Abstract
Curcuminoids, the bioactive compounds with many beneficial effects on human health, exist in Curcuma longa (turmeric). In the present study, the impact of different cell disintegration techniques to enhance total curcuminoid recovery (TC) from fresh and dried turmeric was investigated. The impact of thermal pretreatment (TP), ultrasound pretreatment (UP), enzyme pretreatment (EP), and pulsed electric field pretreatment (PEF) on the recovery of curcumin (CUR), demethoxycurcumin (DMC), and bis-demethoxycurcumin (BDMC) from fresh and dried turmeric were studied. The cell disintegration index (Zp) and high-performance liquid chromatography (HPLC) analysis of curcuminoids were performed to evaluate the efficiency of the applied techniques. With fresh turmeric, the highest curcuminoid recovery was 83.6 mg/g dry basis with EP. The highest structural tissue damage was obtained with UP achieving a cell disintegration level of 92.5%. The technology with the highest time-saving and low specific energy input was PEF with a total curcuminoid recovery of 80.9 mg/g dry basis. Working with dried turmeric, the drying required high specific energy input for 72 h at 50 °C; however, the untreated dried sample reached 125.3 mg/g dry basis of TC without further pretreatment after drying.
1. Introduction
The genus Curcuma belongs to the Zingiberaceae family, and it has more than seventy species of rhizome herbs worldwide. Those species were classified into Curcuma longa, Curcuma amada, Curcuma aromatica, and Curcuma zedoria [1]. Asian countries have a wide distribution of turmeric species, particular for tropics and subtropics countries.
Curcuminoids are a mixture of three different components in turmeric (Curcuma longa). They are the main compounds responsible for the biological activity of turmeric. Curcumin (CUR) is a yellow-colored crystalline polyphenol in purified form. Besides curcumin, curcuminoids contain two other vital derivatives, i.e., demethoxycurcumin (DMC) and bis-demethoxycurcumin (BDMC) [2]. In turmeric rhizome, curcuminoids occupy around 30–150 mg/g depending on geographical origin [3].
Curcuminoids are non-polar compounds; hence, curcuminoid solubility in an organic solvent is significantly higher than in the aqueous solutions. Establishing a selective extraction procedure for curcuminoids is the main goal in some research activities. According to Kadam, et al. [4], the conventional protocols generally use solvents and solid-liquid extraction techniques such as steam distillation, maceration, and Soxhlet extraction. For the conventional methods, the techniques generally involve high temperature (usually higher than 60 °C), long extraction time (up to 72 h for maceration), organic solvents, and less selective extraction [5].
Furthermore, these methods use a high amount of solvent and energy input, which leads to low practical application possibilities. In curcuminoids extraction, conventional extraction methods usually lead to degeneration by light and high temperature during the long exposure.
In the literature, a wide range of extraction techniques for curcuminoids extraction has been presented. With conventional extraction, heating or steaming could be used, but it has a low extraction yield [6,7]. Organic solvents are widely applied, such as ethanol, acetone, and hexane [8]. Furthermore, non-conventional techniques were applied for curcuminoids extraction, such as supercritical carbon dioxide [9], microwave-assisted extraction [9,10], ultrasound-assisted extraction [11].
Thermal pretreatment (TP) was considered conventional, fast, and low operational costs [12]. The starch content in turmeric reaches 35% w/w of dry basis [13], changing the hydrophilic phase’s viscosity and affecting total curcuminoids recovery. Relevant studies used TP as a pretreatment method for further extraction [14] or reference treatment compared to non-conventional extraction techniques [15].
According to Shirsath, Sable, Gaikwad, Sonawane, Saini and Gogate [1], high temperature during extraction enhances curcumin extraction yields because temperature changed different physical characteristics, including viscosity, diffusivity, solubility, and surface tension. In this research, the effect of TP is compared to non-conventional pretreatments such as ultrasound pretreatment (UP), enzyme pretreatment (EP), pulsed electric field pretreatment (PEF).
Ultrasound pretreatment (UP) belongs to non-conventional methods, and it was used to enhance the extraction of polyphenols in various materials. Ultrasound is considered an efficient technique with intensive impact [16]. Yue, et al. [17] concluded that ultrasound could be considered promising and practical for bioactive compounds extraction, with higher yields and short processing time. For curcuminoids extraction, UP is highly recommended due to the increased extraction yield of curcuminoids [9,18,19]. When applying ultrasound during the extraction, the mass transfer can be significantly improved by rupture of plant cells and particle size decrease and improved penetration of the solvent into the plant material [1]. However, the effect of UP on cell disruption in the absence of an effective solvent has been little researched.
Enzymatic pretreatment (EP) is widely applied to increase the extraction yield of bioactive compounds [20]. In curcuminoid extraction, EP with α-amylase and glucoamylase is a selective and effective method [21]. In another study, α-amylase, glucoamylase, and a commercial mixture of enzymes containing cellulase and xylanase were considered to assist curcuminoids recovery [20,21,22]. The amount of pectin in turmeric reaches up to 6.3% w/w [23]; nonetheless, so far, pectinases have been little considered to improve the curcuminoids recovery from turmeric.
The pulsed electric field (PEF) is the most recent applicant to compare with other methods. PEF application in food has been broadly researched. [24]. The extraction yield of polyphenol is much dependent on the material matrix [25]. When applying PEF to plant tissues and cells, electroporation will enhance the membrane permeability by rearranging lipids on the cell membrane; this resulted in greater access to a solvent to bioactive compounds inside the cell cytoplasm [26]. For that reason, PEF was mostly used to enhance the sensitive bioactive extraction because of ultra-short treatment time and non-thermal effect [27].
