Feasibility of a Drug-Releasing Radiofrequency Ablation System in a Porcine Liver Model
Abstract
1. Introduction
2. Materials and Methods
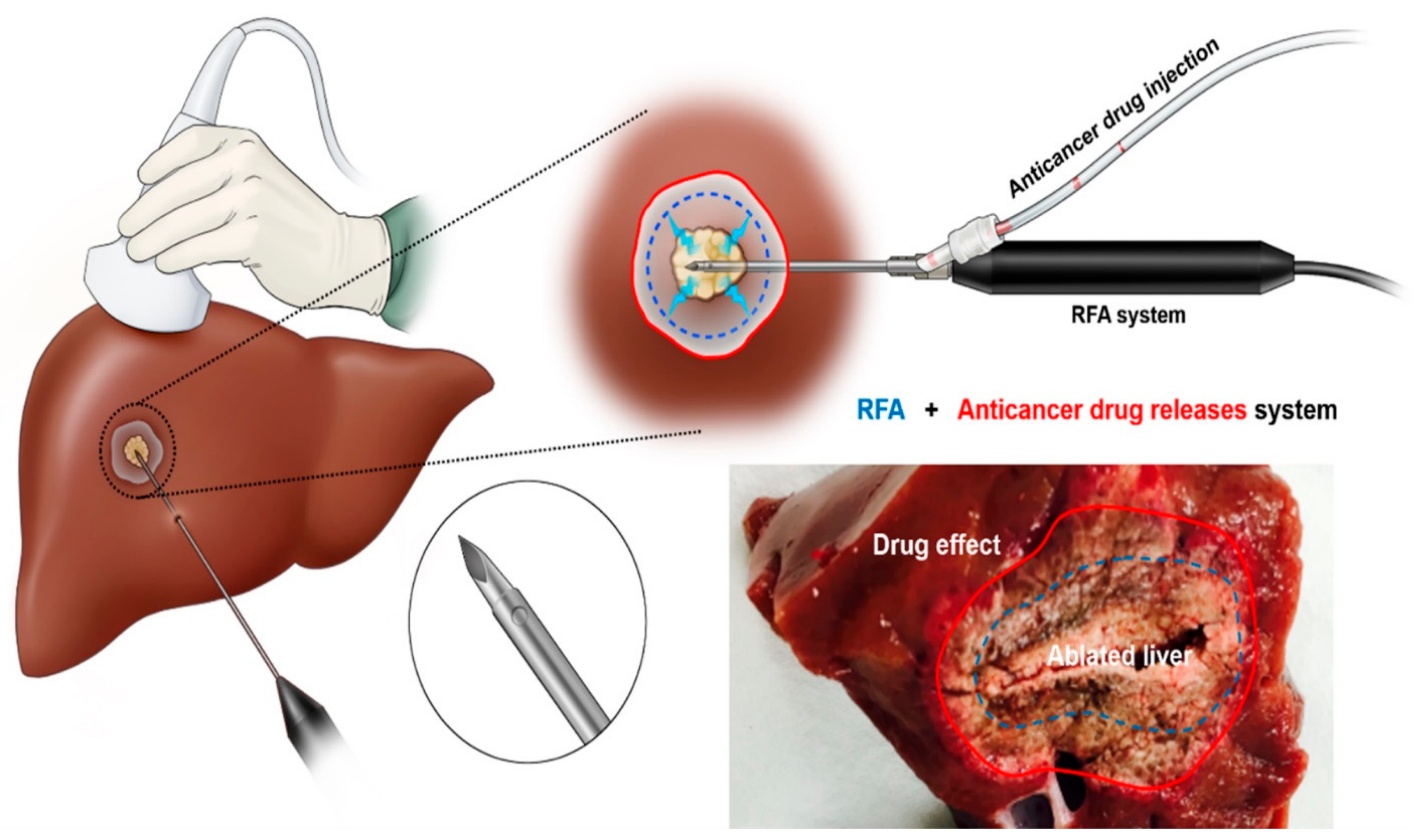
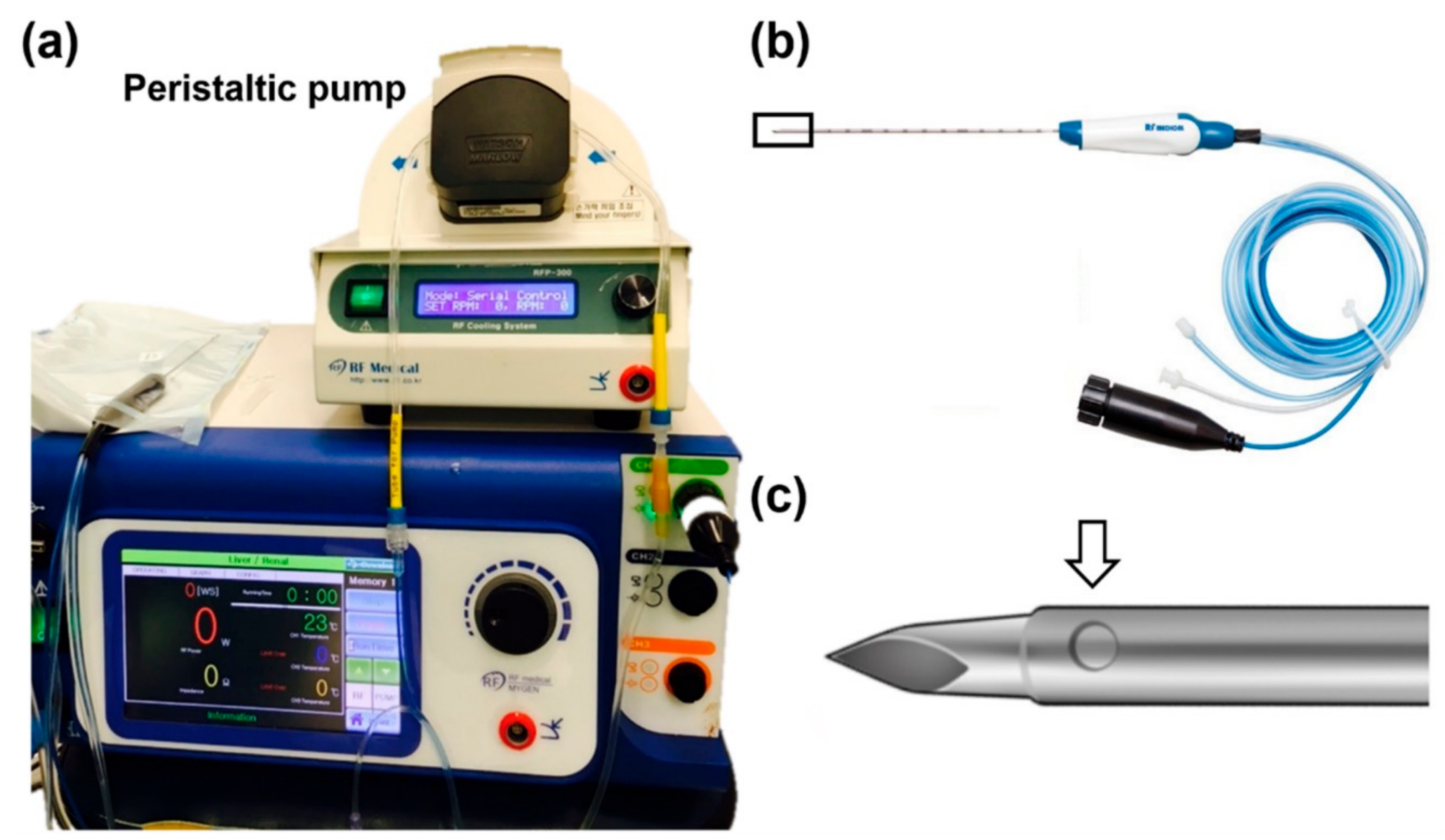
2.1. Drug-Releasing RFA System
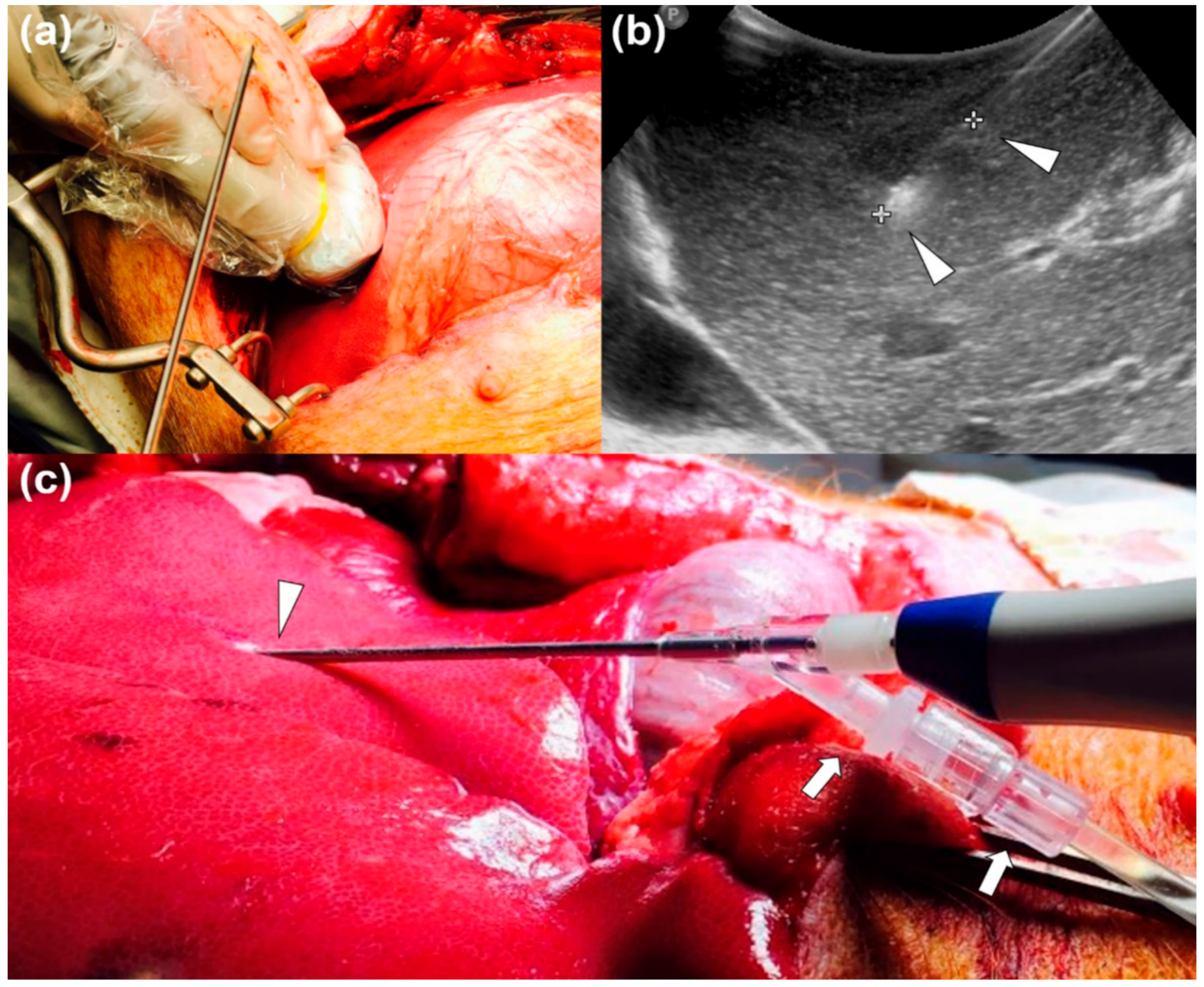
2.2. In Vivo Drug-Releasing RFA Procedure
2.3. Ex Vivo PET/MRI Examination
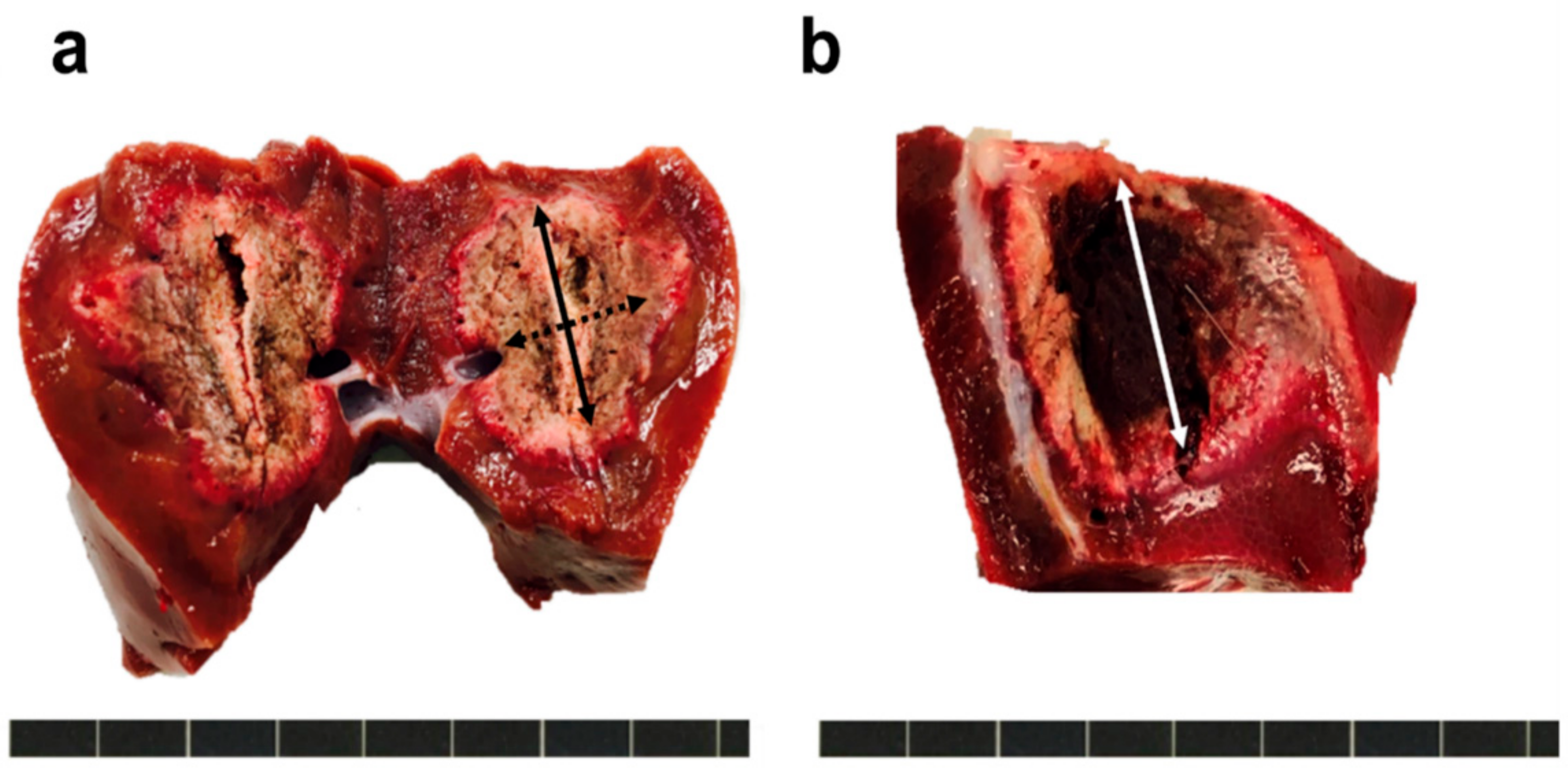
2.4. Measurement of the Ablation Zone
2.5. Statistical Analysis
3. Results
3.1. Procedural Outcomes
3.2. Analysis of Ablation Zone of the Liver
4. Discussion
Limitations and Future Direction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wah, T.M.; Arellano, R.S.; Gervais, D.A.; Saltalamacchia, C.A.; Martino, J.; Halpern, E.F.; Michael, M.; Mueller, P.R. Image-guided percutaneous radiofrequency ablation and incidence of post–radiofrequency ablation syndrome: Prospective survey. Radiology 2005, 237, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Lee, J.M.; Lee, D.H.; Yoon, J.-H.; Kim, Y.J.; Lee, J.-H.; Yu, S.J.; Cho, E.J. Radiofrequency ablation using internally cooled wet electrodes in bipolar mode for the treatment of recurrent hepatocellular carcinoma after locoregional treatment: A randomized prospective comparative study. PLoS ONE 2020, 15, e0239733. [Google Scholar] [CrossRef] [PubMed]
- Curley, S.A.; Izzo, F.; Delrio, P.; Ellis, L.M.; Granchi, J.; Vallone, P.; Fiore, F.; Pignata, S.; Daniele, B.; Cremona, F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: Results in 123 patients. Ann. Surg. 1999, 230, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Decadt, B.; Siriwardena, A.K. Radiofrequency ablation of liver tumours: Systematic review. Lancet Oncol. 2004, 5, 550–560. [Google Scholar] [CrossRef]
