Random Forests Highlight the Combined Effect of Environmental Heavy Metals Exposure and Genetic Damages for Cardiovascular Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Description
- 34 subjects in Class MR;
- 18 subjects in Class HR;
- 38 subjects in Class VHR.
2.2. Methods
2.2.1. Data Sampling
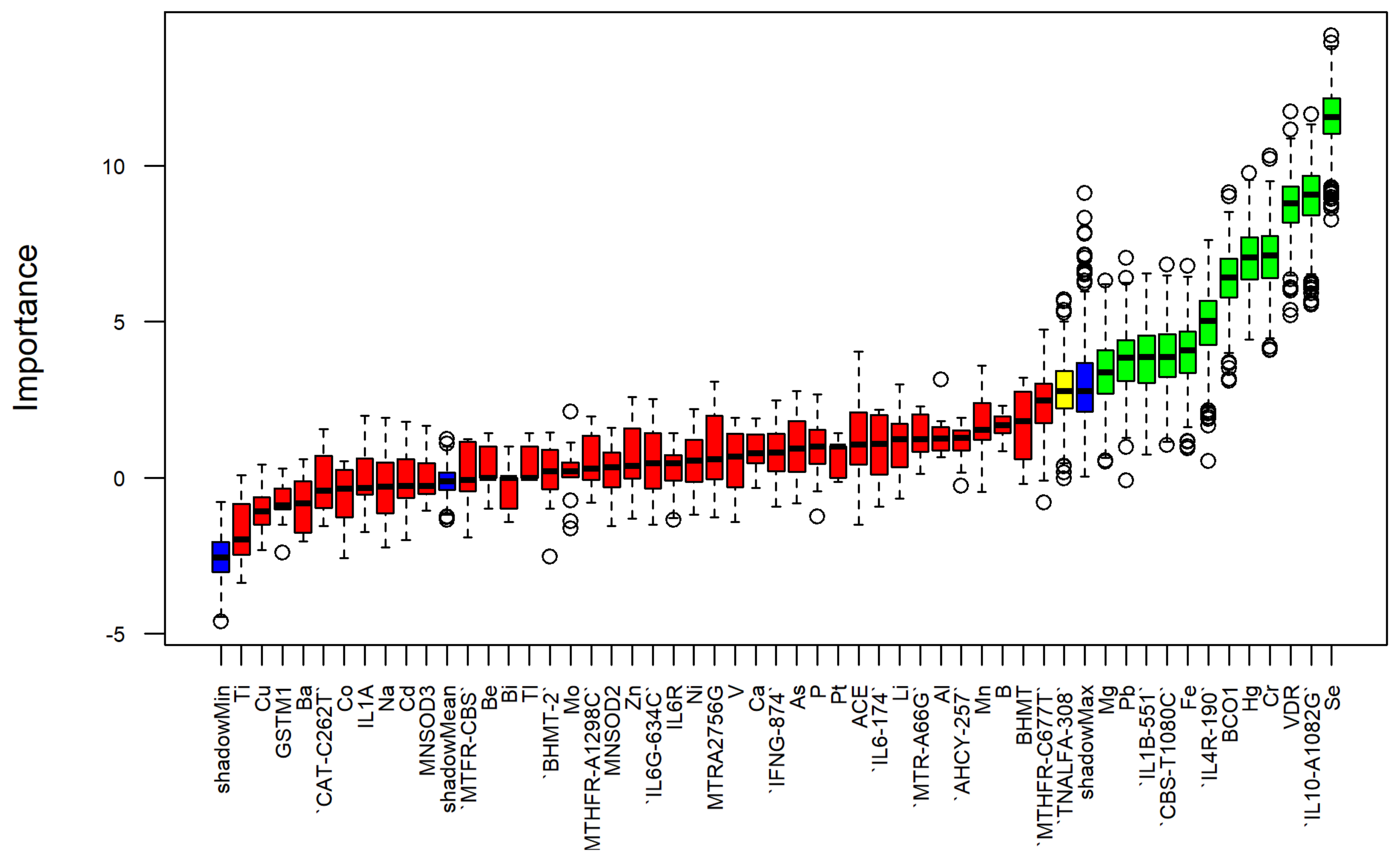
2.2.2. Feature Selection
2.2.3. Classification Model
- A random component that specifies the conditional distribution of the dependent variable composed of n independent observations in relation to the values of the independent variables of the model.
