Helium Atmospheric Pressure Plasma Jet Source Treatment of White Grapes Juice for Winemaking
Abstract
1. Introduction
- the Transylvanian Plateau (Târnave, Alba, Sebeş-Apold, Aiud, Lechinţa vineyards)
- the Moldavian Plateau (Cotnari, Iaşi, Huşi, Dealurile Fălciului, Colinele Tutovei, Zeletin, Dealu Bujorului, Nicoreşti, Iveşti, Covurlui vineyards)
- the Piedmont at the Carpathian’s Curvature (Panciu, Odobeşti, Coteşti, Buzău’s, Dealu Mare vineyards)
- the Getic Plateau (Ştefăneşti-Argeş, Sâmbureşti, Drăgăşani, Dealurile Craiovei, Plaiurile Drâncei, Severin Vineyards)
- The Banat-Crişana-Maramureş (Banat, Miniş-Măderat, Diosig, Valea lui Mihai, Silvaniei vineyards)
- the Sands in the South of Oltenia (Dacilor, Calafat, Sadova-Corabia vineyards)
- the Romanian Plain (Greaca, independent wine-growing centers situated in the Romanian plain)
- The Dobrogea Plateau (Sarica-Niculiţel, Istria-Babadag, Murfatlar, Ostrov vineyards)
2. Materials and Methods
- A set of grapes was collected from a small family wineyard with hybrid white Noble grapes situated in the Bârzeşti-Ştefan cel Mare region (N46°44′40,18″; E27°33′42,63″), Vaslui county. These samples were marked with ‘B’. This studied parcel of 600 m2 was placed close to the house and no soil or leaves treatments were applied. The only agricultural practices were manual cutting and tying of vine shoots and digging.
- A second set of grapes was collected from a vineyard parcel with a mixture of white grapes (Chasselas, Fetească, Busuioacă) situated in Pâhneşti-Arsura-Huşi (N46°46′54,93″; E28°02′38,21″), Vaslui county. These samples were marked with ‘H’. The studied parcel of 2300 m2 was located in a vineyard of around 90 ha, where mechanical ploughing and different treatments for soil and plant were applied. Only cutting and tying of shoots, as well as grapes harvests, were manually made.
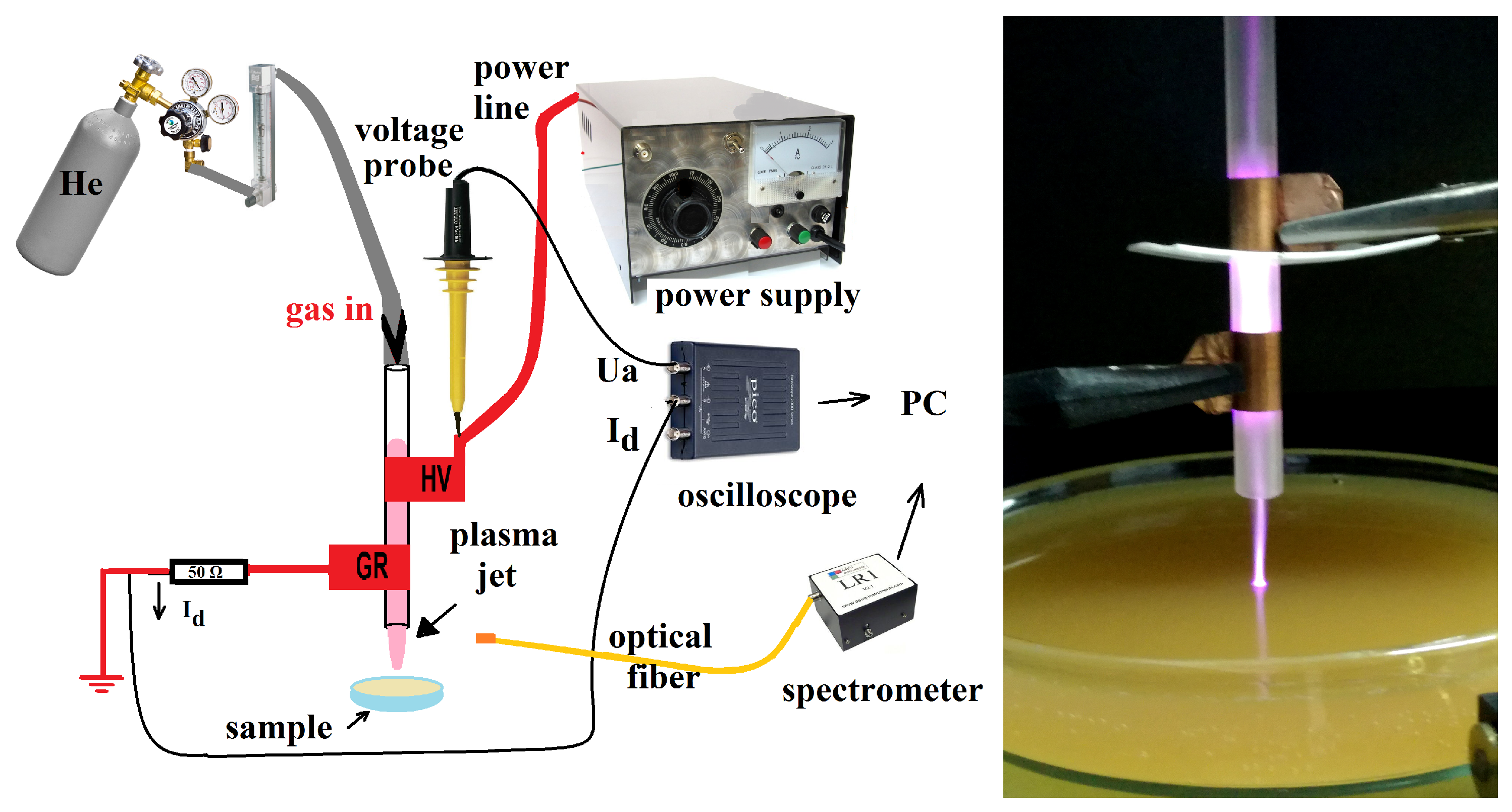
2.1. Plasma Source and Electro-Optical Diagnosis
2.2. Methods for Characterization of Must and Wine
2.2.1. The Ultraviolet (UV) to Infrared (IR) Spectroscopy
2.2.2. The Colour of the Wine and Its Colour Space Parameters (CIELab)
2.2.3. The pH Value, Brix and Potential Alcohol of Must and Wine
- dry, with a sugar content of up to 4 g/L but not less than 2 g/L;
- medium-dry, with a sugar content between 4.01 g/L and 12 g/L, but can go up to 18 g/L;
- mellow or semi-sweet, with a sugar content above 18 g/L and up to 45 g/L inclusive;
- sweet, with a sugar content of more than 45 g/L.