- Shiina, S.; Tateishi, R.; Arano, T.; Uchino, K.; Enooku, K.; Nakagawa, H.; Asaoka, Y.; Sato, T.; Masuzaki, R.; Kondo, Y.; et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 2012, 107, 569–577. [Google Scholar] [CrossRef]
- McGhana, J.P.; Dodd, G.D. Radiofrequency Ablation of the Liver Current Status. Am. J. Roentgenol. 2001, 176, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Shiina, S.; Teratani, T.; Obi, S.; Sato, S.; Tateishi, R.; Fujishima, T.; Ishikawa, T.; Koike, Y.; Yoshida, H.; Kawabe, T.; et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005, 129, 122–130. [Google Scholar] [CrossRef]
- Lin, S.M.; Lin, C.J.; Lin, C.C.; Hsu, C.W.; Chen, Y.C. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma <or =4 cm. Gastroenterology 2004, 127, 1714–1723. [Google Scholar]
- Lencioni, R.A.; Allgaier, H.P.; Cioni, D.; Olschewski, M.; Deibert, P.; Crocetti, L.; Frings, H.; Laubenberger, J.; Zuber, I.; Blum, H.E.; et al. Small hepatocellular carcinoma in cirrhosis: Randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003, 228, 235–240. [Google Scholar] [CrossRef]
- Machi, J.; Uchida, S.; Sumida, K.; Limm, W.M.; Hundahl, S.A.; Oishi, A.J.; Furumoto, N.L.; Oishi, R.H. Ultrasound guided radiofrequency thermal ablation of liver tumors: Percutaneous, laparoscopic, and open surgical approaches. J. Gastrointest. Surg. 2001, 5, 477–489. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Gazelle, G.S.; Solbiati, L.; Rittman, W.J.; Mueller, P.R. Radiofrequency tissue ablation: Increased lesion diameter with a perfusion electrode. Acad. Radiol. 1996, 3, 636–644. [Google Scholar] [CrossRef]