Energy consumption is one of the most important criteria when applying a new technique to the food industry [28]. An applied technique must meet the requirement to adopt legally, product safety, cost reduction, and enhance food quality [29]. PEF was considered the promising innovation method with ultra-fast treatment time (millisecond) and low energy consumption compared to UP and EP.
The current research focused on curcuminoid recovery by applying different cell disintegration techniques on fresh and dried turmeric. The cell disintegration index (Zp) after pretreatment was measured by impedance measurement according to the method of Knorr and Angersbach [30]. Curcuminoids were further extracted by a rapid shaking procedure and quantification took place by high-performance liquid chromatography (HPLC) to compare the effect of different pretreatments on the extraction yield of CUR, DMC and BDMC. In order to consider the energy consumption of all pretreatments, the specific energy input was discussed to compare the efficiency of the applied techniques and applicability in the food industry.
2. Materials and Methods
2.1. Materials
Fresh rhizomes of C. longa were obtained from a local market in Vienna, Austria. Fresh rhizomes were rinsed and ground to the size of about 1 mm (diameter) × 10 mm (length). For dried turmeric preparation, rhizomes of C. longa were dried at 50 °C for 72 h by hot air drying according to the method of Singh, et al. [31]. After that, dried turmeric was frozen 5 min in liquid nitrogen and milled to the powder and sieved with a mesh width of 2 mm. The mean particle size of dried powder was determined by laser diffraction measurement (357.23 ± 12.24 µm). All solvents and chemicals used for the extraction and HPLC analysis were analytical grades (Merck Specialities Private Limited, Germany).
Analytical CUR (CAS 458-37-7), DMC (CAS 22608-11-3) and BDMC (CAS 22608-11-3) standards from Sigma-Aldrich (Germany) with purity ≥95% (HPLC).
2.2. Dry Matter Content
Dry matter content was determined for all raw materials, including fresh turmeric, dried turmeric and sediment turmeric obtained, after centrifugation. The dry matter content was used to calculate the total curcuminoid recovery based on a dry basis. Around 1 g of the turmeric was weighted in an aluminum pan and dried at 105 °C for 4 h.
The dry matter was calculated based on Equation (1)
DM: dry matter [%]; mp: weight [g] of the empty pan; m0: sample weight [g] before drying; m1: weight [g] of the sample and the pan after drying.
All analyses were performed in triplicates. The dry matter of fresh turmeric and dried turmeric were 13.09 ± 0.12% and 90.56 ± 0.85%, respectively.
2.3. Pretreatment of Turmeric
General: Dried and fresh samples were subjected to pretreatments in deionized water. Treated samples were further separated from the water by centrifugation and analyzed for their dry matter content. In a subsequent step, curcuminoids were extracted from the samples through a rapid extraction method. All the pretreatments were performed in triplicate.
2.3.1. Thermal Pretreatment (TP)
The temperature change may change the hydrophilic phase’s viscosity and affect total curcuminoid recovery. Thus, the effect of temperature was studied. TP was performed according to the method of El Darra, et al. [32] with small modifications. Thermal pretreatment was set at 50 °C, 70 °C and 90 °C. Soaking time was 5 min for all samples. A total of 25 g of dried/ fresh turmeric and 50 mL of deionized H2O were used for each batch. The temperatures in the center of the samples were measured twice before and immediately after thermal pretreatment.
2.3.2. Ultrasound Pretreatment (UP)
UP was performed according to the method of Shirsath, Sable, Gaikwad, Sonawane, Saini and Gogate [1] with small modifications. A total of 25 g of dried/ fresh turmeric and 50 g of deionized H2O were put in a 100 mL glass flask for all treatments. Ultrasound was applied to the system using the sonotrode immersed in the water approximately 10 cm for 1 min (UP200St, Hielscher ultrasonic processor, Germany). Ultrasonic power was applied at three different energy levels: 50 W, 100 W and 150 W. The energy input was converted into kJ/kg.
2.3.3. Pulsed Electric Field Pretreatment (PEF)
The effect of the pulsed electric field (PEF) was investigated by using the batch PEF system (DIL, Elcrack, Quakenbruck, Germany). PEF treatment was conducted according to the method of Fauster, et al. [33] with slight modifications. PEF chamber had an electrode distance of 5 cm. The chamber was filled with 25 g of dried/ fresh turmeric and 50 mL of deionized H2O for each batch. The field strength was set to 2 kV/cm, exponential decay pulses, pulse width 25 µs and the voltage was 10 kV. The specific energy inputs were 3.5 kJ/kg, 7 kJ/kg and 14 kJ/kg. The selection of the minimum specific energy input was based on the preliminary test. With these desired specific energy inputs, the number of needed pulses was calculated based on the Equation (2):
The number of pulses to reach the desired energy inputs were 11, 21 and 42 pulses, respectively.
2.3.4. Enzyme Pretreatment (EP)
C. longa contains up to 6.3% w/w of pectic in the plant cell [23], so it is necessary to investigate pectinase’s effect on curcuminoids recovery. EP was carried out according to the method of Kurmudle, Kagliwal, Bankar and Singhal [20] with small modifications. Pectinase used was obtained from Fructozym P (Geisenheim, Germany) with activity 50.000 U/mL. A stock solution of the pectinase was prepared in the pH 5.0 buffer solution at 25 °C. The optimal concentration of pectinase was selected in preliminary experiments by testing the impact of different enzyme concentrations on the yield of curcuminoids extraction at pH 5.0 and 50 °C for 60 min (data not shown). A total of 25 g of turmeric was transferred to a 250 mL flask and 50 mL of pectinase solution was added to reach the activity of 20 U/g turmeric. The enzyme solution was rewarmed to the operating temperature (50 °C) before being added. The reaction times were 1 h, 3 h and 5 h. After completion of EP, the flask was immediately cooldown by an ice bath. Once the mixture’s temperature had reduced to 20 °C, the pectinase was removed by centrifugation (3208 Relative Centrifugal Force (RCF) for 5 min).