- A linear function of regressors.
- A linearizing link function that converts the expectation of , in .
2.2.4. Cross-Validation and Performance Metrics
3. Results
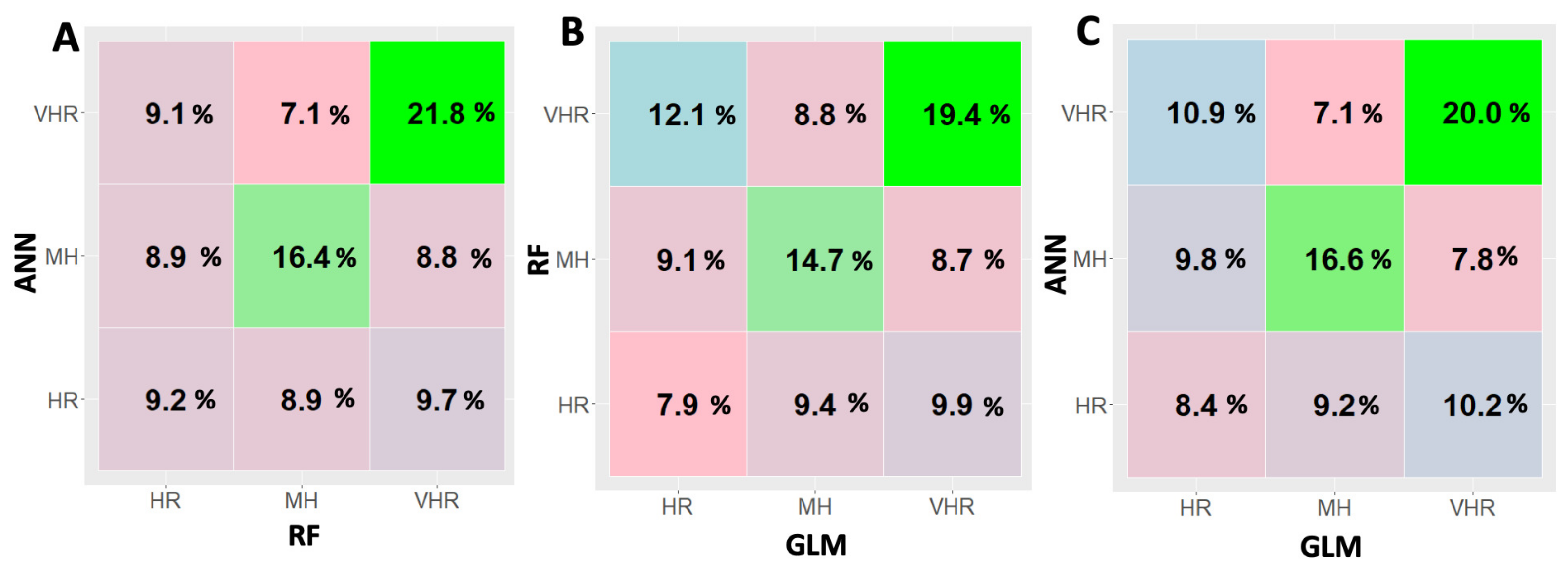
3.1. Comparing Different Machine Learning Strategies
3.2. Clinical Features Affecting CVDs
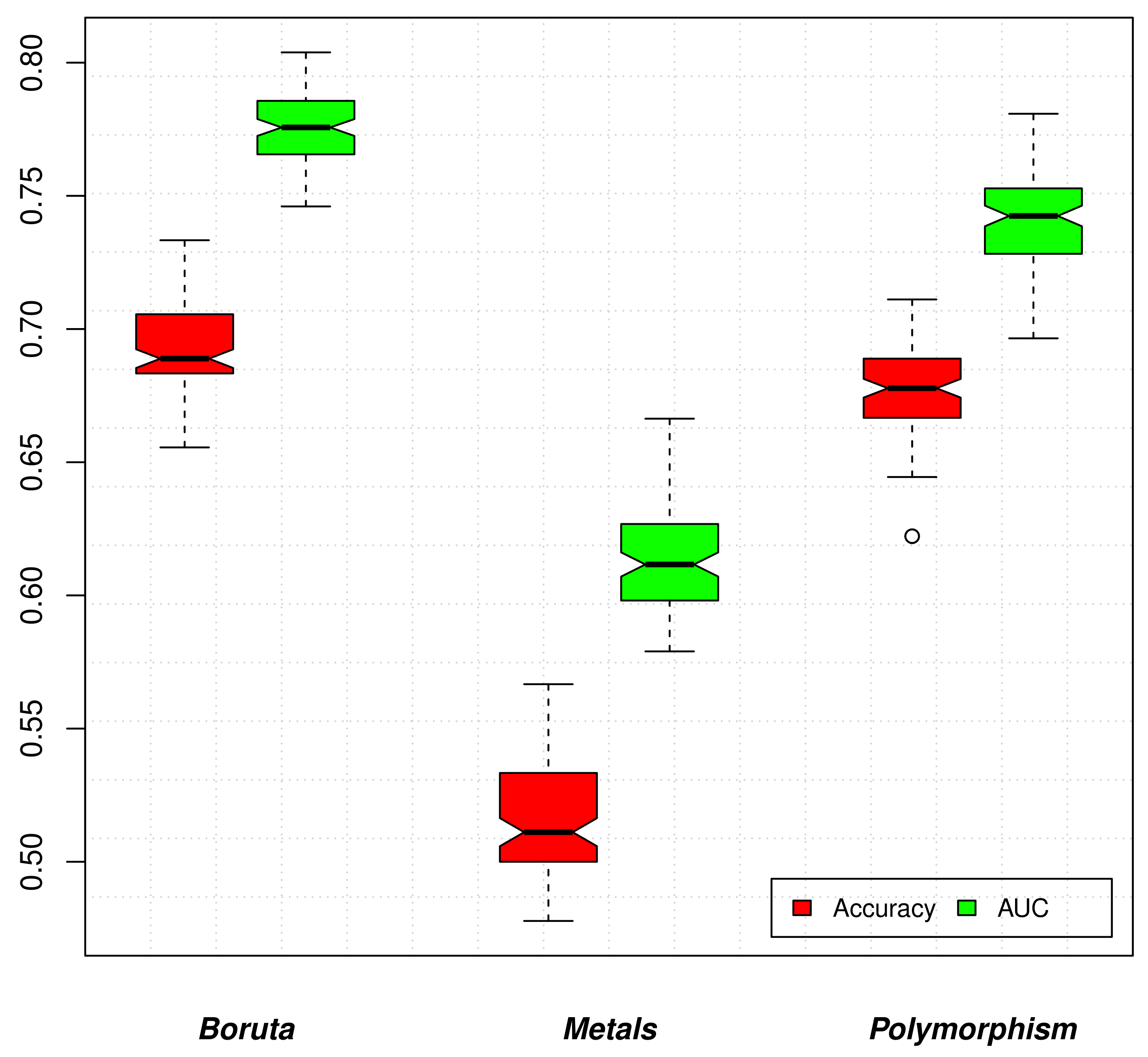
3.3. Assessing the CVD Severity
3.4. Heavy Metals vs. SNPs Informative Power
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in the Terrestrial Environment; Springer: Berlin, Germany, 1982. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Selenium; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2001.
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Copper; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2004.
- ATSDR (Agency of Toxic Substances and Disease Registry). Toxicological profile for Antimony; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 1992.
- ATSDR (Agency of Toxic Substances and Disease Registry). Toxicological profile for Beryllium Atlanta; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2002.
- ATSDR (Agency of Toxic Substances and Disease Registry). Toxicological Profile for Cobalt; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2004.
- ATSDR (Agency of Toxic Substances and Disease Registry). Toxicological Profile for Chromium; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2008.
- ATSDR (Agency of Toxic Substances and Disease Registry). Toxicological profile for Manganese; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2000.