- Rossi, S.; Buscarini, E.; Garbagnati, F.; Stasi, M.D.; Quaretti, P.; Rago, M.; Zangrandi, A.; Andreola, S.; Silverman, D.; Buscarini, L. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. Am. J. Roentgenol. 1998, 170, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Ni, Y.; Yu, J.; Zhang, H.; Baert, A.; Marchal, G. An ex vivo study on radiofrequency tissue ablation: Increased lesion size by using an “expandable-wet” electrode. Eur. Radiol. 2001, 11, 1841–1847. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Solbiati, L.; Hahn, P.F.; Cosman, E.; Conrad, J.E.; Fogle, R.; Gazelle, G.S. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: Laboratory and clinical experience in liver metastases. Radiology 1998, 209, 371–379. [Google Scholar] [CrossRef]
- Lee, J.M.; Han, J.K.; Lee, J.Y.; Kim, S.H.; Choi, J.Y.; Lee, M.W.; Choi, S.H.; Eo, H.; Choi, B.I. Hepatic radiofrequency ablation using multiple probes: Ex vivo and in vivo comparative studies of monopolar versus multipolar modes. Korean J. Radiol. 2006, 7, 106–117. [Google Scholar] [CrossRef][Green Version]
- Lee, J.M.; Han, J.K.; Kim, H.C.; Choi, Y.H.; Kim, S.H.; Choi, J.Y.; Choi, B.I. Switching monopolar radiofrequency ablation technique using multiple, internally cooled electrodes and a multichannel generator: Ex vivo and in vivo pilot study. Investig. Radiol. 2007, 42, 163–171. [Google Scholar] [CrossRef]