2.4. Rapid Extraction of Curcuminoids
After pretreatment, water phase was removed by centrifugation at 3208 RCF for 5 min. A total 1 g of pretreated sample was weighted in a 10 mL flask tube. Rapid extraction by vortex was performed according to the method of Gómez-Mejía, et al. [34] with modifications. Further, 8 mL of ethanol 96% was added by a volumetric pipette and conducted intensive shaking with vortex shaker (Vortex-Genie 2 Vortex Mixer 12 DFS Item. Manufacturer: Scientific Industries Supplier Diversity Partner SI0236, Chicago, USA) for 15 min in the dark condition. After shaking, the flask tube was centrifuged (3208 RCF, 5 min). The supernatant was filtered through a 0.45 µm Polytetrafluoroethylene (PTFE) membrane filter before quantification by HPLC. The untreated sample was prepared at the same time as treated samples and without applied pretreatment techniques.
Determination of total curcuminoid content in the raw material, Soxhlet extraction was used for characterizing the total amount of curcuminoids in turmeric. Maximum cell disintegration was reached by putting the fresh material in freeze-drying for three days, milling by a cyclone mill (Twister, Retsch, Germany) with 1 mm sieve size. The particle size after milling reached 232 µm. The powder was put in a tight and dark flask in a desiccator until extraction.
The extraction was performed using a Soxhlet apparatus consisting of a distillation flask, thimble holder and condenser. The dried turmeric was placed in a thimble holder and contacted the condensed fresh solvent from the distillation flask. The extraction of curcuminoids takes place in the thimble chamber. When the liquid reaches the thimble overflow level, the fluid moves through the siphon back into the reservoir, carrying extracted solutes into the bulk liquid. Around 0.05 g of turmeric powder was placed in a thimble and mixed with 150 mL of acetone 99% (EC 200-662-2, Sigma-Aldrich GmbH, Darmstadt, Germany). Soxhlet extraction was performed for 6 h, with 390 ± 10 s per circulation. The optimal time of Soxhlet was selected in preliminary experiments by testing the kinetic released curcuminoids from 1–8 h.
The maximum obtained total curcuminoids recovery (TC) was 143.7 ± 0.8 mg/g dry basis (61.7 mg/g of CUR, 24.4 mg/g of DMC and 57.6 mg/g of BDMC), and these values were considered as the maximum curcuminoids content in the raw material.
2.5. Quantification of Curcuminoids
Curcuminoids quantification (CUR, DMC and BDMC) in the extract solution were quantified using a high-performance liquid chromatography system (HPLC–DAD, Shimadzu Cooperation, Kyoto, Japan). The column was Nucleodur C18 Gravity (Macherey-Nagel, Düren, Germany; 125/3 mm, 5.0 μm particle size). HPLC column was initially cleaned up by elution, which typically required 30 min by acetonitrile: water = 50: 50. After the washing step, the column was conditioned by eluting the mobile phase for 10 min and simultaneously.
The mobile phase consisted of acetonitrile (A), 2% acetic acid (B), and acetone (C). Quantitative levels of curcuminoids were determined using the above solvents programmed linearly from 45 to 65% acetonitrile in A for 0–15 min. The gradient then went from 65 to 45% acetonitrile in A for 15–20 min, with a constant of 5% of C.
The ethanol extract from the rapid extraction was filtered through a 0.20 µm PTFE membrane filter before use. The gradient elution flowed through the column at a flow rate of 0.5 mL/min at 33 °C. Sample detection was performed at 425 nm, and the injection volume for the sample analysis was 5 µL.
The linearity of the method was evaluated by analyzing a series of standard curcuminoids. Six concentrations of curcuminoids standard solution were ranged from 5, 10, 25, 50 and 100 ppm of standard CUR, DMC, and BDMC. The calibration range was chosen to be suitable with average curcuminoids concentrations in extracts. Analysis of the calibration curves showed good correlation between concentration and resulting peak area for CUR (R2 = 0.9998), DMC (R2 = 0.9996) and BDMC (R2 = 0.9967).
Curcuminoids concentrations were calculated based on linear calibration functions and concerning the dilution factor. The content of CUR, DMC, and BDMC was expressed as milligram per gram of dry basis (mg/g).
2.6. Determination of Cell Disintegration Index (Zp)
The cell disintegration index (Zp) was determined by the method of Knorr and Angersbach [30]. The electrical conductivity of the pretreated sample was measured with an impedance analyzer (Sigma Check, EloCheck, Berlin, Germany); the electrical current goes through the sample when connected to a 12V DC source. The sample after pretreatment was put in a cylindrical tube (d = 10 mm, l = 10 mm). The tube was placed between two stainless steel electrodes. There were 14 different frequency levels from 1.38 kHz to 11.2 MHz measured. The Zp was calculated by the Equation (3) [30]; therefore, the impedance value at 5.5 and 1400 kHz was used. The untreated sample was considered as the control sample.
where , = electrical conductivity of untreated and treated materials, respectively, in a low-frequency field (1–5 kHz); ; = electrical conductivity of untreated and treated materials, respectively, in a high-frequency field (3–50 MHz).
For Intact Cells, = 0; for Total Cell Disintegration, = 12.7.
2.7. Particle Size
The particle size distribution (PSD) of the powders was measured using a laser diffraction system (LA-960, Horiba, Kyoto, Japan) equipped with a liquid and a dry dispersion unit. PSD was measured according to the method of Haas, et al. [35] with modifications. The particle size of samples was measured in the liquid dispersion system filled with water (150 mL), ultrasound for 15 s was used to avoid an agglomeration of the particles. Agitation was run during the measurement. All the evaluations were performed in triplicate.