3. Results and Discussion
3.1. Plasma Source Electro-Optical Characterization
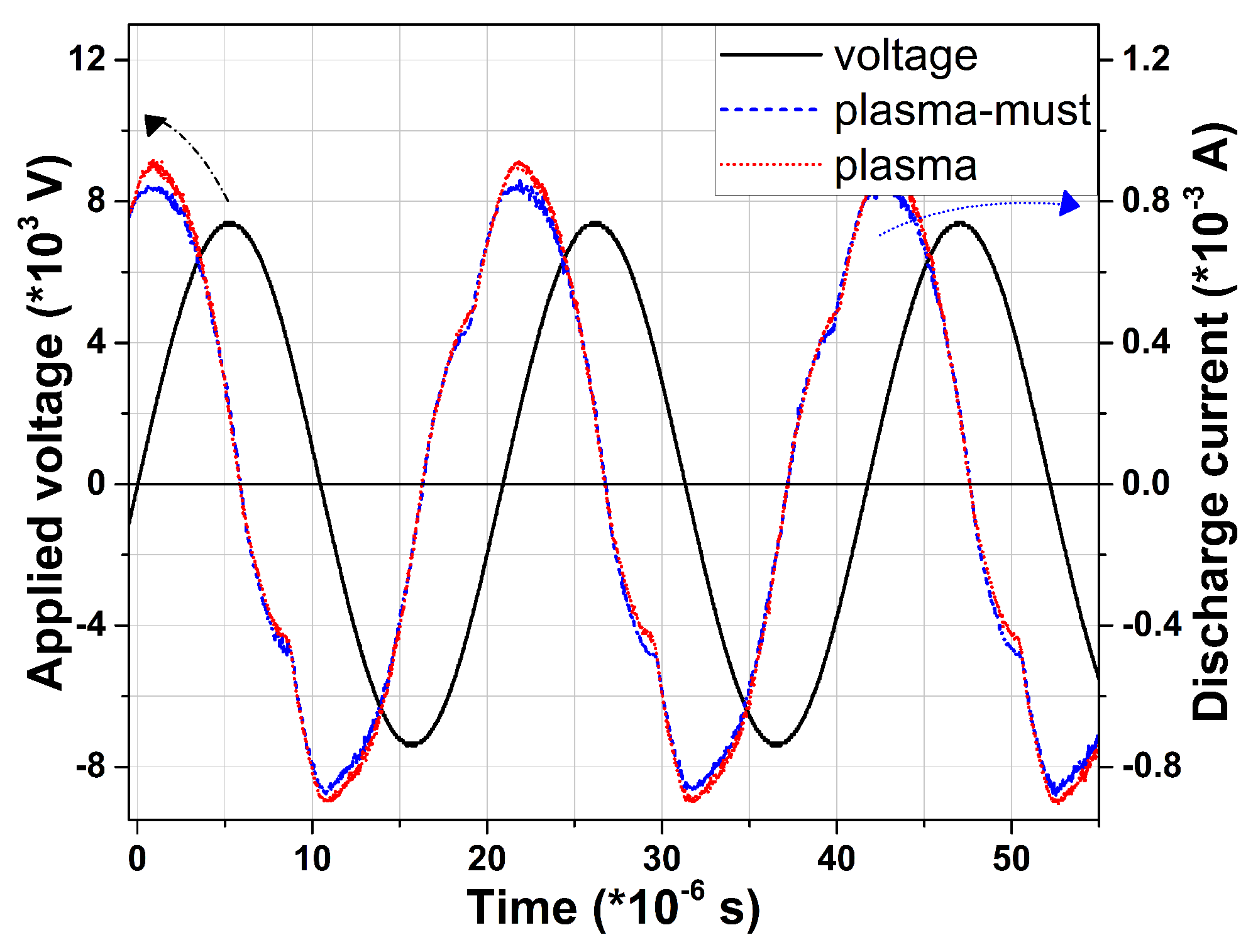
3.1.1. Plasma Source Electrical Diagnosis
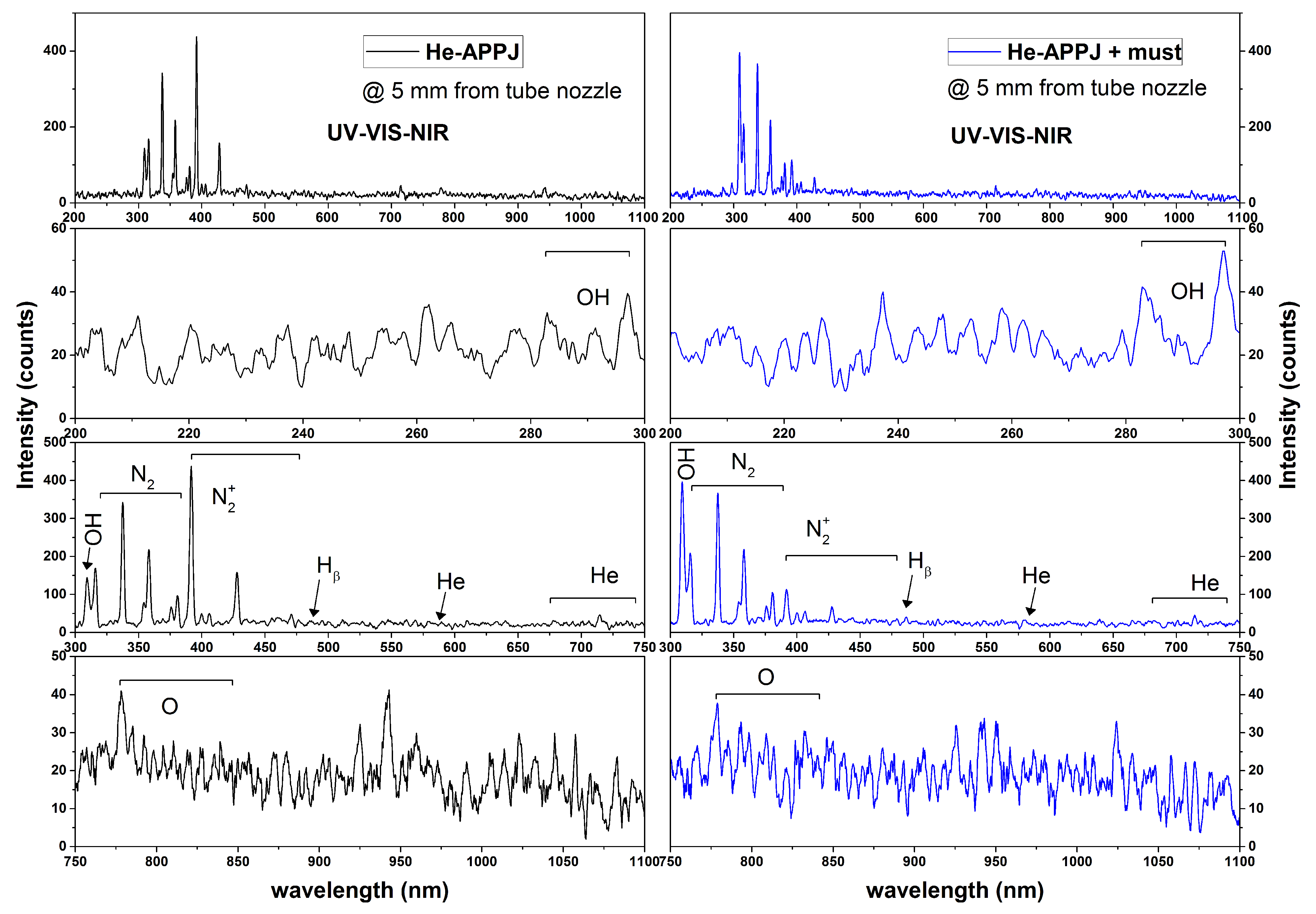
3.1.2. Plasma Source Optical Diagnosis
3.2. Characterization of White Grapes Juice and Wine
3.2.1. UV-Vis Spectroscopy of White Grapes Juice and Wine
3.2.2. ATR-FTIR Spectroscopy of White Grapes Juice and Wine
3.2.3. Colour of Must and Wine
3.2.4. pH, Brix and Densitometry Measurements of Must and Wine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kogelschatz, U. Dielectric-barrier discharges: Their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Kim, H.H. Nonthermal plasma processing for air-pollution control: A historical review, current issues, and future prospects. Plasma Process. Polym. 2004, 1, 91–110. [Google Scholar] [CrossRef]
- Kogelschatz, U. Atmospheric-pressure plasma technology. Plasma Phys. Control. Fusion 2004, 46, B63. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Nastuta, A.; Rusu, G.; Topala, I.; Chiper, A.; Popa, G. Surface modifications of polymer induced by atmospheric DBD plasma in different configurations. J. Optoelectron. Adv. Mater 2008, 10, 2038–2042. [Google Scholar]
- Von Woedtke, T.; Kramer, A.; Weltmann, K.D. Plasma sterilization: What are the conditions to meet this claim? Plasma Process. Polym. 2008, 5, 534–539. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Kindel, E.; Brandenburg, R.; Meyer, C.; Bussiahn, R.; Wilke, C.; Von Woedtke, T. Atmospheric pressure plasma jet for medical therapy: Plasma parameters and risk estimation. Contrib. Plasma Phys. 2009, 49, 631–640. [Google Scholar] [CrossRef]
- Nastuta, A.V.; Topala, I.; Grigoras, C.; Pohoata, V.; Popa, G. Stimulation of wound healing by helium atmospheric pressure plasma treatment. J. Phys. D Appl. Phys. 2011, 44, 105204. [Google Scholar] [CrossRef]
- Topala, I.; Nastuta, A.V. Helium atmospheric pressure plasma jet: Diagnostics and application for burned wounds healing. In Plasma for Bio-Decontamination, Medicine and Food Security. NATO Science for Peace and Security Series A: Chemistry and Biology; Machala, Z., Hensel, K., Akishev, Y., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 335–345. [Google Scholar] [CrossRef]
- Han, J. Review of major directions in non-equilibrium atmospheric plasma treatments in medical, biological, and bioengineering applications. Plasma Med. 2013, 3. [Google Scholar] [CrossRef]
- Nastuta, A.V.; Pohoata, V.; Topala, I. Atmospheric pressure plasma jet—Living tissue interface: Electrical, optical, and spectral characterization. J. App. Phys. 2013, 113, 183302. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Keidar, M. Plasma for cancer treatment. Plasma Sources Sci. Technol. 2015, 24, 033001. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma–liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Bekeschus, S.; Wende, K.; Hefny, M.M.; Rödder, K.; Jablonowski, H.; Schmidt, A.; von Woedtke, T.; Weltmann, K.D.; Benedikt, J. Oxygen atoms are critical in rendering THP-1 leukaemia cells susceptible to cold physical plasma-induced apoptosis. Sci. Rep. 2017, 7, 2791. [Google Scholar] [CrossRef] [PubMed]
- Nastuta, A.V.; Topala, I.; Pohoata, V.; Mihaila, I.; Agheorghiesei, C.; Dumitrascu, N. Atmospheric pressure plasma jets in inert gases: Electrical, optical and mass spectrometry diagnosis. Rom. Rep. Phys. 2017, 69, 407. [Google Scholar]
- Dai, X.; Bazaka, K.; Richard, D.J.; Thompson, E.R.W.; Ostrikov, K.K. The emerging role of gas plasma in oncotherapy. Trends Biotechnol. 2018, 36, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Favia, P.; Robert, E.; von Woedtke, T. White paper on plasma for medicine and hygiene: Future in plasma health sciences. Plasma Process. Polym. 2019, 16, 1800033. [Google Scholar] [CrossRef]
- Brandenburg, R.; Bogaerts, A.; Bongers, W.; Fridman, A.; Fridman, G.; Locke, B.R.; Miller, V.; Reuter, S.; Schiorlin, M.; Verreycken, T.; et al. White paper on the future of plasma science in environment, for gas conversion and agriculture. Plasma Process. Polym. 2019, 16, 1700238. [Google Scholar] [CrossRef]
- Cvelbar, U.; Walsh, J.L.; Černák, M.; de Vries, H.W.; Reuter, S.; Belmonte, T.; Corbella, C.; Miron, C.; Hojnik, N.; Jurov, A.; et al. White paper on the future of plasma science and technology in plastics and textiles. Plasma Process. Polym. 2019, 16, 1700228. [Google Scholar] [CrossRef]