- Lee, J.M.; Han, J.K.; Chang, J.M.; Chung, S.Y.; Kim, S.H.; Lee, J.Y.; Lee, M.W.; Choi, B.I. Radiofrequency ablation of the porcine liver in vivo: Increased coagulation with an internally cooled perfusion electrode. Acad. Radiol. 2006, 13, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.H.; Lee, J.Y.; Jun, S.R.; Kim, K.W.; Kim, S.H.; Han, J.K.; Choi, B.I. Radiofrequency Ablation with an Internally Cooled Monopolar Directional Electrode: Ex Vivo and in Vivo Experimental Studies in the Liver. Radiology 2016, 278, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Brace, C.L.; Sampson, L.A.; Hinshaw, J.L.; Sandhu, N.; Lee, F.T. Radiofrequency ablation: Simultaneous application of multiple electrodes via switching creates larger, more confluent ablations than sequential application in a large animal model. J. Vasc. Interv. Radiol. 2009, 20, 118–124. [Google Scholar] [CrossRef]
- Kinahan, P.E.; Fletcher, J.W. PET/CT Standardized Uptake Values (SUVs) in Clinical Practice and Assessing Response to Therapy. Semin. Ultrasound CT MR 2010, 31, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Brendle, C.; Kupferschläger, J.; Nikolaou, K.; Fougère, C.L.; Gatidis, S.; Pfannenberg, C. Is the standard uptake value (SUV) appropriate for quantification in clinical PET imaging?—Variability induced by different SUV measurements and varying reconstruction methods. Eur. J. Radiol. 2015, 84, 158–162. [Google Scholar] [CrossRef]
- Cho, K.J.; Choi, N.K.; Shin, M.H.; Chong, A.R. Clinical usefulness of FDG-PET in patients with hepatocellular carcinoma undergoing surgical resection. Ann. Hepatobiliary Pancreat. Surg. 2017, 21, 194–198. [Google Scholar] [CrossRef][Green Version]
- Chan, A.C.Y.; Chan, S.C.; Chok, K.S.H.; Cheung, T.T.; Chiu, D.W.; Poon, R.T.P.; Fan, S.T.; Lo, C.M. Treatment strategy for recurrent hepatocellular carcinoma: Salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl. 2013, 19, 411–419. [Google Scholar] [CrossRef]