- Godfrey, M.E.; Wojcik, D.P.; Krone, C.A. Apolipoprotein E genotyping as a potential biomarker for mercury neurotoxicity. J. Alzheimers Dis. 2003, 5, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Experientia Supplementum; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101. [Google Scholar]
- Flora, S.J.S.; Mittal, M.; Mehta, A. Heavy metal induced oxidative stress and its reversal by chelation therapy. Ind. J. Med. Res. 2018, 128, 501–523. [Google Scholar]
- Morales, M.E.; Derbes, R.S.; Ade, C.M.; Ortego, J.C.; Stark, J.; Deininger, P.L.; Roy-Engel, A.M. Heavy metal exposure influences double strand break DNA repair outcomes. PLoS ONE 2016, 11, e0151367. [Google Scholar]
- Jadoon, S.; Malik, A. DNA Damage by Heavy Metals in Animals and Human Beings: An Overview. Biochem. Pharmacol. 2017, 6, 3. [Google Scholar] [CrossRef]
- Benoff, S.; Hauser, R.; Marmar, J.L.; Hurley, I.R.; Napolitano, B.; Centola, G.M. Cadmium concentrations in blood and seminal plasma: Correlations with sperm number and motility in three male populations (infertility patients, artificial insemination donors, and unselected volunteers). Mol. Med. 2009, 15, 248–262. [Google Scholar] [CrossRef]
- AHA (American Heart Association). Heart disease and stroke statistics—2007 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007, 115, e169–e171. [Google Scholar]
- Mokdad, A.H.; Mensah, G.A.; Krish, V.; Glenn, S.D.; Miller-Petrie, M.K.; Lopez, A.D.; Murray, C. Global, Regional, National, and Subnational Big Data to Inform Health Equity Research: Perspectives from the Global Burden of Disease Study 2017. Ethn. Dis. 2019, 29 (Suppl. S1), 159–172. [Google Scholar] [CrossRef]
- CDC (Centers for Disease Control and Prevention). The Burden of Chronic Diseases and Their Risk Factors—National and State Perspectives; CDC: Washington, DC, USA, 2004.
- Planchart, A.; Green, A.; Hoyo, C.; Mattingly, C.J. Heavy metal exposure and metabolic syndrome: Evidence from human and model system studies. Curr. Environ. Health Rep. 2018, 5, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.F.; Singh, K.; Chan, H.M. Mercury exposure, blood pressure, and hypertension: A systematic review and dose–response meta-analysis. Environ. Health Perspect. 2018, 126, 076002. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.Y.; Kim, S.J.; Kim, H.G.; Lee, J.H.; Choi, Y.; Lee, H.; Kim, Y. Evaluation of estrogenicity of major heavy metals. Sci. Total Environ. 2003, 312, 15–21. [Google Scholar] [CrossRef]
- Zierold, K.M.; Knobeloch, L.; Anderson, H. Prevalence of chronic diseases in adults exposed to arsenic-contaminated drinking water. Am. J. Public Health 2004, 94, 1936–1937. [Google Scholar] [CrossRef] [PubMed]
- Meliker, J.R.; Wahl, R.L.; Cameron, L.L.; Nriagu, J.O. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: A standardized mortality ratio analysis. Environ. Health 2007, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.H.; Jeng, J.S.; Yip, P.K.; Chen, C.L.; Hsu, L.I.; Hsueh, Y.M.; Chiou, H.Y.; Wu, M.M.; Chen, C.J. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation 2002, 105, 1804–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, C.M.; Meliker, J.R. Blood and urine cadmium, blood pressure, and hypertension: A systematic review and meta-analysis. Environ. Health Perspect. 2010, 118, 1676–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salonen, J.T.; Seppänen, K.; Nyyssönen, K.; Korpela, H.; Kauhanen, J.; Kantola, M.; Tuomilehto, J.; Esterbauer, H.; Tatzber, F.; Salonen, R. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in Eastern Finnish men. Circulation 1995, 91, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Plaza, M.; Guallar, E.; Navas-Acien, A. Environmental metals and cardiovascular disease. BMJ 2018, 362, k3435. [Google Scholar] [CrossRef]