- Nastuta, A.V.; Popa, G. Surface oxidation and enhanced hydrophilization of polyamide fiber surface after He/Ar atmospheric pressure plasma exposure. Rom. Rep. Phys. 2019, 71, 1–16. [Google Scholar]
- Šimek, M.; Černák, M.; Kylián, O.; Foest, R.; Hegemann, D.; Martini, R. White paper on the future of plasma science for optics and glass. Plasma Process. Polym. 2019, 16, 1700250. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Kolb, J.F.; Holub, M.; Uhrlandt, D.; Šimek, M.; Ostrikov, K.; Hamaguchi, S.; Cvelbar, U.; Černák, M.; Locke, B.; et al. The future for plasma science and technology. Plasma Process. Polym. 2019, 16, 1800118. [Google Scholar] [CrossRef]
- Branỳ, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef]
- Keidar, M. Introduction: Plasma for Cancer Therapy. In Plasma Cancer Therapy; Keidar, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–13. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; Colonna, W.; Keener, K. Effect of high voltage atmospheric cold plasma on white grape juice quality. J. Sci. Food Agric. 2017, 97, 4016–4021. [Google Scholar] [CrossRef] [PubMed]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.; Skotti, E. Novel application and industrial exploitation of winery by-products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Perinban, S.; Orsat, V.; Raghavan, V. Nonthermal Plasma—Liquid Interactions in Food Processing: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1985–2008. [Google Scholar] [CrossRef] [PubMed]
- Sainz-García, E.; López-Alfaro, I.; Múgica-Vidal, R.; López, R.; Escribano-Viana, R.; Portu, J.; Alba-Elías, F.; González-Arenzana, L. Effect of the Atmospheric Pressure Cold Plasma Treatment on Tempranillo Red Wine Quality in Batch and Flow Systems. Beverages 2019, 5, 50. [Google Scholar] [CrossRef]
- Huang, C.; Wu, J.; Wu, J.; Ting, Y. Effect of novel atmospheric-pressure jet pretreatment on the drying kinetics and quality of white grapes. J. Sci. Food Agric. 2019, 99, 5102–5111. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Cheng, J.H.; Sun, D.W. Chemical, physical and physiological quality attributes of fruit and vegetables induced by cold plasma treatment: Mechanisms and application advances. Crit. Rev. Food Sci. Nutr. 2020, 60, 2676–2690. [Google Scholar] [CrossRef]
- Ozen, E.; Singh, R. Atmospheric cold plasma treatment of fruit juices: A review. Trends Food Sci. Technol. 2020, 103, 144–151. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Domínguez, R.; Pateiro, M.; Lorenzo, J.M. Influence of Plasma Treatment on the Polyphenols of Food Products—A Review. Foods 2020, 9, 929. [Google Scholar] [CrossRef]
- Santamera, A.; Escott, C.; Loira, I.; del Fresno, J.M.; González, C.; Morata, A. Pulsed Light: Challenges of a Non-Thermal Sanitation Technology in the Winemaking Industry. Beverages 2020, 6, 45. [Google Scholar] [CrossRef]
- Sainz-García, A.; González-Marcos, A.; Múgica-Vidal, R.; Muro-Fraguas, I.; Escribano-Viana, R.; González-Arenzana, L.; López-Alfaro, I.; Alba-Elías, F.; Sainz-García, E. Application of atmospheric pressure cold plasma to sanitize oak wine barrels. LWT 2020, in press. [Google Scholar] [CrossRef]
- Sarangapani, C.; Scally, L.; Gulan, M.; Cullen, P.J. Dissipation of Pesticide Residues on Grapes and Strawberries Using Plasma-Activated Water. Food Bioproc. Tech. 2020, 13, 1728–1741. [Google Scholar] [CrossRef]
- Song, Y.; Fan, X. Cold plasma enhances the efficacy of aerosolized hydrogen peroxide in reducing populations of Salmonella Typhimurium and Listeria innocua on grape tomatoes, apples, cantaloupe and romaine lettuce. Food Microbiol. 2020, 87, 103391. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, Z.; Tounekti, T.; Khemira, H. Cold plasma treatment to release dormancy and improve growth in grape buds: A promising alternative to natural chilling and rest breaking chemicals. Sci. Rep. 2020, 10, 2667. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhang, R.; Fan, L.; Ma, Y.; Wu, D.; Li, K.; Bai, Y. Microbial inactivation and quality of grapes treated by plasma-activated water combined with mild heat. LWT 2020, 109336. [Google Scholar] [CrossRef]
