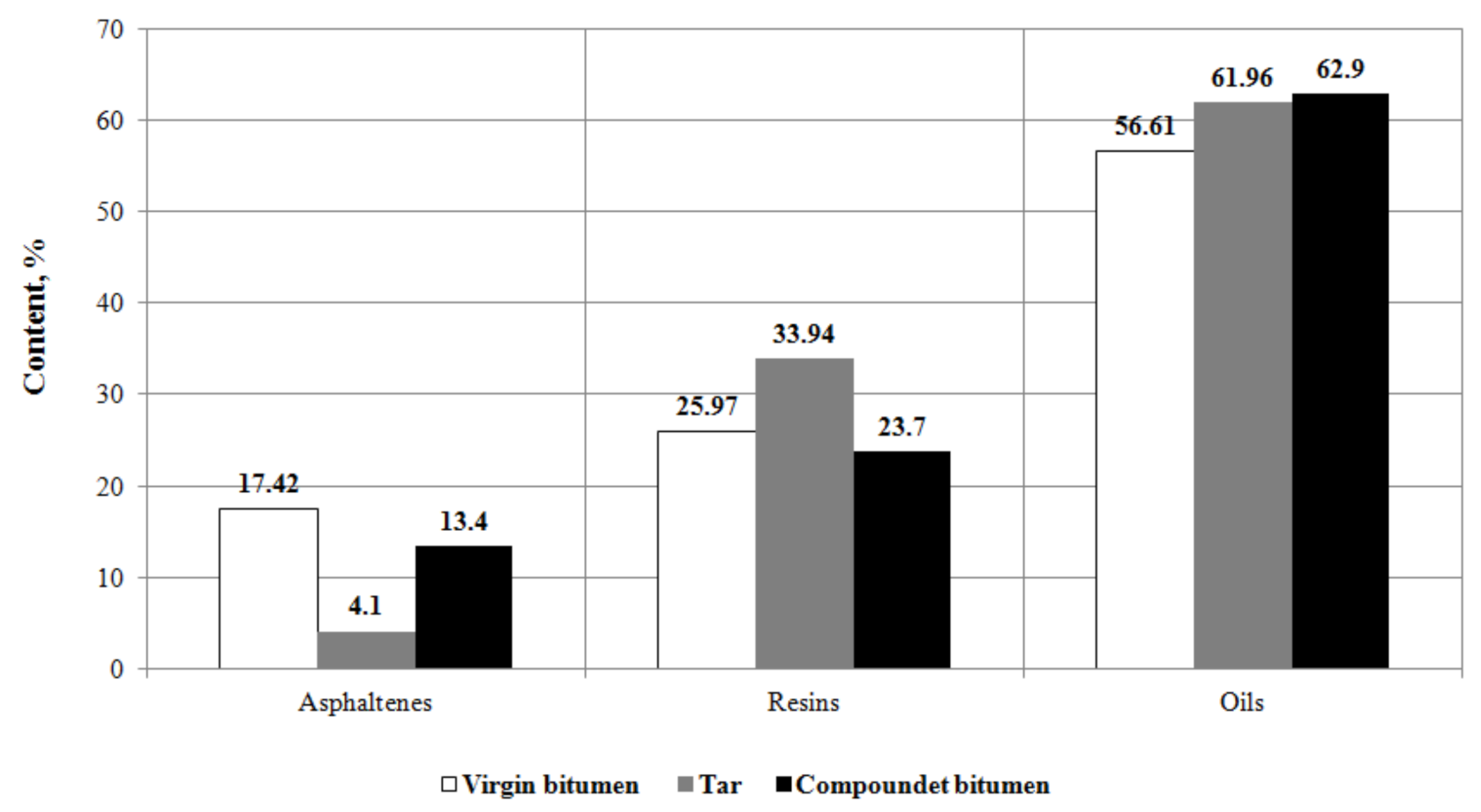
3.3.1. Group Chemical Composition
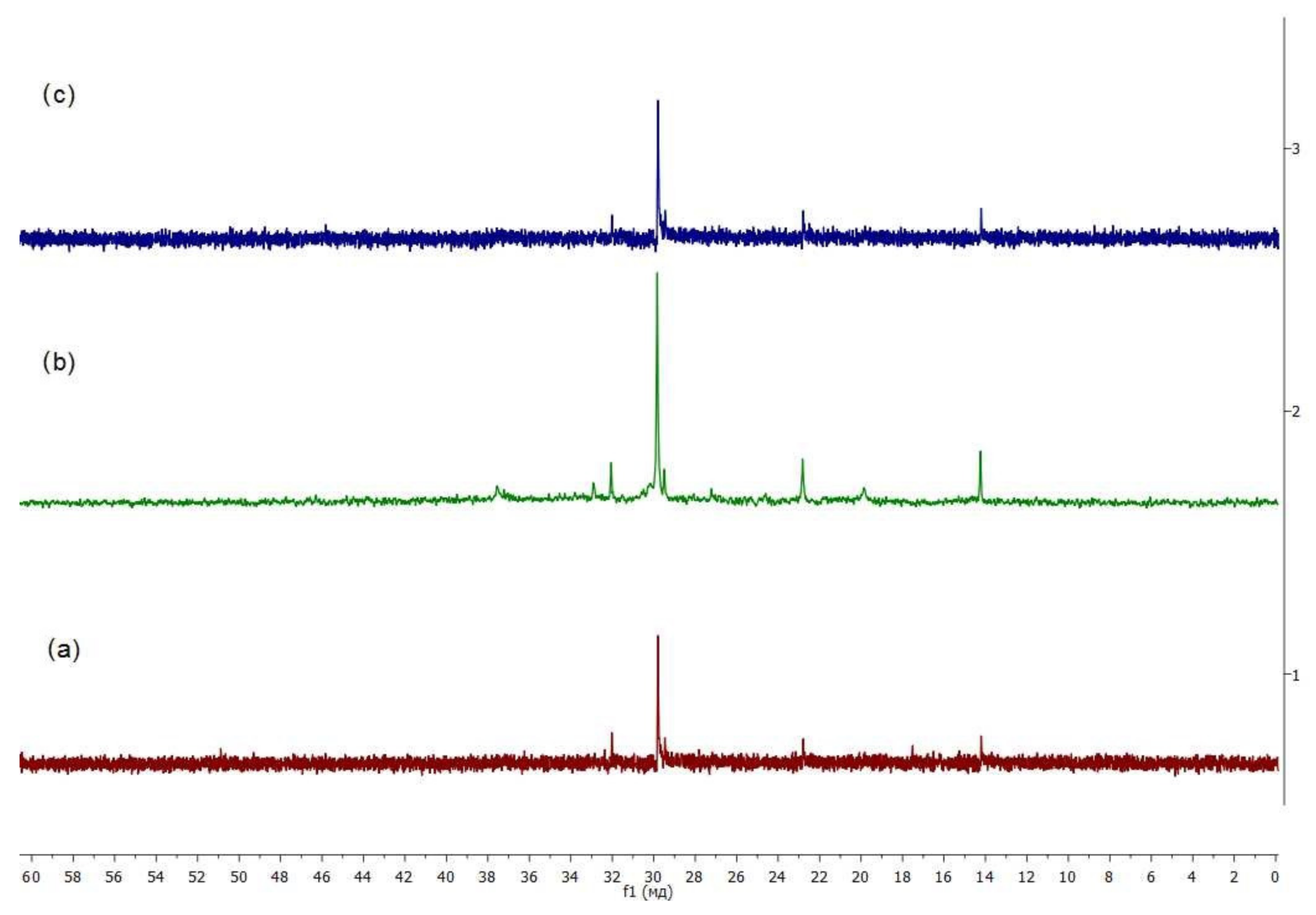
3.3.2. Fragments of Molecules
4. Conclusions
- The effect of compounding is greater with the decrease in temperature. The tar content equal to 20% by weight is considered as an optimal one when compounding the road bitumen with penetration grade 100–130.
- At all the tested temperatures (−24 °C, −30 °C and −36 °C), the stiffness of the compounded bitumen is significantly (from 18% to 34%) lower than that of the virgin bitumen, i.e., the compounded bitumen has the increased low temperature stability.