- Alissa, E.M.; Ferns, G.A. Heavy Metal Poisoning and Cardiovascular Disease. J. Toxicol. 2011, 2011, 870125. [Google Scholar] [CrossRef]
- Hammer, D.I.; Finklea, J.F.; Hendricks, R.H.; Shy, C.M.; Horton, R.J. Hair trace metal levels and environmental exposure. Am. J. Epidemiol. 1971, 93, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Folin, M.; Contiero, E.; Vaselli, G.M. Trace element determination in humans. Biol. Trace Elem. Res. 1991, 31, 147–158. [Google Scholar] [CrossRef]
- Mehra, R.; Juneja, M. Elements in scalp hair and nails indicating metal body burden in polluted environment. J. Sci. Ind. Res. 2005, 64, 119–124. [Google Scholar]
- Saiki, R.K.; Scharf, S.; Faloona, F.; Mullis, K.B.; Horn, G.T.; Erlich, H.A.; Arnheim, N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 1985, 230, 1350–1354. [Google Scholar] [CrossRef]
- Senior, K.; Mazza, A. Italian Triangle of death linked to waste crisis. Lancet Oncol. 2004, 5, 525–527. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L. European Guidelines on CVD Prevention in Clinical Practice 2016. Eur. J. Prev. Cardiol. 2016, 23, NP1–NP96. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 341–378. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. Feature selection with the Boruta package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef] [Green Version]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Chapman and Hall: London, UK, 1988. [Google Scholar]
- Nelder, J.A.; Wedderburn, R.W.M. Generalized Linear Models. J. R. Stat. Soc. Ser. A (Gen.) 1972, 135, 370–384. [Google Scholar] [CrossRef]
- Hoffman, J.P. Generalized Linear Models: An Applied Approach; Pearson, Allyn, and Bacon: Boston, MA, USA, 2003. [Google Scholar]
- Müller, M. Generalized Linear Models. In Springer Handbook of Computational Statistics; Gentle, J., Härdle, W., Mori, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Hardin, J.W.; Hilbe, J.M. Generalized Linear Models and Extensions; StataCorp LP: College Station, TX, USA, 2007. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L. Bagging predictors. Mach. Learn. 1996, 24, 123–140. [Google Scholar] [CrossRef] [Green Version]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Buononato, E.V.; De Luca, D.; Galeandro, I.C.; Congedo, M.L.; Cavone, D.; Intranuovo, G.; Guastadisegno, C.M.; Corrado, V.; Ferri, G.M. Assessment of environmental and occupational exposure to heavy metals in Taranto and other provinces of Southern Italy by means of scalp hair analysis. Environ. Monit. Assess. 2016, 188, 337. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Ramond, A.; O’Keeffe, L.M.; Shahzad, S.; Kunutsor, S.K.; Muka, T.; Gregson, J.; Willeit, P.; Warnakula, S.; Khan, H.; et al. Environmental toxic metal contaminants and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2018, 362, k3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batuman, V.; Landy, E.; Maesaka, J.K.; Wedeen, R.P. Contribution of lead to hypertension with renal impairment. N. Engl. J. Med. 1983, 309, 17–21. [Google Scholar] [CrossRef]
- Feigin, R.D.; Shannon, D.C.; Reynolds, S.L.; Shapiro, L.W. Lead poisoning in children. Clin. Pediatr. (Phila) 1965, 4, 38–45. [Google Scholar] [CrossRef]
- Moncrieff, A.A.; Koumides, O.P.; Clayton, B.E.; Patrick, A.D.; Renwick, A.G.C.; Roberts, G.E. Lead poisoning in children. Arch. Dis. Child. 1964, 39, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Silbergeld, E.K. Preventing lead poisoning in children. Annu. Rev. Public Health 1997, 18, 187–210. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, J.L. Iron and the sex difference in heart disease risk. Lancet 1981, 13, 1293–1294. [Google Scholar] [CrossRef]
- Sullivan, J.L. The iron paradigm of ischemic heart disease. Am. Heart J. 1989, 117, 1177–1188. [Google Scholar] [CrossRef]