- Laurita, R.; Contaldo, N.; Zambon, Y.; Bisag, A.; Canel, A.; Gherardi, M.; Laghi, G.; Bertaccini, A.; Colombo, V. The use of plasma-activated water in viticulture: Induction of resistance and agronomic performance in greenhouse and open field. Plasma Process. Polym. 2020, 18, e2000206. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Patange, A.; Sun, D.W.; Tiwari, B. Plasma-activated water: Physicochemical properties, microbial inactivation mechanisms, factors influencing antimicrobial effectiveness, and applications in the food industry. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3951–3979. [Google Scholar] [CrossRef] [PubMed]
- Ashtiani, S.H.M.; Rafiee, M.; Morad, M.M.; Khojastehpour, M.; Khani, M.; Rohani, A.; Shokri, B.; Martynenko, A. Impact of gliding arc plasma pretreatment on drying efficiency and physicochemical properties of grape. Innov. Food Sci. Emerg. Technol. 2020, 63, 102381. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J. Enhancement of phenolic compounds extraction from grape pomace by high voltage atmospheric cold plasma. LWT 2020, 133, 109970. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Ojha, S.; Šeremet, D.; Nutrizio, M.; Maltar-Strmečki, N.; Valić, S.; Kljusurić, J.G.; Tiwari, B. Free radical detection in water after processing by means of high voltage electrical discharges and high power ultrasound. J. Food Process. Preserv. 2020, 45, e15176. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Xiang, Q.; Liao, X.; Liu, D.; Ding, T. Understanding the impact of nonthermal plasma on food constituents and microstructure—A review. Food Bioprocess Technol. 2018, 11, 463–486. [Google Scholar] [CrossRef]
- López, M.; Calvo, T.; Prieto, M.; Múgica-Vidal, R.; Muro-Fraguas, I.; Alba-Elías, F.; Alvarez-Ordóñez, A. A review on non-thermal atmospheric plasma for food preservation: Mode of action, determinants of effectiveness, and applications. Front. Microbiol. 2019, 10, 622. [Google Scholar] [CrossRef]
- White, R.E. Understanding Vineyard Soils, 2nd ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Basalekou, M.; Pappas, C.; Tarantilis, P.; Kallithraka, S. Wine Authenticity and Traceability with the Use of FT-IR. Beverages 2020, 6, 30. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.; Buica, A.; Nieuwoudt, H.; Aleixandre, J.; du Toit, W. Spectrophotometric analysis of phenolic compounds in grapes and wines. J. Agric. Food Chem. 2017, 65, 4009–4026. [Google Scholar] [CrossRef]
- Guilford, J.M.; Pezzuto, J.M. Wine and health: A review. Am. J. Enol. Vitic. 2011, 62, 471–486. [Google Scholar] [CrossRef]
- Golan, R.; Gepner, Y.; Shai, I. Wine and Health—New Evidence. Eur. J. Clin. Nutr. 2019, 72, 55–59. [Google Scholar] [CrossRef]
- Albu, C.; Radu, L.E.; Radu, G.L. Assessment of Melatonin and Its Precursors Content by a HPLC-MS/MS Method from Different Romanian Wines. ACS Omega 2020, 5, 27254–27260. [Google Scholar] [CrossRef] [PubMed]
- Ferraz da Costa, D.C.; Pereira Rangel, L.; Quarti, J.; Santos, R.A.; Silva, J.L.; Fialho, E. Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy. Molecules 2020, 25, 3531. [Google Scholar] [CrossRef]
- OIV. State of the World Vitivinicultural Sector in 2019. 2020. Available online: http://www.oiv.int/public/medias/7298/oiv-state-of-the-vitivinicultural-sector-in-2019.pdf (accessed on 1 December 2020).
- OIV. 2020 Wine Production First Estimates 27/10/2020. 2020. Available online: http://www.oiv.int/public/medias/7541/en-oiv-2020-world-wine-production-first-estimates.pdf (accessed on 1 December 2020).
- Cotea, D.V.; Barbu, N.; Grigorescu, C.; Cotea, V.V. Vineyards and Wines of Romania; Academiei Române: Bucureşti, Romania, 2005. [Google Scholar]
- Ionesi, L.; Ionesi, B.; Lungu, A.; Rosca, V.; Ionesi, V. Sarmaţianul mediu şi superior de pe Platforma Moldovenească; Middle and Upper Sarmatian on Moldavian Platform; Academiei Române: Bucureşti, Romania, 2005; pp. 1–439. [Google Scholar]
- OIV. International Standard for the Labelling of Wines-Edition 2015. Available online: http://www.oiv.int/public/medias/4776/oiv-wine-labelling-standard-en-2015.pdf (accessed on 1 December 2020).
- OIV. Compendium of International Methods of Wine and Must Analysis. 2020. Available online: http://www.oiv.int/public/medias/7372/oiv-compendium-volume-1-2020.pdf (accessed on 1 December 2020).