- The optimal compounding (20% of the tar by weight) increased bitumen penetration by 54 decimillimeters, which changed its original grade 100–130 to 130–200; softening point and viscosity have decreased by 5 °C and 65%, respectively; Fraas point remained practically unchanged; and ductilityincreased by 10.4%.
- It has been found by adsorption chromatography that the optimal compounding significantly changes the group chemical composition of bitumen: asphaltenes and resins decreased by 23% and 8.8%, respectively, and oils increased by 11.1%.
- Reduction in compounded bitumen of solid (asphaltenes) and high-viscosity (resins) components, increase in low-viscosity components (oils), as well as the decrease in the content of methyl groups in them (35.1%), and the increase in the content of quaternary aliphatic carbon atoms (13.4%) increase the mobility of molecules and supramolecular structures, which increases the low-temperature stability of the compounded bitumen.
- To increase high temperature resistance of the obtained compounded bitumen, it is recommended to modify it with a polymer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kutiin, Y.; Telyashev, E. Bitumens and Bitumen Materials. Standards, Quality, Technologies; Publisher GUE PCP RB: Ufa, Russia, 2018. [Google Scholar]
- The Shell Bitumen Handbook, 6th ed.; Thomas Telford Publishing: London, UK, 2015.
- Papagiannakis, A.; Masad, E. Pavement Design and Materials; Willey & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- The Asphalt Handbook, MS–4, 7th ed.; Asphalt Institute: Lexington, KY, USA, 2007.
- Radovskiy, B.; Teltayev, B. Viscoelastic Properties of Asphalts Based on Penetration and Softening Point; Springer International Publishing AG: Cham, Switzerland, 2018. [Google Scholar]
- Teltayev, B.B.; Amirbayev, E.D.; Radovskiy, B.S. Viscoelastic characteristics of blown bitumen at low temperatures. Constr. Build. Mater. 2018, 189, 54–61. [Google Scholar] [CrossRef]
- Teltayev, B.B.; Radovskiy, B.S. Predicting thermal cracking of asphalt pavements from bitumen and mix properties. RMPD 2018, 19, 1832–1847. [Google Scholar] [CrossRef]
- Honarmand, M.; Tanzadeh, J.; Beiranvand, M. Bitumen and its modifier for use in pavement engineering. In Sustainable Construction and Building Materials; IntechOpen: London, UK, 2019; pp. 249–270. [Google Scholar]
- Bala, N.; Kamaruddin, I.; Napiah, M. The influence of polymer on rheological and thermo oxidative aging properties of modified bitumen binders. J. Tekn. 2017, 79, 69–73. [Google Scholar] [CrossRef]
- Abdullin, A.I.; Emelyanycheva, E.A. A study of properties of road petroleum bitumen modified with polymer additives. J. Chem. Techn. Metal. 2018, 53, 422–429. [Google Scholar]
- Akkouri, N.; Baba, K.; Simou, S.; Elfarissi, L.; Nounah, A. Recycled thermoplastics modified bitumen improved with thermoplastic elastomer. In Proceedings of the Seventh International Congress “Water, Waste and Environment” (EDE7-2019), online, 20–22 November 2019; Volume 150, p. 02015. [Google Scholar]
- Dekhli, S.; Mokhtar, K.A.; Hammoum, F.; Bachir, D.S. Rheological behaviour of ethylene–vinyl–acetate (EVA) modified road bitumen. J. Appl. Sci. 2015, 15, 445–455. [Google Scholar] [CrossRef][Green Version]