- Muaddi, H.; Al-Adra, D.P.; Beecroft, R.; Ghanekar, A.; Moulton, C.-A.; Doyle, A.; Selzner, M.; Wei, A.; McGilvray, I.D.; Gallinger, S.; et al. Liver Transplantation is Equally Effective as a Salvage Therapy for Patients with Hepatocellular Carcinoma Recurrence Following Radiofrequency Ablation or Liver Resection with Curative Intent. Ann. Surg. Oncol. 2018, 25, 991–999. [Google Scholar] [CrossRef]
- Mulier, S.; Ni, Y.; Jamart, J.; Ruers, T.; Marchal, G.; Michel, L. Local Recurrence After Hepatic Radiofrequency Coagulation: Multivariate Meta-Analysis and Review of Contributing Factors. Ann. Surg. 2005, 242, 158–171. [Google Scholar] [CrossRef]
- Poon, R.T.P.; Fan, S.T.; Wong, J. Risk Factors, Prevention, and Management of Postoperative Recurrence After Resection of Hepatocellular Carcinoma. Ann. Surg. 2000, 232, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Yasuno, M.; Uetake, H.; Ishiguro, M.; Mizunuma, N.; Komori, T.; Miyata, G.; Shiomi, A.; Kagimura, T.; Sugihara, K. mFOLFOX6 plus bevacizumab to treat liver-only metastases of colorectal cancer that are unsuitable for upfront resection (TRICC0808): A multicenter phase II trial comprising the final analysis for survival. Int. J. Clin. Oncol. 2019, 24, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Goldberg, S.N.; Lazzaroni, S.; Meloni, F.; Ierace, T.; Solbiati, L.; Gazelle, G.S. Hepatocellular carcinoma: Radiofrequency ablation of medium and large lesions. Radiology 2000, 214, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Kim, Y.S.; Rhim, H.; Lim, H.K.; Lee, M.W.; Choi, D. A