- Tzachristas, A.; Pasvanka, K.; Calokerinos, A.; Proestos, C. Polyphenols: Natural antioxidants to be used as a quality tool in wine authenticity. Appl. Sci. 2020, 10, 5908. [Google Scholar] [CrossRef]
- Sanna, R.; Piras, C.; Marincola, F.; Lecca, V.; Maurichi, S.; Scano, P. Multivariate statistical analysis of the UV-vis profiles of wine polyphenolic extracts during vinification. J. Agric. Sci. 2014, 6, 152. [Google Scholar] [CrossRef][Green Version]
- Aleixandre-Tudo, J.L.; du Toit, W. The Role of UV-Visible Spectroscopy for Phenolic Compounds Quantification in Winemaking. In Frontiers and New Trends in the Science of Fermented Food and Beverages; Solís-Oviedo, R.L., de la Cruz Pech-Canul, A., Eds.; IntechOpen: Rijeka, Croatia, 2018; pp. 1–22. [Google Scholar] [CrossRef]
- Yalçın, O.; Tekgündüz, C.; Öztürk, M.; Tekgündüz, E. Investigation of the traditional organic vinegars by UV–VIS spectroscopy and rheology techniques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 246, 118987. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Azcarate, S.M.; Camiña, J.; Callejón, R.M. Assessment of UV–visible spectroscopy as a useful tool for determining grape-must caramel in high-quality wine and balsamic vinegars. Food Chem. 2020, 323, 126792. [Google Scholar] [CrossRef]
- Louw, L.; Roux, K.; Tredoux, A.; Tomic, O.; Naes, T.; Nieuwoudt, H.; Van Rensburg, P. Characterization of selected South African young cultivar wines using FTMIR spectroscopy, gas chromatography, and multivariate data analysis. J. Agric. Food Chem. 2009, 57, 2623–2632. [Google Scholar] [CrossRef]
- Sen, I.; Ozturk, B.; Tokatli, F.; Ozen, B. Combination of visible and mid-infrared spectra for the prediction of chemical parameters of wines. Talanta 2016, 161, 130–137. [Google Scholar] [CrossRef]
- Basalekou, M.; Kallithraka, S.; Tarantilis, P.A.; Kotseridis, Y.; Pappas, C. Ellagitannins in wines: Future prospects in methods of analysis using FT-IR spectroscopy. LWT 2019, 101, 48–53. [Google Scholar] [CrossRef]
- Topala, C.M.; Tataru, L.D. ATR-FTIR Spectroscopy Coupled with Chemical and Chemometric Analysis to Distinguish Between Some Sweet Wines. Rev. Chim. 2019, 70, 2355–2361. [Google Scholar] [CrossRef]
- Álvarez, Á.; Yáñez, J.; Neira, Y.; Castillo-Felices, R.; Hinrichsen, P. Simple distinction of grapevine (Vitis vinifera L.) genotypes by direct ATR-FTIR. Food Chem. 2020, 328, 127164. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Gallego, R.; Rodríguez-Pulido, F.; Toci, A.; García-Estevez, I. Phenolic Composition, Quality and Authenticity of Grapes and Wines by Vibrational Spectroscopy. Food Rev. Int. 2020, 1–29. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Kiefer, J.; Santini, A.; Lombardi-Boccia, G.; Souto, E.; Romani, A.; Lampe, A.; Nicoli, F.; Gabrielli, P.; et al. Grape seeds: Chromatographic profile of fatty acids and phenolic compounds and qualitative analysis by FTIR-ATR spectroscopy. Foods 2020, 9, 10. [Google Scholar] [CrossRef]
- Coimbra, M.; Gonçalves, F.; Barros, A.; Delgadillo, I. Fourier transform infrared spectroscopy and chemometric analysis of white wine polysaccharide extracts. J. Agric. Food Chem. 2002, 50, 3405–3411. [Google Scholar] [CrossRef]
- Banc, R.; Loghin, F.; Miere, D.; Fetea, F.; Socaciu, C. Romanian wines quality and authenticity using FT-MIR spectroscopy coupled with multivariate data analysis. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2014, 42, 556–564. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.; Nieuwoudt, H.; Olivieri, A.; Aleixandre, J.; du Toit, W. Phenolic profiling of grapes, fermenting samples and wines using UV-Visible spectroscopy with chemometrics. Food Control 2018, 85, 11–22. [Google Scholar] [CrossRef]
- Minute, F.; Giotto, F.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Alternative Methods for Measuring the Susceptibility of White Wines to Pinking Alteration: Derivative Spectroscopy and CIEL*a*b* Colour Analysis. Foods 2021, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Moshe, R.; Deutsch, J.; Wolfed, F.; Tilis, K.; Stiller, A.; Flam, I.; Gat, Y. Determination of basic components in white wines by HPLC, FT-IR spectroscopy, and electrophoretic techniques. J. Food Compos. Anal. 1992, 5, 236–245. [Google Scholar] [CrossRef]
- Todasca, M.C.; Chira, N.; Deleanu, C.; Rosca, S. Romanian wine study using IR spectroscopy in comparison with 1 HNMR. UPB Sci. Bull. B Chem. Mater. Sci. 2007, 69, 3–10. [Google Scholar]
- Olejar, K.J.; Ricci, A.; Swift, S.; Zujovic, Z.; Gordon, K.; Fedrizzi, B.; Versari, A.; Kilmartin, P. Characterization of an antioxidant and antimicrobial extract from cool climate, white grape marc. Antioxidants 2019, 8, 232. [Google Scholar] [CrossRef]
- Bañuelos, M.A.; Loira, I.; Guamis, B.; Escott, C.; Del Fresno, J.M.; Codina-Torrella, I.; Quevedo, J.M.; Gervilla, R.; Chavarría, J.M.R.; de Lamo, S.; et al. White wine processing by UHPH without SO2. Elimination of microbial populations and effect in oxidative enzymes, colloidal stability and sensory quality. Food Chem. 2020, 332, 127417. [Google Scholar] [CrossRef]
- Dienes-Nagy, Á.; Marti, G.; Breant, L.; Lorenzini, F.; Fuchsmann, P.; Baumgartner, D.; Zufferey, V.; Spring, J.; Gindro, K.; Viret, O.; et al. Identification of putative chemical markers in white wine (Chasselas) related to nitrogen deficiencies in vineyards. OENO One 2020, 54, 583–599. [Google Scholar] [CrossRef]
- Mendes, E.; Duarte, N. Mid-Infrared Spectroscopy as a Valuable Tool to Tackle Food Analysis: A Literature Review on Coffee, Dairies, Honey, Olive Oil and Wine. Foods 2021, 10, 477. [Google Scholar] [CrossRef]
- Ţârdea, C. Chimia şi Analiza Vinului [Chemistry and Wine Analysis]; Ion Ionescu de la Brad: Iaşi, Romania, 2007. [Google Scholar]
- Delgado-González, M.; Carmona-Jiménez, Y.; Rodríguez-Dodero, M.; García-Moreno, M. Color Space Mathematical Modeling Using Microsoft Excel. J. Chem. Educ. 2018, 95, 1885–1889. [Google Scholar] [CrossRef]
- García-Marino, M.; Escudero-Gilete, M.; Escribano-Bailón, M.; González-Miret, M.; Rivas-Gonzalo, J.; Heredia, F. Colorimetric characteristics of the phenolic fractions obtained from Tempranillo and Graciano wines through the use of different instrumental techniques. Anal. Chim. Acta 2012, 732, 153–161. [Google Scholar] [CrossRef]
- Rolle, L.; Giordano, M.; Giacosa, S.; Vincenzi, S.; Segade, S.; Torchio, F.; Perrone, B.; Gerbi, V. CIEL*a*b* parameters of white dehydrated grapes as quality markers according to chemical composition, volatile profile and mechanical properties. Anal. Chim. Acta 2012, 732, 105–113. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Perez-Wom, M.; Habib, G.; Giovagnoli-Vicuña, C.; Cañas-Sarazua, R.; Tabilo-Munizaga, G.; Salazar, F.N. Oenological and quality characteristic on young white wines (sauvignon blanc): Effects of high hydrostatic pressure processing. J. Food Qual. 2017, 2017, 8524073. [Google Scholar] [CrossRef]
- Jacobson, J.L. Introduction to Wine Laboratory Practices and Procedures, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2006; p. 135. [Google Scholar] [CrossRef]
- Bührle, F.; Gohl, A.; Weber, F. Impact of Xanthylium Derivatives on the Color of White Wine. Molecules 2017, 22, 1376. [Google Scholar] [CrossRef] [PubMed]
- Ewart, A. White wines. In Fermented Beverage Production; Lea, A.G.H., Piggott, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 89–106. [Google Scholar] [CrossRef]
- Musther, J. VinoCalc. 2020. Available online: https://www.vinolab.hr/calculator/gravity-density-sugar-conversions-en19 (accessed on 1 May 2020).