2.8. Statistical Analysis
All pretreatments and analyses were conducted in triplicates. Statistical analyses were performed using Statgraphics Centurion XVII, version 17.1.04 (Statpoint Technologies, Inc., Warrenton, VA, USA). Results are expressed as mean ± standard deviations of three single determinations. One-way ANOVA (analysis of variance with α = 0.05) and Fisher’s least significance test was used to establish the significance of differences among the mean values.
3. Results and Discussion
3.1. Effect of Thermal Pretreatment on Total Curcuminoids Recovery of Fresh Turmeric
The effect of pretreatment on cell disintegration index and total curcuminoid recovery are shown in Figure 1 and Figure 2, respectively. In high starch content material like C. Longa, suitable temperature usage may create beneficial effects for extraction. For instance, the changing of viscosity, diffusivity, solubility, and surface tension [1].
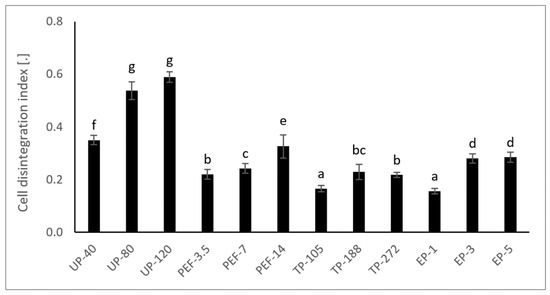
Figure 1.
Influence of pretreatment on cell disintegration index (Zp) of fresh turmeric. Means with different lowercase letters indicate significant differences (p < 0.05). Ultrasound pretreatment (UP), Pulsed electric field pretreatment (PEF), Thermal pretreatment (TP), Enzyme pretreatment (EP).
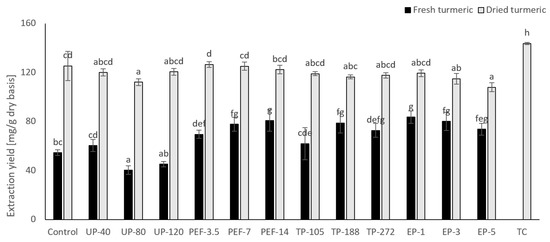
Figure 2.
Effect of pretreatment on total curcuminoid recovery. Means with a different letter are significantly different (p < 0.05).
It was reported that turmeric starch content might reach 35% on a wet basis [13], and amylose content in isolated turmeric starch was up to 48.4% w/w [36]. In addition, fresh turmeric contains 86.91% of water and was treated in hot water (50–90 °C) so the behavior of starch is the crucial point and should be taken into account. In the literature, it was revealed that turmeric starch showed no change in rheological behavior change up to 60 °C; however, in the range of 60–80 °C, the starch starts to swell, and the water-holding capacity of the starch increased considerably after 80 °C, and finally lead to pasting or gelatinization [36].
In the research of Maskooki and Eshtiaghi [27], thermal treatment should be higher than the temperature of 70 °C to achieve the destruction of the cell membrane. For those reasons, in the present investigation, the effect of temperature was examined at 50 °C, 70 °C and 90 °C to gain insight into TP’s influence on different cell disintegration levels.The main effect of varying pretreatment methods in the present investigation is mainly on the turmeric cell and tissue. Fauster, et al. [37] implied that Zp is the vital parameter to evaluate the rate of damaged cells within the plant after treatment.
In this work, the obtained results indicated that a preheating of fresh turmeric to 50 °C (TP-105) did not significantly increase the TC than the untreated sample (Figure 2). Nevertheless, 70 °C (TP-188) and 90 °C (TP-272) increased the Zp up to 39% and 32%, respectively (Figure 1); hence the TC raised to 27% and 17%, respectively (Figure 2). Albeit most intensive TP treatment, TP-272 increased TC (Figure 2) with no significant difference with TP-188.
Lestari and Indrayanto [38] reported that CUR was stabled when exposed at 70 °C in 10 min. In the case of higher temperatures, CUR starts to decompose lightly. For this reason, applying high temperatures can lead to the decomposition of curcuminoids.
3.2. Effect of Ultrasound Pretreatment on Total Curcuminoids Recovery of Fresh Turmeric
The effects of UP to total curcuminoids recovery were summarized in Figure 2. Applied ultrasound may significantly increase the mass transfer by destroying the plant cells and decrease the size of materials [1]. Besides shock waves and cavitation effects, ultrasound may create a thermal effect [39].
Ultrasound can increase extraction yield, but high ultrasound levels can also lead to a degradation of curcuminoid power exceeding a particular value [40]. In the present work, the UP effect was investigated at different specific energy inputs (WT): 40 kJ/kg (UP-40), 80 kJ/kg (UP-80) and 120 kJ/kg (UP-80).
UP treatment did not lead to a significantly higher TC than the untreated sample (Figure 2) and TP. However, the disintegration level of UP-treatment was considerably higher than the untreated sample, shown as Zp value (Figure 1). Moreover, UP-40 had the lowest WT, but TC was 50% and 34% higher than UP-80 and UP-120 treatment, respectively. Even the amount of curcuminoids in UP-80 treatment was lower than the untreated sample. It is clearly shown that higher WT does not necessarily contribute to increasing the number of curcuminoids in the extract.