- Salas, M.A.; Perez–Acebo, H.; Calderon, V.; Gonzalo–Orden, H. Bitumen modified with recycled polyurethane foam for employment in hot mix asphalt. Ing. Investig. 2018, 38, 60–66. [Google Scholar] [CrossRef]
- Porto, M.; Caputo, P.; Loise, V.; Eskandarsefat, S.; Teltayev, B.B.; Rossi, C.O. Bitumen and bitumen modification: A review on latest advances. Appl. Sci. 2019, 9, 742. [Google Scholar] [CrossRef]
- Caputo, P.; Porto, M.; Loise, V.; Teltayev, B.B.; Rossi, C.O. Analysis of mechanical performance of bitumen modified with waste plastic and rubber (SBR) additives by rheology and PGSE NMR experiments. Eur. Chemico-Tech. J. 2019, 21, 235–239. [Google Scholar] [CrossRef]
- Speight, J.G. Chapter 12—Environmental Aspects of Asphalt Use. In Asphalt Materials Science and Technology; Speight, J.G., Ed.; Butterworth-Heinemann Elselvier Ltd.: Oxford, UK, 2016; pp. 475–511. [Google Scholar]
- Menzie, C.A.; Potocki, B.B.; Santodonato, J. Exposure to carcinogenic PAHs in the environment. Environ. Sci. Technol. 1992, 26, 1278–1284. [Google Scholar] [CrossRef]
- Varjani, S.J.; Joshi, R.R.; Kumar, P.S.; Srivastava, V.K.; Kumar, V.; Banerjee, C.C.; Kumar, R.P. Polycyclic aromatic hydrocarbons from petroleum oil industry activities: Effect on human health and their biodegradation. In Waste Bioremediation; Varjani, S.J., Gnansounou, E., Gurunathan, B., Pant, D., Zakaria, A.Z., Eds.; Springer Nature: Singapore, 2018; pp. 185–199. [Google Scholar]
- Patent for Invention. Method of Production of Compounded Bitumen. No. RU 2302447 C, 10 July 2007. [Google Scholar]
- Patent for Invention. Method for Bitumen Production. No. RU 2405807 C, 10 December 2010. [Google Scholar]
- Patent for Invention. Method of Producing Asphaltite–Containing Road Bitumen. No. RU 2552469 C, 10 June 2015. [Google Scholar]
- Imanbayev, Y.; Akkenzheyeva, A.; Bussurmanova, A.; Serikbayeva, A.; Boranbayeva, A. Preparation of polymer bitumen binder in the presence of a stabilizer. Processes 2021, 9, 182. [Google Scholar] [CrossRef]
- Bitumens and Bituminous Binders. Oil Road Viscous Bitumens. Technical Specifications; ST RK 1373; JSC “KazdorNII”: Astana, Kazakhstan, 2013. [Google Scholar]
- Teltayev, B.B.; Rossi, C.O.; Izmailova, G.G.; Amirbayev, Y.D.; Elshibayev, A.O. Evaluating the effect of asphalt binder modification on the low–temperature cracking resistance of hot mix asphalt. CSCM 2019, 11, 1–13. [Google Scholar] [CrossRef]
- Tar. Technical Specifications; ST RK 3337; JSC “KazdorNII”: Astana, Kazakhstan, 2018.
- Standard Specification for Performance Graded Asphalt Binder; AASHTO M 320; AASHTO: Washington, DC, USA, 2017.
- Standard Specification for Performance Graded Asphalt Binder; ASTM D 6373; ASTM International: West Conshohocken, PA, USA, 2016.
- Performance Graded Asphalt Binder Specification and Testing, 3rd ed.; Superpave Series No. 1 (SP–1); Asphalt Institute Inc.: Lexington, KY, USA, 2003; pp. 1–59.
- Standard Test Method for Effect of Heat and Air on Moving Film of Asphalt Binder (Rolling Thin–Film Oven Test); AASHTO T 240; AASHTO: Washington, DC, USA, 2013.
- Standard Practice for Accelerated Aging of Asphalt Binder Using Pressurized Aging Versel (PAV); ASTM D 6521; ASTM International: West Conshohocken, PA, USA, 2008.
- Standard Test Method for Determining the Flexural Creep Stiffness of Asphalt Binder Using the Bending Beam Rheometer (BBR); ASTM D 6648; ASTM International: West Conshohocken, PA, USA, 2008.