- Gerber, I.; Mihaila, I.; Hein, D.; Nastuta, A.V.; Jijie, R.; Pohoata, V.; Topala, I. Time behaviour of helium atmospheric pressure plasma jet electrical and optical parameters. Appl. Sci. 2017, 7, 812. [Google Scholar] [CrossRef]
- Guo, J.; Huang, K.; Wang, X.; Lyu, C.; Yang, N.; Li, Y.; Wang, J. Inactivation of yeast on grapes by plasma-activated water and its effects on quality attributes. J. Food Prot. 2017, 80, 225–230. [Google Scholar] [CrossRef]
- Lukić, K.; Vukušić, T.; Tomašević, M.; Ćurko, N.; Gracin, L.; Ganić, K.K. The impact of high voltage electrical discharge plasma on the chromatic characteristics and phenolic composition of red and white wines. Innov. Food Sci. Emerg. Technol. 2019, 53, 70–77. [Google Scholar] [CrossRef]
- Nishime, T.; Wannicke, N.; Horn, S.; Weltmann, K.D.; Brust, H. A Coaxial Dielectric Barrier Discharge Reactor for Treatment of Winter Wheat Seeds. Appl. Sci. 2020, 10, 7133. [Google Scholar] [CrossRef]
- Starek-Wójcicka, A.; Sagan, A.; Terebun, P.; Kwiatkowski, M.; Kiczorowski, P.; Pawlat, J. Influence of a Helium–Nitrogen RF Plasma Jet on Onion Seed Germination. Appl. Sci. 2020, 10, 8973. [Google Scholar] [CrossRef]
- Wang, J.; Han, R.; Liao, X.; Ding, T. Application of plasma-activated water (PAW) for mitigating methicillin-resistant Staphylococcus aureus (MRSA) on cooked chicken surface. LWT 2020, 137, 110465. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, J.; Sun, D. Inhibition of fruit softening by cold plasma treatments: Affecting factors and applications. Crit. Rev. Food Sci. Nutr. 2020, 61, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Fan, L.; Li, Y.; Dong, S.; Li, K.; Bai, Y. A review on recent advances in plasma-activated water for food safety: Current applications and future trends. Crit. Rev. Food Sci. Nutr. 2020, 1–19. [Google Scholar] [CrossRef]
- Fan, L.; Liu, X.; Ma, Y.; Xiang, Q. Effects of plasma-activated water treatment on seed germination and growth of mung bean sprouts. J. Taibah Univ. Sci. 2020, 14, 823–830. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Vidyarthi, S.K.; Zhong, C.S.; Zheng, Z.A.; An, Y.; Wang, J.; Wei, Q.; Xiao, H.W. Cold plasma enhances drying and color, rehydration ratio and polyphenols of wolfberry via microstructure and ultrastructure alteration. LWT 2020, 134, 110173. [Google Scholar] [CrossRef]
- Gerling, T.; Nastuta, A.V.; Bussiahn, R.; Kindel, E.; Weltmann, K.D. Back and forth directed plasma bullets in a helium atmospheric pressure needle-to-plane discharge with oxygen admixtures. Plasma Sources Sci. Technol. 2012, 21, 034012. [Google Scholar] [CrossRef][Green Version]
- Gerling, T.; Wild, R.; Nastuta, A.V.; Wilke, C.; Weltmann, K.D.; Stollenwerk, L. Correlation of phase resolved current, emission and surface charge measurements in an atmospheric pressure helium jet. Eur. Phys. J. Appl. Phys. 2015, 71, 20808. [Google Scholar] [CrossRef]
- Luque, J.; Crosley, D.R. LIFBASE: Database and spectral simulation program (version 1.5). SRI Int. Rep. MP 1999, 99. Available online: https://ci.nii.ac.jp/naid/10011750245 (accessed on 5 August 2021).
- Navrátil, Z.; Trunec, D.; Šmíd, R.; Lazar, L. A software for optical emission spectroscopy-problem formulation and application to plasma diagnostics. Czechoslov. J. Phys. 2006, 56, B944–B951. [Google Scholar] [CrossRef]
- Radzig, A.A.; Smirnov, B.M. Reference Data on Atoms, Molecules, and Ions; Springer: Berlin/Heidelberg, Germany, 1985; pp. 230–235. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y.; Reader, J.; Team, N.A. NIST Atomic Spectra Database (version 5.8). 2020. Available online: https://physics.nist.gov/asd (accessed on 1 December 2020). [CrossRef]