In the report of Lou, Wang, Zhang and Wang [40], the author showed that when the ultrasonic power is too high, the extracted compounds might degrade, and the yield may exhibit a slight decrease. It is also essential to understand that the ultrasound applying temperature cannot be controlled adequately at significantly high power levels [41]. In our study, the sample was applied UP-120 increased temperature than 20 °C at the end of the pretreatment (Table 1). It may lead to the degradation of curcuminoids because UP can create bubbles with a temperature of up to 5000 K, 1000 atm, and heating and cooling rate above 1010 K/s [42].

Table 1.
Treatment time, specific energy input [kJ/kg], start and end temperature [°C] of different pretreatment on fresh turmeric. Ultrasound pretreatment (UP), Pulsed electric field pretreatment (PEF), Thermal pretreatment (TP), Enzyme pretreatment (EP).
As a result, destroying plant cells in the UP does not result in higher amounts of curcuminoids leaching into the extract solution during rapid extraction.
3.3. Effect of Enzyme Pretreatment on Total Curcuminoids Recovery of Fresh Turmeric
Enzyme pretreatment was considered an environmental and non-thermal treatment, increasing selective extraction without the side effect of temperature with bioactive compounds [43]. The effect of EP on total curcuminoid recovery was shown in Figure 2.
In the present study, enzyme soaking time was 1 h, 3 h and 5 h. EP-1 showed TC 53% higher than the untreated sample, and there was no significant increase in the TC observed beyond 1 h of soaking (Figure 1). This phenomenon can be explained by the watery contacting time of EP is considerably higher than that of UP. Even more, curcuminoids were also reported to be unstable in water [38], and they could be decomposed into smaller molecules. The degradation products found were vanillin, p-hydroxybenzaldehyde, ferulic aldehyde, p-hydroxybenzoic acid, ferulic acid and vanillic acid. Muniglia, Claisse, Baudelet and Ricochon [43] reported that extraction time, however, may become a hindering factor as prolonged durations frequently cause bioactive compound disintegration. Besides, extended exposure at elevated temperatures or oxidation processes. In this case, 1 h of soaking with pectinase was the suitable time.
3.4. Effect of PEF Pretreatment on Total Curcuminoids Recovery of Fresh Turmeric
The pulsed electric field is a non-thermal method used recently to enhance the plant’s recovery of sensitive compounds. The treated plant cells experience an increase in membrane permeability, which leads to a more significant mass transfer during extraction. In the current study, the effect of PEF was investigated at three levels of specific energy input. The impact of PEF pretreatment on total curcuminoid recovery of fresh turmeric was shown in Figure 2.
With PEF-3.5, TC is higher than the untreated sample at 27%. Nevertheless, when doubled WT, resulting in no significant difference between samples treated with PEF-3.5 and the control (Figure 2).
Barba, Zhu, Koubaa, Sant’Ana, Orlien and Technology [25] reported that when the treatment remains moderate, a local and reversible membrane rupture can take place. When stopping the treatment, the membrane can reverse to initial properties. Hence, the WT must be higher than a certain level to get irreversible changes.
The present investigation has enabled confirming that phenomenon, PEF-14, increased TC value by 16.2% in comparison with PEF-3.5 (Figure 2). The Zp value of PEF-14 was higher 48% than PEF-3.5 (Figure 1). This result is strongly claimed a considerable change in the number of irreversible cells when increasing WT from 3.5 kJ/kg to 14 kJ/kg.
Nonetheless, further increase of the treatment time caused additional cell damage until the Zp became constant, indicating complete saturation of cell breakdown in the current treatment. Similar saturation results have been reported by other authors [44,45,46].
Treatment time of PEF was 0.54–2.05 millisecond, significantly shorter than UP (1 min), TP (5 min), EP (1 h); howbeit, PEF did not show a lower yield than other treatments (Figure 2).
In addition, the temperature of the sample after PEF treatment did not change. In the current research, all PEF models did not increase the temperature to higher than 2 °C (Table 1). Similar results were found by Maskooki and Eshtiaghi [27]; PEF can integrate the cell with a non-thermal effect.
3.5. Effect of Pretreatment Time on BDMC, DMC, CUR Recovery of Fresh Turmeric
In the current investigation, the effect of pretreatment time on BDMC, DMC and CUR will be further discussed. Processing time is an essential parameter that should be considered when applying a method in practice. Prolonging the treatment time may lead to increasing in the cost and reducing the quality of materials. However, the handling time of TP, UP, PEF were all lower than 5 min, so there was less evidence to claim the correlation between treatment time and the deterioration of curcuminoids.
During pretreatment implementation, curcuminoids may be affected by environmental conditions such as oxygen, light and temperature. Depending on the structure of each molecule, different levels of degradation might be reached. The difference in molecular structure between BDMC, DMC and CUR is the number of methoxy group links with phenol rings; this characteristic can lead to different transformations undergo oxidation. CUR is a molecule with two methoxy groups, so it is exposed strongly to impact factors; however, DMC has one methoxy group remaining, and BDMC has no methoxy groups; hence they are less sensitive against oxygen [47]. With the difference in molecular structure, prolonged treatment time has varying effects on the extract compounds. In the current study, the EP has the longest processing time, varies from 1–5 h, while for other pretreatments, the duration maximum is 5 min.
With enzymatic pretreatment, the TC was not changed when increased treatment time (Figure 3); howbeit, the extracted amount of each curcuminoid had slightly decreased after 5 h of soaking (Figure 3), while BDMC and DMC did not change considerably (Figure 3b,c). In the observation of [47], in 2 h of soaking, the amount of CUR reduced 75%, while BDMC and DMC had less reduction than CUR (Figure 3a).