- Teltayev, B.B.; Seilkhanov, T.M. NMR–spectroscopy determination of fragmentary composition of bitumen and its components. Eurasian Chemico-Tech. J. 2018, 20, 153–158. [Google Scholar] [CrossRef]
- Rakhmatullin, I.Z.; Efimov, S.V.; Margulis, B.Y.; Klochkov, V.V. Qualitative and quantitative analysis of oil samples extracted from some Bashkortostan and Tatarstan oilfields based on NMR spectroscopy data. J. Pet. Sci. Eng. 2017, 156, 12–18. [Google Scholar] [CrossRef]
- Jameel, A.G.; Khateeb, A.; Elbaz, A.M.; Emwas, A.-H.; Zhang, W.; William, L.; Robertsa, W.L.; Sarathy, S.M. Characterization of deasphalted heavy fuel oil using APPI (+) FT–ICR massspectrometry and NMR spectroscopy. Fuel 2019, 253, 950–963. [Google Scholar] [CrossRef]
- Yen, T.F.; Erdman, J.A.; Pollak, S.S. Investigation of the structure of petroleum asphaltenes by X–ray diffraction. Anal. Chem. 1961, 33, 1587–1594. [Google Scholar] [CrossRef]
- Dickie, J.P.; Yen, T.F. Microstructures of the asphaltic fractions by various instrumentals methods. Anal. Chem. 1967, 39, 1847–1852. [Google Scholar] [CrossRef]
- Mullins, O.C. The modified Yen model. Energy Fuels 2010, 24, 2179–2207. [Google Scholar] [CrossRef]
- Mullins, O.C. The asphaltenes. Annu. Rev. Anal. Chem. 2011, 4, 339–418. [Google Scholar] [CrossRef]
- Rossi, C.O.; Caputo, P.; De Luca, G.; Maiuolo, L.; Eskandarsefat, S.; Sangiorgi, C. 1H–NMR spectroscopy: A possible approach to advanced bitumen characterization for industrial and paving applications. Appl. Sci. 2018, 8, 1–14. [Google Scholar]
- Borisova, T.I.; Mikhailov, G.P. Investigation of dielectric relaxation in co–polymers of methylmetacrilate with sterol. High Mol. Com. 1959, 4, 563–573. [Google Scholar]
- Mikhailov, G.P.; Krasner, L.V. Investigation of temperature dependence of dielectric losses of polymers of homological ranges for methyl acrilate and vinyl acetate. High Mol. Com. 1962, 7, 1071–1075. [Google Scholar]
- Bartenev, G.M.; Zelenev, Y.V. Physics and Mechanics of Polymers; Higher School: Moscow, Russia, 1983. [Google Scholar]
- Ferry, J.D. Viscoelastic Properties of Polymers, 3rd ed.; Willey: New York, NY, USA, 1980. [Google Scholar]
- Tager, A.A. Physico–Chemistry of Polymers; Scientific World: Moscow, Russia, 2007. [Google Scholar]
- Mikhailov, G.P.; Borisova, T.I. Regarding some peculiarities dipole–elastic losses in polymers in relation to their construction. High Mol. Com. 1964, 10, 1778–1784. [Google Scholar]
- Ouellette, R.J.; Rawn, J.D. Organic Chemistry: Structure, Mechanism, Synthesis, 2nd ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–39. [Google Scholar]
- Ouellette, R.J.; Rawn, D. Alkanes and Cycloalkanes: Structures and Reactions. In Organic Chemistry, 2nd ed.; Elsevier Inc.: Baltimore, MD, USA, 2018; pp. 87–133. [Google Scholar]



| Indicator | Measurement Unit | Standard | Value |
|---|---|---|---|
| Penetration (25 °C) | 0.1 mm | ST RK 1226-2003 | 116 |
| Ductility (25 °C) | cm | ST RK 1374-2005 | 115 |
| Softening point | °C | ST RK 1227-2003 | 45 |
| Fraas point | °C | ST RK 1229-2003 | −27 |
| Penetration index | - | ST RK 1226-2003 | −0.312 |
| Dynamic viscosity | Pa∙s | ST RK 1211-2003 | 138 |
| Indicator | Measurement Unit | Standard | Value |
|---|---|---|---|
| Conditional viscosity (80 °C) | s | ST RK 1683-2007 | 82 |