- Junqua, R.; Carullo, D.; Ferrari, G.; Pataro, G.; Ghidossi, R. Ohmic heating for polyphenol extraction from grape berries: An innovative prefermentary process. OENO One 2021, 55, 39–51. [Google Scholar] [CrossRef]
- Azcarate, S.M.; Cantarelli, M.Á.; Pellerano, R.G.; Marchevsky, E.J.; Camiña, J. Classification of Argentinean Sauvignon blanc wines by UV spectroscopy and chemometric methods. J. Food Sci. 2013, 78, C432–C436. [Google Scholar] [CrossRef]
- Kerslake, F.; Longo, R.; Dambergs, R. Discrimination of juice press fractions for sparkling base wines by a UV-Vis spectral phenolic fingerprint and chemometrics. Beverages 2018, 4, 45. [Google Scholar] [CrossRef]
- Scutaru, I.; Balanuta, A.; Zgardan, D. The determination of oxidation behavior of white wines produced from local and european grape varieties using spectrophotometric method. J. Eng. Sci. 2018, XXV, 82–93. [Google Scholar] [CrossRef]
- Martelo-Vidal, M.J.; Vazquez, M. Evaluation of ultraviolet, visible, and near infrared spectroscopy for the analysis of wine compounds. Czech J. Food Sci. 2014, 32, 37–47. [Google Scholar] [CrossRef]
- Yu, J.; Wang, H.; Zhan, J.; Huang, W. Review of recent UV–Vis and infrared spectroscopy researches on wine detection and discrimination. Appl. Spectrosc. Rev. 2018, 53, 65–86. [Google Scholar] [CrossRef]
- Banc, R.; Loghin, F.; Miere, D.; Ranga, F.; Socaciu, C. Phenolic composition and antioxidant activity of red, rosé and white wines originating from Romanian grape cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2020, 48, 716–734. [Google Scholar] [CrossRef]

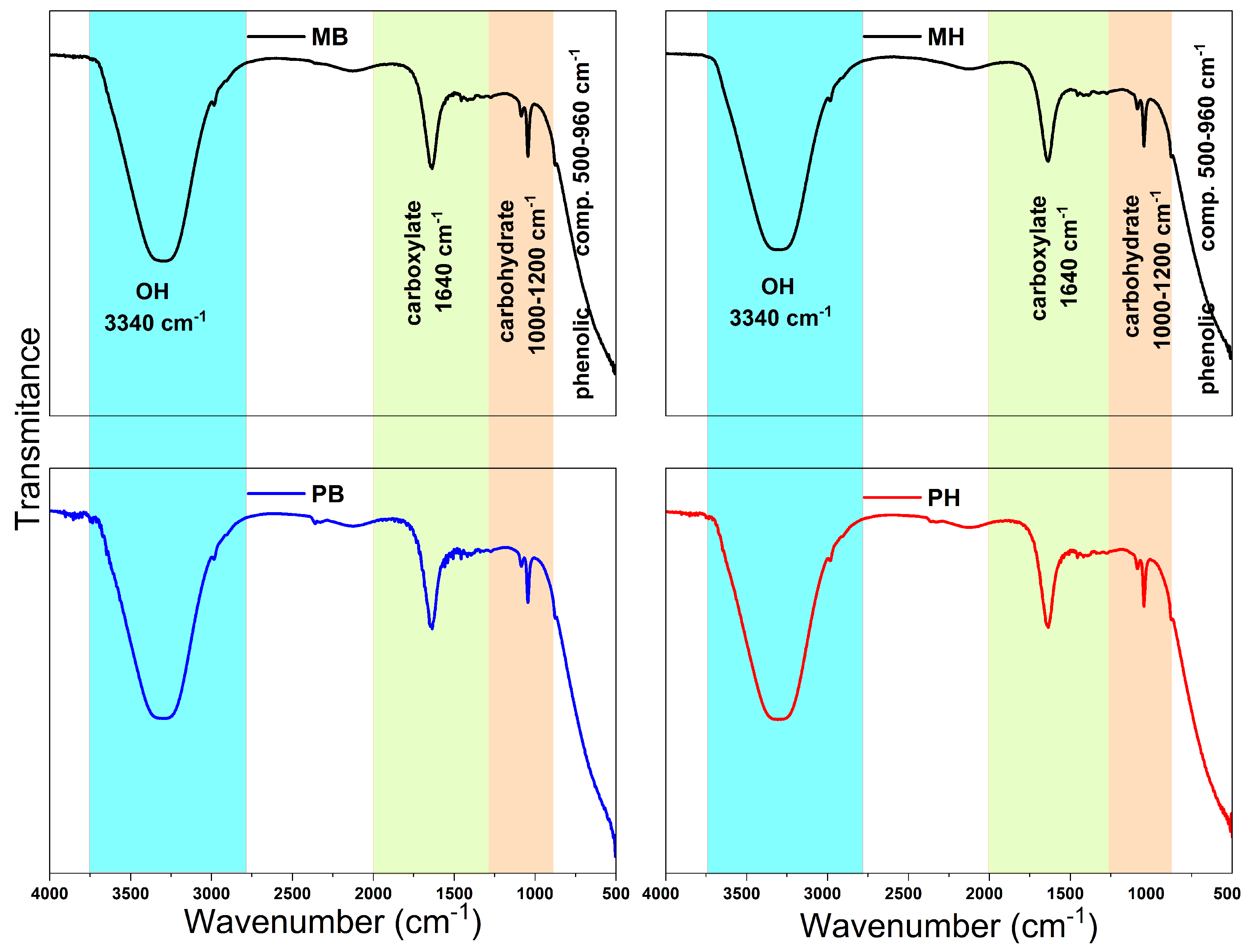


| Wavenumber Regions (cm−1) | Groups | Assignment |
|---|---|---|
| <1000 | stretching and bending vibrations | phosphates, phenolics, mono-substituted phenyl derivates, unsaturated lipids, carotenoids |
| 1068–1065 1107–1110 1200 | stretching vibration of C-O O-H stretch second overtones | sugars and organic acids |
| 950–1250 | stretching and bending vibrations | hydrolyzable and condensed tannins glucose, oligo- and polysaccharides, alcohols |
| 1457–1288 | C=O, C=C, -CH2-, C-H, -CH3, O-H | aldehydes, carboxy |
| 1530–1600 | C-N | amino acids and derivatives |
| 1516–1519 1610–1614 | C=C | aromatic compounds, flavonoids |
| 1700 | C=O | organic acids |
| 1704–1712 | C=O | esters of hydrolyzable tannins, derivatives of gallic acid and flavors |
| 1600–1900 | O-H stretching C-H3 stretch first overtone C-H2, C-H stretch first overtones | water, ethanol, glucose |
| 2100–2300 | C-H combinations vibrations and overtones | sugar and ethanol |
| 2800–3000 | C-H stretching of hydrocarbons -CH3 asymmetric stretching vibration O-H stretching of carboxylic acids | glycerol, catechins and free phenolic acids |
| 3000–3500 | -OH | alcohols, phenols, water |
[nm] | Transition | Normalized Intensity | Ek [eV] | |
|---|---|---|---|---|
| in Midair | with Must | |||
| 308 OH | ← | 0.333 | 1 | 4 |
| 337 N2 | ← | 0.782 | 0.927 | 11.0 |
| 391 | ← | 1 | 0.299 | 18.7 |
| 486 H | 4 ← 2 | 0.070 | 0.117 | 12.7 |
| 587 He | ← | 0.067 | 0.078 | 23.1 |
| 667 He | ← | 0.058 | 0.091 | 23.1 |
| 706 He | ← | 0.073 | 0.088 | 22.7 |
| 728 He | ← | 0.067 | 0.076 | 22.9 |
| 777 O | ← | 0.097 | 0.111 | 10.7 |
| 844 O | ← | 0.054 | 0.087 | 11.0 |
| Method | Trot (OH) [K] | Trot () [K] | Tv () [K] | Tgas [°C] |
|---|---|---|---|---|