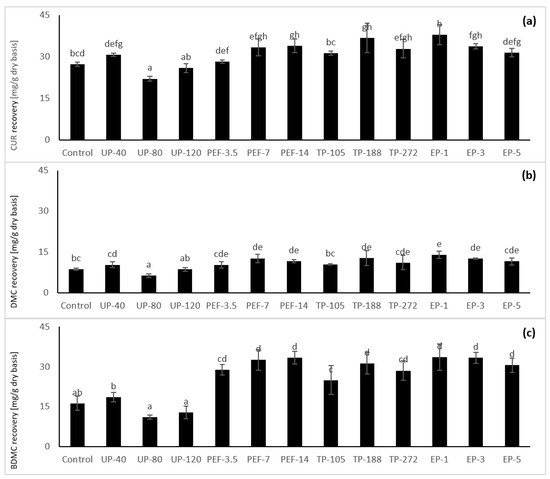
Figure 3.
Effect of pretreatment techniques on Curcumin (CUR) (a), Demethoxycurcumin (DMC) (b) and Bis-demethoxycurcumin (BDMC) (c) recovery of fresh turmeric. In a panel, values with the same lowercase letter are not considering as significantly different (p < 0.05).
Furthermore, when soaking, the impact of oxygen on CUR could not be ignored; Ref. [47] concluded that DMC and BDMC are less affected by autooxidation than CUR. Besides, BDMC and DMC could increase CUR stability.
With the same observation, ultrasound treatment has resulted in the decline of CUR, DMC and BDMC recovery with increasing power (Figure 3a–c). According to Izadifar, Babyn and Chapman [39], the more intensive ultrasound power, the more deteriorated bioactive compounds. On the other hand, turmeric’s pretreatments with PEF or thermal pretreatment did not reduce TC value when increasing WT (Figure 3). This occurrence might be explained as PEF was carried out within several µs and without thermal effect. Thermal pretreatment in the current research was being done in 5 min, while curcuminoids can stable with a temperature of 70 °C in 10 min [38].
3.6. Effect of Different Pretreatment on Total Curcuminoids Recovery of Dried Turmeric
The effect of the EP on the total curcuminoid recovery is shown in Figure 2. The trend was different from fresh turmeric; pretreatment approaches did not show considerable improvement for the extraction of dried turmeric. Noteworthy, with EP-3 and EP-5, the TC value was lower than the untreated dried turmeric sample, 114.9 mg/g and 107.95 mg/g, respectively (Figure 2). According to Gordon, Luis, Ashley, Osheroff and Schneider [47], water may destroy a vast amount of curcuminoids after 2 h of soaking at neutral pH.
In the current research, EP had a long exposure time, enhancing the disintegration of sensitive compounds. In fresh turmeric, TC was 54.6 mg/g with the untreated sample, and the highest recovery was 83.6 mg/g with the EP sample. Nonetheless, with dried turmeric, the untreated sample reached 125.3 mg/g of TC; this value may be a critical point. The water content of fresh turmeric was around seven times higher than dried turmeric. High amount of water could become a barrier to curcuminoids diffusion because curcuminoids are non-polar compounds.
Although pretreatment on dried turmeric showed a higher result than fresh turmeric, the needed energy input for drying was considerably high and time-consuming (72 h at 50 °C), which is an essential factor when applying processing concepts in the industry. The comparison of energy consumption will be further discussed in Section 3.7.
3.7. Comparison of Total Specific Energy Input of the Pretreatments
About the pretreatments on fresh turmeric, specific energy input (WT) was ordered as: TP > UP > EP > PEF (Table 1). TP was considered as the conventional method to assist extraction. The thermal treatment has advantages such as fast and no modern equipment requirements; however, TP’s energy consumption is too high. The main mechanism of UP is creating extremely high local temperature and pressure in the ultra-short time [42]. Moreover, this method has many industrial applications [48]. Nevertheless, the major disadvantage of UP is that it consumes much energy, ultrasonic waves are limited in penetration depth, may generate high temperatures, and cannot be completely controlled regarding a uniform distribution, resulting in the loss of some sensitive components.
EP is a low energy consumption technique than other pretreatments (Table 1). According to Puri, et al. [49], enzymatic pretreatment is a highly selective and effective method. However, the disadvantage is that the processing time can be prolonged, and the investment and operation costs are expensive [50]. PEF was considered a non-thermal treatment widely investigated in the last two decades [51] and low energy consumption, high efficiency, no temperature increasing, so it is very suitable for the extraction of temperature-sensitive compounds. Table 1 shows that PEF is the least energy-consuming pretreatment compared to the other treatments used in this study.
The moisture content of fresh and dried turmeric was 86.91% and 9.44%, respectively. To dry the fresh turmeric, the drying was last 72 h at 50 °C. Nonetheless, with dried material, the Zp value reached a critical point, and TC did not increase anymore under different cell disintegration techniques.
4. Conclusions
The effect of different pretreatments on the extraction yield of curcuminoids from turmeric was evaluated. For fresh turmeric pretreatment, PEF, TP and EP showed good performance. Nevertheless, EP requires 1 h for soaking, while TP and PEF need a processing time of 5 min and several milliseconds, respectively. UP showed to be the most effective in cell disintegration with 92.5% of disintegrated cells. PEF showed a non-thermal cell disintegration effect on the material and was the lowest in energy consumption compared with TP, UP and EP. For dried turmeric samples, none of the pretreatment increased the extraction yield compared to the reference sample. The TC value reached 125.3 mg/g dry basis without further pretreatment on dried turmeric. Hence, the lower water content and some cell damage already occurring during the drying may contribute to a facilitated extraction.