| Density (20 ± 2 °C) | kg/m3 | ST RK 2114-2011 | 956 |
| Flash point | °C | ST RK 1804-2008 | 280 |
| Water content | % | ST RK 1375-2005 | 0 |
| δ (1H), ppm | Notation of Atoms | Functional Group |
|---|---|---|
| 0.5–1.0 | Hγ | Methyl groups of saturated compounds. Methyl groups in γ and further positions to an aromatic ring. |
| 1.0–2.0 | Hβ | Methylene and methine groups of saturated compounds. Atoms of hydrogen in methyl groups in β position to an aromatic ring. Atoms of hydrogen of methylene and methine groups in β and further positions to the aromatic ring. |
| 2.0–4.0 | Hα | Atoms of hydrogen in α position to aromatic and carbonyl carbons, heteroatoms |
| 4.5–6.3 | Haℓ | Atoms of hydrogen of olefin groups |
| 6.3–9.0 | Har | Atoms of hydrogen of aromatic nuclei and phenol hydroxils |
| δ (13C), ppm | Notation of Atoms | Functional Group |
|---|---|---|
| 7–17 | Cpm | Atoms of carbon of methyl groups connected with a methylene group. |
| 17–25 | Cpa | Atoms of carbon of methyl groups connected with a methine group or an aromatic ring. |
| 25–50 | Caℓ | Quaternary aliphatic atoms of carbon. |
| Tar Content, % | 0 | 5 | 10 | 15 | 20 | 25 | 27 | 30 |
| Penetration at 25 °C, 0.1 mm | 116 | 118 | 128 | 160 | 170 | 182 | 203 | 216 |
| Indicator | Measurement Unit | Standard | Value | |
|---|---|---|---|---|
| Virgin Bitumen | Compounded Bitumen | |||
| Penetration (25 °C) | 0.1 mm | ST RK 1226-2003 | 116 | 170 |
| Ductility (25 °C) | cm | ST RK 1374-2005 | 115 | 127 |
| Softening point | °C | ST RK 1227-2003 | 45 | 40 |
| Fraas point | °C | ST RK 1229-2003 | −27 | −28 |
| Penetration index | - | ST RK 1226-2003 | −0.312 | −0.962 |
| Dynamic viscosity | Pa∙s | ST RK 1211-2003 | 138 | 49 |
| Tar Content, % | 0 | 5 | 10 | 15 | 20 | 25 | 27 | 30 |
| Measurement Temperature, °C | 45.0 | 44.5 | 43.0 | 40.5 | 40.0 | 38.0 | 37.8 | 36.6 |
| Type of Atoms | Bitumen | Tar | Compounded Bitumen |
|---|---|---|---|
| Hα | 9.0 | 9.8 | 9.2 |
| Hβ | 60.0 | 59.9 | 60.1 |
| Hγ | 25.3 | 24.3 | 25.0 |
| Haℓ | 94.3 | 94.0 | 94.3 |
| Har | 5.7 | 6.0 | 5.7 |
| Type of Atoms | Bitumen | Tar | Compounded Bitumen |
|---|---|---|---|
| Cpm | 10.5 | 8.3 | 9.2 |
| Cpa | 17.1 | 15.1 | 8.7 |
| Caℓ | 72.4 | 76.6 | 82.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teltayev, B.; Seilkhanov, T.; Oliviero Rossi, C.; Amirbayev, Y.; Begaliyeva, S. Low Temperature Resistance Increase for Bitumen by Compounding with Tar. Appl. Sci. 2021, 11, 8579. https://doi.org/10.3390/app11188579
Teltayev B, Seilkhanov T, Oliviero Rossi C, Amirbayev Y, Begaliyeva S. Low Temperature Resistance Increase for Bitumen by Compounding with Tar. Applied Sciences. 2021; 11(18):8579. https://doi.org/10.3390/app11188579
Chicago/Turabian StyleTeltayev, Bagdat, Tulegen Seilkhanov, Cesare Oliviero Rossi, Yerik Amirbayev, and Sakhypzhamal Begaliyeva. 2021. "Low Temperature Resistance Increase for Bitumen by Compounding with Tar" Applied Sciences 11, no. 18: 8579. https://doi.org/10.3390/app11188579
APA StyleTeltayev, B., Seilkhanov, T., Oliviero Rossi, C., Amirbayev, Y., & Begaliyeva, S. (2021). Low Temperature Resistance Increase for Bitumen by Compounding with Tar. Applied Sciences, 11(18), 8579. https://doi.org/10.3390/app11188579