| Spectrum Analyzer | x | x | 2265 − 2566 ± 190 | x |
| Lifbase | 340 − 350 ± 2 | 345 − 355 ± 2 | x | x |
| IR camera | x | x | x | 40 ± 1.5 |
| k-type probe | x | x | x | 35 ± 3.0 |
| Wine Samples | Region 1 500–960 (cm−1) | Region 2 1000–1200 (cm−1) | Region 3 1250–2000 (cm−1) | Region 4 2800–3500 (cm−1) |
|---|---|---|---|---|
| MB | 865, 903 | 1044, 1084 | 1318, 1385, 1636 | 2983, 3290 |
| PB | 876 | 1044, 1088 | 1317, 1397, 1456, 1636 | 2984, 3303 |
| MH | 876 | 1044, 1085 | 1274, 1392, 1455, 1636 | 2983, 3290 |
| PH | 875 | 1044, 1084 | 1320, 1388, 1456, 1636 | 2980, 3313 |
| Sample | L* | a* | b* | WI | h | R | G | B | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1y MB | 85.49 ± 0.03 | 6.43 ± 0.02 | 39.19 ± 0.03 | 57.72 ± 0.06 | 39.71 ± 0.06 | 80.68 ± 0.09 | - | 250 | 208 | 138 |
| 1y PB | 95.26 ± 0.02 | 1.20 ± 0.05 | 22.00 ± 0.08 | 77.46 ± 0.05 | 22.03 ± 0.09 | 86.88 ± 0.01 | 20.45 ± 0.03 | 255 | 239 | 198 |
| 2y MB | 78.22 ± 0.07 | 12.18 ± 0.04 | 48.34 ± 0.06 | 45.60 ± 0.02 | 49.85 ± 0.01 | 75.85 ± 0.06 | - | 241 | 183 | 102 |
| 2y PB | 91.13 ± 0.06 | 3.01 ± 0.03 | 25.89 ± 0.01 | 72.46 ± 0.09 | 26.07 ± 0.06 | 83.36 ± 0.03 | 27.47 ± 0.05 | 254 | 226 | 179 |
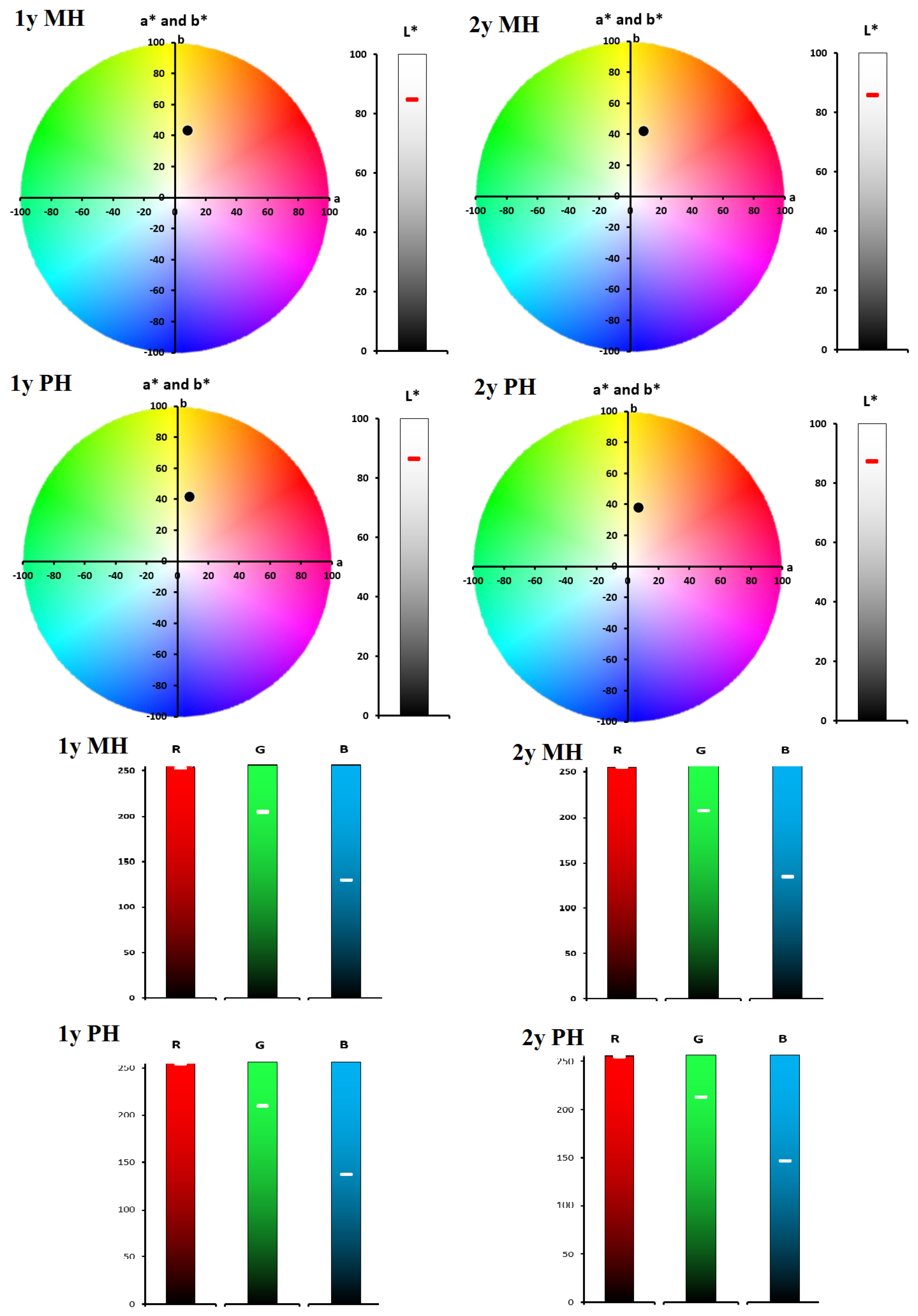
| 1y MH | 84.75 ± 0.05 | 8.48 ± 0.02 | 42.98 ± 0.09 | 53.62 ± 0.05 | 43.80 ± 0.04 | 78.83 ± 0.07 | - | 252 | 204 | 129 |
| 1y PH | 86.26 ± 0.08 | 7.98 ± 0.05 | 41.58 ± 0.04 | 55.49 ± 0.08 | 42.34 ± 0.08 | 79.13 ± 0.05 | 2.12 ± 0.02 | 255 | 209 | 136 |
| 2y MH | 85.64 ± 0.04 | 8.95 ± 0.08 | 41.75 ± 0.07 | 54.95 ± 0.03 | 42.70 ± 0.05 | 77.90 ± 0.01 | - | 255 | 206 | 134 |
| 2y PH | 87.32 ± 0.09 | 7.19 ± 0.06 | 37.83 ± 0.04 | 59.46 ± 0.07 | 38.51 ± 0.09 | 79.24 ± 0.03 | 4.62 ± 0.05 | 255 | 212 | 146 |
| Sample | pH | Brix (%) | Dissolved Sugar (g/L) | Density (g/L) | Potential Alcohol (% v/v) |
|---|---|---|---|---|---|
| MB | 3.3 ± 0.1 | 10.5 ± 0.5 | 109.4 | 1042 | 5.55 |
| PB | 3.3 ± 0.1 | 10.5 ± 0.5 | 109.4 | 1042 | 5.55 |
| MH | 3.4 ± 0.1 | 15.0 ± 0.5 | 159.2 | 1061 | 8.13 |
| PH | 3.4 ± 0.1 | 15.0 ± 0.5 | 159.2 | 1061 | 8.13 |
| Sample | pH | Brix (%) | Dissolved Sugar) (g/L) | Density (g/L) |
Alcohol (% v/v) |
|---|---|---|---|---|---|
| 1y MB | 3.5 ± 0.1 | 6.0 ± 0.5 | 61.4 | 1023 | 16.5 ± 1 |
| 1y PB | 3.5 ± 0.1 | 6.0 ± 0.5 | 61.4 | 1023 | 15.0 ± 1 |
| 2y MB | 3.6 ± 0.1 | 7.0 ± 0.5 | 71.9 | 1027 | 17.0 ± 1 |
| 2y PB | 3.6 ± 0.1 | 6.0 ± 0.5 | 61.4 | 1023 | 16.0 ± 1 |
| 1y MH | 3.5 ± 0.1 | 10.0 ± 0.5 | 104.5 | 1040 | 13.0 ± 1 |
| 1y PH | 3.5 ± 0.1 | 10.0 ± 0.5 | 104.5 | 1040 | 11.0 ± 1 |
| 2y MH | 3.4 ± 0.1 | 6.5 ± 0.5 | 66.6 | 1025 | 16.0 ± 1 |
| 2y PH | 3.4 ± 0.1 | 6.0 ± 0.5 | 61.4 | 1023 | 15.5 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huzum, R.; Nastuta, A.V. Helium Atmospheric Pressure Plasma Jet Source Treatment of White Grapes Juice for Winemaking. Appl. Sci. 2021, 11, 8498. https://doi.org/10.3390/app11188498
Huzum R, Nastuta AV. Helium Atmospheric Pressure Plasma Jet Source Treatment of White Grapes Juice for Winemaking. Applied Sciences. 2021; 11(18):8498. https://doi.org/10.3390/app11188498
Chicago/Turabian StyleHuzum, Ramona, and Andrei Vasile Nastuta. 2021. "Helium Atmospheric Pressure Plasma Jet Source Treatment of White Grapes Juice for Winemaking" Applied Sciences 11, no. 18: 8498. https://doi.org/10.3390/app11188498
APA StyleHuzum, R., & Nastuta, A. V. (2021). Helium Atmospheric Pressure Plasma Jet Source Treatment of White Grapes Juice for Winemaking. Applied Sciences, 11(18), 8498. https://doi.org/10.3390/app11188498