Author Contributions
Conceptualization, H.L.-T., T.F., K.H. and H.J.; methodology, H.L.-T., T.F. and K.H.; investigation, H.L.-T., J.V. and T.G.; formal analysis, H.L.-T.; resources, H.L.-T. and H.J.; validation, H.L.-T. and T.F.; visualization, H.L.-T.; writing—original draft preparation, H.L.-T., K.H.; writing—review and editing, H.L.-T., T.F., K.H. and H.J.; project administration, H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the University of Natural Resources and Life Sciences Vienna (BOKU) in the frame of the OeAD Grant No. Ref. ICM-2019-13805.
Data Availability Statement
Data is included within the article.
Acknowledgments
The authors thank GNT-Europa GmbH (Germany) for providing turmeric powder used for trials. In addition, the authors would like to thank Brian Gallogly and Mary Violet Berger for their support and advice during the technical trials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shirsath, S.; Sable, S.; Gaikwad, S.; Sonawane, S.; Saini, D.; Gogate, P. Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach: Effect of different operating parameters. Ultrason. Sonochem. 2017, 38, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.I.; Uliana, M.R.; Costa, S.M.; Magro, M.; Vianello, F.; Ming, L.C.; Lima, G.P.P. Exclusion of solar UV radiation increases the yield of curcuminoid in Curcuma longa L. Ind. Crop. Prod. 2016, 89, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, P.; Babu, K.N.; Sivaraman, K. Turmeric: The Genus Curcuma; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction of biomolecules from seaweeds. In Seaweed Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 243–269. [Google Scholar]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Ahmad, S.; Ali, M.; Ansari, S.H.; Ahmed, F. Phytoconstituents from the rhizomes of Curcuma aromatica Salisb. J. Saudi Chem. Soc. 2011, 15, 287–290. [Google Scholar] [CrossRef] [Green Version]
- Govindarajan, V.; Stahl, W.H. Turmeric—Chemistry, technology, and quality. Crit. Rev. Food Sci. Nutr. 1980, 12, 199–301. [Google Scholar] [CrossRef]
- Sonawane, S.; Nirmal, S.; Patil, A.; Pattan, S. Development and validation of HPTLC method to detect curcumin and gallic acid in polyherbal formulation. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2664–2673. [Google Scholar] [CrossRef]
- Wakte, P.S.; Sachin, B.; Patil, A.; Mohato, D.; Band, T.; Shinde, D. Optimization of microwave, ultra-sonic and supercritical carbon dioxide assisted extraction techniques for curcumin from Curcuma longa. Sep. Purif. Technol. 2011, 79, 50–55. [Google Scholar] [CrossRef]
- Dandekar, D.V.; Gaikar, V. Microwave assisted extraction of curcuminoids from Curcuma longa. Sep. Sci. Technol. 2002, 37, 2669–2690. [Google Scholar] [CrossRef]
- Mandal, V.; Dewanjee, S.; Sahu, R.; Mandal, S.C. Design and optimization of ultrasound assisted extraction of curcumin as an effective alternative for conventional solid liquid extraction of natural products. Nat. Prod. Commun. 2009, 4, 1934578X0900400121. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, L.O.; Pereira, R.N.; Tonon, R.V.; Cabral, L.M.C.; Santiago, M.C.P.; Vicente, A.A.; Teixeira, J.A.C.; Matta, V.M.; Freitas, S.P. Antioxidant compounds recovery from juçara residue by thermal assisted extraction. Plant Foods Hum. Nutr. 2018, 73, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Braga, M.E.; Leal, P.F.; Carvalho, J.E.; Meireles, M.A.A. Comparison of yield, composition, and antioxidant activity of turmeric (Curcuma longa L.) extracts obtained using various techniques. J. Agric. Food Chem. 2003, 51, 6604–6611. [Google Scholar] [CrossRef]
- Raj, A.S.; Chakraborty, S.; Rao, P.S. Thermal assisted high-pressure processing of Indian gooseberry (Embilica officinalis L.) juice–Impact on colour and nutritional attributes. LWT 2019, 99, 119–127. [Google Scholar] [CrossRef]
- Mane, S.; Bremner, D.H.; Tziboula-Clarke, A.; Lemos, M.A. Effect of ultrasound on the extraction of total anthocyanins from Purple Majesty potato. Ultrason. Sonochem. 2015, 27, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ngadi, M.O.; Ma, Y. Optimisation of pulsed ultrasonic and microwave-assisted extraction for curcuminoids by response surface methodology and kinetic study. Food Chem. 2014, 165, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Zhang, F.; Wang, Z. Study on Ultrasonic Extraction of Gastrodin from Gastrodia elata Bl. Sep. Sci. Technol. 2010, 45, 832–838. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction of curcumin by sample–solvent dual heating mechanism using Taguchi L9 orthogonal design. J. Pharm. Biomed. Anal. 2008, 46, 322–327. [Google Scholar] [CrossRef]
- Rouhani, S.; Alizadeh, N.; Salimi, S.; Haji-Ghasemi, T. Ultrasonic Assisted Extraction of Natural Pigments from Rhizomes of Curcuma Longa, L. Prog. Color. Colorants Coat. 2009, 2, 103–113. [Google Scholar]
- Kurmudle, N.; Kagliwal, L.D.; Bankar, S.B.; Singhal, R.S. Enzyme-assisted extraction for enhanced yields of turmeric oleoresin and its constituents. Food Biosci. 2013, 3, 36–41. [Google Scholar] [CrossRef]
- Kurmudle, N.N.; Bankar, S.B.; Bajaj, I.B.; Bule, M.V.; Singhal, R.S. Enzyme-assisted three phase partitioning: A novel approach for extraction of turmeric oleoresin. Process. Biochem. 2011, 46, 423–426. [Google Scholar] [CrossRef]