- Salonen, J.T.; Nyyssönen, K.; Korpela, H.; Tuomilehto, J.; Seppënen, R.; Salonen, R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation 1992, 86, 803–811. [Google Scholar] [CrossRef] [Green Version]
- Sempos, C.T.; Gillum, R.F.; Looker, A.C. Iron and Heart Disease. In Preventive Nutrition. Nutrition and Health; Bendich, A., Deckelbaum, R.J., Eds.; Humana Press: Totowa, NJ, USA, 2001. [Google Scholar]
- Genchi, G.; Sinicropi, M.; Carocci, A.; Lauria, G.; Catalano, A. Mercury exposure and heart diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef] [Green Version]
- Salonen, J.T.; Seppënen, K.; Lakka, T.A.; Salonen, R.; Kaplan, G.A. Mercury accumulation and accelerated progression of carotid atherosclerosis: A population-based prospective 4-year follow-up study in men in eastern Finland. Atherosclerosis 2000, 148, 265–273. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Afshari, R.; Mehrpour, O.; Samarghandian, S. Mercury and Atherosclerosis: Cell Biology, Pathophysiology, and Epidemiological Studies. Biol. Trace Elem. Res. 2020, 196, 27–36. [Google Scholar] [CrossRef]
- Benstoem, C.; Goetzenich, A.; Kraemer, S.; Borosch, S.; Manzanares, W.; Hardy, G.; Stoppe, C. Selenium and Its Supplementation in Cardiovascular Disease—What do We Know? Nutrients 2015, 7, 3094–3118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Mateo, G.; Navas-Acien, A.; Pastor-Barriuso, R.; Guallar, E. Selenium and coronary heart disease: A meta-analysis. Am. J. Clin. Nutr. 2006, 84, 762–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laczmanski, L.; Milewicz, A.; Lwow, F.; Puzianowska-Kuznicka, M.; Pawlak, M.; Kolackov, K.; Jedrzejuk, D.; Krzyzanowska-Swiniarska, B.; Bar-Andziak, E.; Chudek, J.; et al. Vitamin D receptor gene polymorphism and cardiovascular risk variables in elderly Polish subjects. Gynecol. Endocrinol. 2013, 29, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.; Seeger, F.H.; von Quernheimd, Q.F.; Ramos-Lopez, E. Vitamin D gene polymorphisms and risk of acute cardiovascular events. Clin. Epidemiol. Glob. Health 2020, 8, 1371–1376. [Google Scholar] [CrossRef]
- Cai, X.; Lian, F.; Kong, Y.; Huang, L.; Xu, L.; Wu, Y.; Ma, H.; Yang, L. Carotenoid metabolic (BCO1) polymorphisms and personal behaviors modify the risk of coronary atherosclerosis: A nested case-control study in Han Chinese with dyslipidaemia (2013–2016). Asia Pac. J. Clin. Nutr. 2019, 28, 192–202. [Google Scholar] [PubMed]
- Nicaud, V.; Raoux, S.; Poirier, O.; Cambien, F.; O’Reilly, D.S.; Tiret, L. The TNF alpha/G-308A polymorphism influences insulin sensitivity in offspring of patients with coronary heart disease: The European Atherosclerosis Research Study II. Atherosclerosis 2002, 161, 225–317. [Google Scholar] [CrossRef]
- Ma, L.; Su, H.; Wang, Y.; Zhou, Y.; Kang, Z.; Xu, Y.; Gao, J. Interleukin-1β (IL-1β) C-511T polymorphism is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Eur. J. Inflamm. 2020, 18, 2058739220918047. [Google Scholar] [CrossRef]
- Jiménez-Luna, J.; Grisoni, F.; Schneider, G. Drug discovery with explainable artificial intelligence. Nat. Mach. Intell. 2021, 2, 573–584. [Google Scholar] [CrossRef]




| Heavy Metals | |||
|---|---|---|---|
| Aluminum | Arsenic | Barium | Beryllium |
| Bismuth | Boron | Cadmium | Calcium |
| Chromium | Cobalt | Copper | Iron |
| Lead | Lithium | Magnesium | Manganese |
| Mercury | Molybdenum | Nickel | Phosphorus |
| Platinum | Selenium | Sodium | Thallium |
| Titanium | Vanadium | Zinc | |
| Polymorphisms | |||
| IL10 A1082G | TNF /G-308A | CBS T1080C | IL-1C-551T |
| IL4R 190A/G | BCO1 | VDR | ACE |
| IL-1-899 (+4845) C > T | GSTM1 | IL-6 (-174)G/C | BHMT (GA AA AG) |
| MTFR CBS C6997 | MTR A2756G | MTR A66G | BHMT (GG AG AA) |