comparison of US-guided percutaneous radiofrequency ablation of medium-sized hepatocellular carcinoma with a cluster electrode or a single electrode with a multiple overlapping ablation technique. J. Vasc. Interv. Radiol. 2011, 22, 771–779. [Google Scholar] [CrossRef]
- Goldberg, S.N.; Gazelle, G.S.; Mueller, P.R. Thermal ablation therapy for focal malignancy: A unified approach to underlying principles, techniques, and diagnostic imaging guidance. Am. J. Roentgenol. 2000, 174, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Beiderwellen, K.; Gomez, B.; Buchbender, C.; Hartung, V.; Poeppel, T.D.; Nensa, F.; Kuehl, H.; Bockisch, A.; Lauenstein, T.C. Depiction and characterization of liver lesions in whole body 18F-FDG PET/MRI. Eur. J. Radiol. 2013, 82, e669–e675. [Google Scholar] [CrossRef] [PubMed]
- Beiderwellen, K.; Geraldo, L.; Ruhlmann, V.; Heusch, P.; Gomez, B.; Nensa, F.; Umutlu, L.; Lauenstein, T.C. Accuracy of 18F-FDG PET/MRI for the Detection of Liver Metastases. PLoS ONE 2015, 10, e0137285. [Google Scholar] [CrossRef] [PubMed]

| Dmin (cm) | Dmax (cm) | Dv (cm) | AV (cm3) | IV (cm3) | Circularity | Injection Dose (mCi/30 mL) | Estimated Dose (mCi/30 mL) | Retention Rate (%) | SUV | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.1 | 2.32 | 3.24 | 3.1 | 12.20 | 14.12 | 0.72 | 6.94 | 5.02 | 72.33 | 0.458 |
| 1.2 | 2.46 | 3.74 | 3.14 | 15.19 | 17.21 | 0.66 | 7.45 | 5.70 | 76.51 | 0.541 |
| 1.3 | 2.22 | 3.51 | 3.04 | 12.40 | 14.31 | 0.63 | 5.46 | 3.86 | 70.70 | 0.484 |
| 2.1 | 3.01 | 3.41 | 3.17 | 17.03 | 18.91 | 0.88 | 7.03 | 5.27 | 74.96 | 0.375 |