- Sahne, F.; Mohammadi, M.; Najafpour, G.D.; Moghadamnia, A.A. Enzyme-assisted ionic liquid extraction of bioactive compound from turmeric (Curcuma longa L.): Isolation, purification and analysis of curcumin. Ind. Crop. Prod. 2017, 95, 686–694. [Google Scholar] [CrossRef]
- Harsha, M.R.; Chandra Prakash, S.V.; Dharmesh, S.M. Modified pectic polysaccharide from turmeric (Curcuma longa): A potent dietary component against gastric ulcer. Carbohydr. Polym. 2016, 138, 143–155. [Google Scholar] [CrossRef]
- Lebovka, N.; Bazhal, M.; Vorobiev, E. Simulation and experimental investigation of food material breakage using pulsed electric field treatment. J. Food Eng. 2000, 44, 213–223. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Neumann, E.; Kakorin, S.; Toensing, K. Principles of membrane electroporation and transport of macromolecules. In Electrochemotherapy, Electrogenetherapy, and Transdermal Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–35. [Google Scholar]
- Maskooki, A.; Eshtiaghi, M.N. Impact of pulsed electric field on cell disintegration and mass transfer in sugar beet. Food Bioprod. Process. 2012, 90, 377–384. [Google Scholar] [CrossRef]
- Chemat, F.; Zill e, H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Périno-Issartier, S.; Abert-Vian, M.; Chemat, F. Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products. Food Bioprocess. Technol. 2011, 4, 1020–1028. [Google Scholar] [CrossRef]
- Knorr, D.; Angersbach, A. Impact of high-intensity electric field pulses on plant membrane permeabilization. Trends Food Sci. Technol. 1998, 9, 185–191. [Google Scholar] [CrossRef]
- Singh, G.; Arora, S.; Kumar, S. Effect of mechanical drying air conditions on quality of turmeric powder. J. Food Sci. Technol. 2010, 47, 347–350. [Google Scholar] [CrossRef] [Green Version]
- El Darra, N.; Grimi, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed electric field, ultrasound, and thermal pretreatments for better phenolic extraction during red fermentation. Eur. Food Res. Technol. 2013, 236, 47–56. [Google Scholar] [CrossRef]
- Fauster, T.; Schlossnikl, D.; Rath, F.; Ostermeier, R.; Teufel, F.; Toepfl, S.; Jaeger, H. Impact of pulsed electric field (PEF) pretreatment on process performance of industrial French fries production. J. Food Eng. 2018, 235, 16–22. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.; Obernberger, J.; Zehetner, E.; Kiesslich, A.; Volkert, M.; Jaeger, H. Impact of powder particle structure on the oxidation stability and color of encapsulated crystalline and emulsified carotenoids in carrot concentrate powders. J. Food Eng. 2019, 263, 398–408. [Google Scholar] [CrossRef]
- Kuttigounder, D.; Lingamallu, J.R.; Bhattacharya, S. Turmeric powder and starch: Selected physical, physicochemical, and microstructural properties. J. Food Sci. 2011, 76, C1284–C1291. [Google Scholar] [CrossRef]
- Fauster, T.; Philipp, C.; Hanz, K.; Scheibelberger, R.; Teufl, T.; Nauer, S.; Scheiblhofer, H.; Jaeger, H. Impact of a combined pulsed electric field (PEF) and enzymatic mash treatment on yield, fermentation behaviour and composition of white wine. Eur. Food Res. Technol. 2020, 246, 609–620. [Google Scholar] [CrossRef] [Green Version]
- Lestari, M.L.; Indrayanto, G. Curcumin. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 39, pp. 113–204. [Google Scholar]
- Izadifar, Z.; Babyn, P.; Chapman, D. Mechanical and Biological Effects of Ultrasound: A Review of Present Knowledge. Ultrasound Med. Biol. 2017, 43, 1085–1104. [Google Scholar] [CrossRef] [Green Version]
- Lou, Z.; Wang, H.; Zhang, M.; Wang, Z. Improved extraction of oil from chickpea under ultrasound in a dynamic system. J. Food Eng. 2010, 98, 13–18. [Google Scholar] [CrossRef]
- Balachandran, S.; Kentish, S.E.; Mawson, R.; Ashokkumar, M. Ultrasonic enhancement of the supercritical extraction from ginger. Ultrason. Sonochem. 2006, 13, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Doktycz, S.J.; Suslick, K.S. Interparticle collisions driven by ultrasound. Science 1990, 247, 1067–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muniglia, L.; Claisse, N.; Baudelet, P.-H.; Ricochon, G. Enzymatic Aqueous Extraction (EAE). In Alternative Solvents for Natural Products Extraction; Springer: Berlin/Heidelberg, Germany, 2014; pp. 167–204. [Google Scholar]
- Eshtiaghi, M.; Knorr, D. High electric field pulse pretreatment: Potential for sugar beet processing. J. Food Eng. 2002, 52, 265–272. [Google Scholar] [CrossRef]
- Lebovka, N.; Bazhal, M.; Vorobiev, E. Estimation of characteristic damage time of food materials in pulsed-electric fields. J. Food Eng. 2002, 54, 337–346. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Gordon, O.N.; Luis, P.B.; Ashley, R.E.; Osheroff, N.; Schneider, C. Oxidative transformation of demethoxy-and bisdemethoxycurcumin: Products, mechanism of formation, and poisoning of human topoisomerase IIα. Chem. Res. Toxicol. 2015, 28, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Sowbhagya, H.B.; Chitra, V.N. Enzyme-assisted extraction of flavorings and colorants from plant materials. Crit. Rev. Food Sci. Nutr. 2010, 50, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Vorobiev, E.; Lebovka, N. Pulse electric field-assisted extraction. In Enhancing Extraction Processes in the Food Industry; CRC Press: Boca Raton, FL, USA, 2011; pp. 25–28. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).