| IL6 634C/G and 174C/G | IL6R > Asp358Ala; rs2228145 A > C | MNSOD2 | MNSOD3 |
| MTHFR C677T | MTHFR A1298C | IFNG 874T/A | AHCY 257A/G |
| CAT C262T | |||
| Class | Clinical Status |
|---|---|
| MR | ESC SCORE RISK < 5% and LDL threshold < 115 mg/dL |
| HR | Patients with familial dyslipidemia or severe hypertension, diabetics without cardiovascular risk factors and without organ damage and patients with moderate chronic renal failure (GFR > 30–59 mL/min/1.73 m2). 5% < ESC SCORE RISK < 10% and LDL threshold < 100% mg/dL |
| VHR | Patients with documented cardiovascular disease (from coronary angiography, stress echocardiography, radionuclide imaging, ultrasound evidence of carotid plaque), previous myocardial infarction, previous acute coronary syndrome, previous coronary revascularization surgery with aorto-coraric bypass or percutaneous transluminal coronary angioplasty or peripheral, previous ischemic stroke and peripheral arterial disease, diabetic with one or more cardiovascular risk factors and/or markers of organ damage (and microalbuminuria) and with severe renal insufficiency (GFR < 30–59 mL/min/173 m2). ESC SCORE RISK > 10% and LDL threshold < 70 mg/dL |
| Sensitivity | |||
|---|---|---|---|
| Class | GLM | RF | ANN |
| MR | (43.35 ± 6.62)% | (63.21 ± 5.60)% | (41.77 ± 8.74)% |
| HR | (30.00 ± 10.27)% | (47.94 ± 7.88)% | (36.00 ±11.80)% |
| VHR | (44.26 ± 6.46)% | (65.21 ± 4.68)% | (45.63 ± 7.88)% |
| Precision | |||
| Class | GLM | RF | ANN |
| MR | (49.34 ± 6.64% | (59.12 ± 4.19)% | (47.35 ± 6.19)% |
| HR | (19.88 ± 5.68)% | (45.16 ± 6.80)% | (23.54 ± 7.01)% |
| VHR | (50.18 ± 5.80)% | (72.30 ± 4.13)% | (53.59 ± 6.51)% |
| F1 | |||
| Class | GLM | RF | ANN |
| MR | (45.36 ± 5.86)% | (60.99 ± 4.18)% | (44.11 ± 7.09)% |
| HR | (23.77 ± 7.13) | (46.28 ± 6.66) | (28.27 ± 8.43)% |
| VHR | (46.87 ± 5.56) | (68.47 ± 3.62)% | (49.02 ± 6.43)% |
| Selected Metals | Selected Polymorphisms |
|---|---|
| Se | IL10 A1082G |
| Cr | VDR |
| Hg | BCO1 |
| Fe | IL4R 190A/G |
| Pb | IL-1βC-551T |
| Mg | CBS T1080C |
| TNFα/G-308A |
| Class | Sensitivity | Precision | F1 |
|---|---|---|---|
| Moderate risk | (77.44 ± 3.04)% | (65.71 ± 2.15)% | (71.08 ± 2.24)% |
| High risk | (46.78 ± 3.88)% | (48.96 ± 3.41)% | (47.79 ± 3.27)% |
| Very high risk | (72.94 ± 1.68)% | (84.84 ± 2.47)% | (78.41 ± 1.44)% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaco, A.; Lacalamita, A.; Amoroso, N.; D’Orta, A.; Del Buono, A.; di Tuoro, F.; Tangaro, S.; Galeandro, A.I.; Bellotti, R. Random Forests Highlight the Combined Effect of Environmental Heavy Metals Exposure and Genetic Damages for Cardiovascular Diseases. Appl. Sci. 2021, 11, 8405. https://doi.org/10.3390/app11188405
Monaco A, Lacalamita A, Amoroso N, D’Orta A, Del Buono A, di Tuoro F, Tangaro S, Galeandro AI, Bellotti R. Random Forests Highlight the Combined Effect of Environmental Heavy Metals Exposure and Genetic Damages for Cardiovascular Diseases. Applied Sciences. 2021; 11(18):8405. https://doi.org/10.3390/app11188405
Chicago/Turabian StyleMonaco, Alfonso, Antonio Lacalamita, Nicola Amoroso, Armando D’Orta, Andrea Del Buono, Francesco di Tuoro, Sabina Tangaro, Aldo Innocente Galeandro, and Roberto Bellotti. 2021. "Random Forests Highlight the Combined Effect of Environmental Heavy Metals Exposure and Genetic Damages for Cardiovascular Diseases" Applied Sciences 11, no. 18: 8405. https://doi.org/10.3390/app11188405
APA StyleMonaco, A., Lacalamita, A., Amoroso, N., D’Orta, A., Del Buono, A., di Tuoro, F., Tangaro, S., Galeandro, A. I., & Bellotti, R. (2021). Random Forests Highlight the Combined Effect of Environmental Heavy Metals Exposure and Genetic Damages for Cardiovascular Diseases. Applied Sciences, 11(18), 8405. https://doi.org/10.3390/app11188405