| 2.2 | 2.93 | 3.88 | 3.21 | 19.10 | 22.67 | 0.76 | 6.47 | 4.98 | 76.97 | 0.398 |
| 2.3 | 2.48 | 3.6 | 3.01 | 14.06 | 15.79 | 0.69 | 7.10 | 5.46 | 76.90 | 0.465 |
| 3.1 | 2.41 | 3.64 | 3.29 | 15.10 | 19.10 | 0.66 | 7.81 | 5.84 | 74.78 | 0.431 |
| 3.2 | 2.67 | 3.49 | 3.02 | 17.43 | 18.30 | 0.77 | 7.43 | 4.93 | 66.35 | 0.396 |
| 3.3 | 2.5 | 3.52 | 3.14 | 14.47 | 14.50 | 0.71 | 8.45 | 5.62 | 66.51 | 0.405 |
| Mean (± SD) | 2.56 ± 0.27 | 3.56 ± 0.19 | 3.12 ± 0.09 | 15.22 ± 2.30 | 17.21 ± 2.85 | 0.72 ± 0.08 | 7.13 ± 0.84 | 5.19 ± 0.60 | 72.89 ± 4.22 | 0.44 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.C.; Lee, K.B.; Ham, S.J.; Jung, J.H.; Park, Y.; Won, D.-S.; Kim, K.W.; Park, J.-H. Feasibility of a Drug-Releasing Radiofrequency Ablation System in a Porcine Liver Model. Appl. Sci. 2021, 11, 8301. https://doi.org/10.3390/app11188301
Cho YC, Lee KB, Ham SJ, Jung JH, Park Y, Won D-S, Kim KW, Park J-H. Feasibility of a Drug-Releasing Radiofrequency Ablation System in a Porcine Liver Model. Applied Sciences. 2021; 11(18):8301. https://doi.org/10.3390/app11188301
Chicago/Turabian StyleCho, Young Chul, Ki Baek Lee, Su Jung Ham, Jin Hwa Jung, Yubeen Park, Dong-Sung Won, Kyung Won Kim, and Jung-Hoon Park. 2021. "Feasibility of a Drug-Releasing Radiofrequency Ablation System in a Porcine Liver Model" Applied Sciences 11, no. 18: 8301. https://doi.org/10.3390/app11188301
APA StyleCho, Y. C., Lee, K. B., Ham, S. J., Jung, J. H., Park, Y., Won, D.-S., Kim, K. W., & Park, J.-H. (2021). Feasibility of a Drug-Releasing Radiofrequency Ablation System in a Porcine Liver Model. Applied Sciences, 11(18), 8301. https://doi.org/10.3390/app11188301







