Featured Application
In this study, we describe the use of a kinematic analysis system which employs three-dimensional motor analysis using an infrared camera to assess hand motor function following a specific algorithm to quantify finger movements.
Abstract
The Brunnstrom recovery stage (BRS) assessment is a frequently used clinical instrument, but does not allow temporal or spatial analysis owing to its use of binary assessments. We developed a kinematic analysis system (Fahrenheit) that employs three-dimensional motor analysis using the Leap Motion Controller as an infrared camera to assess hand motor function in patients post- cerebrovascular disease (CeVD)/stroke, according to the BRS assessment criteria. We investigated whether Fahrenheit could predict the outcome of the BRS assessment of hand motor function in post-CeVD patients with hemiplegia. Thirty-two inpatients with CeVD were recruited in this pilot study. Participants’ hand motor function after CeVD was assessed through their performance of nine tasks according to the BRS assessment. We constructed a receiver operating characteristic (ROC) curve based on each participants’ performance, and compared the results of the BRS assessment and computed the area under the curve (AUC) for each ROC curve. All task values showed significant differences between sufficient and insufficient movements. AUC analyses showed that the nine tasks assessed using Fahrenheit had high predictability (all AUC values ≥ 0.7), which were comparable to those of the therapists’ assessment. Measurements with Fahrenheit showed high predictability with respect to the BRS criteria, indicating that Fahrenheit may have clinical application for assessing post-CeVD finger movement and motor functions. Further verification involving more patients is required to ensure that Fahrenheit becomes a more reliable evaluation tool.
1. Introduction
Cerebrovascular disease (CeVD)/stroke is the leading cause of disability in the elderly and affects 15 million people worldwide annually, according to data from the World Health Organization [1]. In patients with hemiparesis following CeVD, the prevalence of upper extremity (UE) and hand impairment is approximately 80% in the acute phase [2]. Patients with hemiparesis following CeVD often have abnormal muscle synergies, spasticity, and muscle weakness, leading to functional disability. The assessment of UE and hand impairment following CeVD is critical for determining prognosis and evaluating treatment efficacy. The assessment of motor function following CeVD is usually performed using standardized clinical scales. One of the most frequently used clinical instruments for assessing UE and hand impairment in CeVD is the Brunnstrom recovery stage (BRS) assessment, which comprises an ordinal scale with six stages, from flaccid paralysis in stage I to voluntary individual joint movements in stage VI [3]. The BRS assessment of UE function was moderately correlated with neurophysiological measures using the Hoffmann/Muscle (H/M) slope ratio and the H/M max ratio, and was highly correlated with the Modified Ashworth Scale score for the evaluation of motor recovery in CeVD patients [4]. However, BRS scores are influenced by therapists’ abilities to conduct this assessment. Moreover, the BRS assessment does not allow for the identification of finer changes in UE and hand impairment within each stage, given that it is an observer-based ordinal scale that utilizes binary assessments that are either “sufficient” or “insufficient”.
The Fugl-Meyer assessment (FMA) is a clinical instrument that uses the BRS criteria [5] and provides a cluster analysis of UE function in patients with chronic CeVD, enabling the classification of 3 or 4 levels of impairment [6]. The FMA-UE measures motor recovery in terms of the degree of impairment, but this does not accurately describe a patient’s actual ability to accomplish a task. This is because compensatory movements develop in response to abnormal muscle synergies, and these compensatory movements provide an alternative functional form of recovery. Hence, the FMA-UE score does not always predict functional recovery because of the ceiling effect, given that it relies on discrete scoring criteria rather than continuous measurement [7].
Microsoft Kinect, which utilizes infrared (IR) sensors to obtain highly accurate 3D motor analyses of limb motion, without markers, has been used to assess progress during rehabilitation [8]. However, the Kinect skeleton model, with its total of 25 anatomical landmarks, is insufficient for analyzing fine hand movements, as it only analyzes four landmarks in the hand: the tips of the hand and thumb, which are the only two distal points, the midpoint of the hand, and the wrist. Therefore, there is a need for a numeric scale that can be used to assess the specific arm or hand movements that patients can or cannot perform following a CeVD.
Thus, we developed Fahrenheit, a method to record hand movements using three-dimensional (3D) motor analysis, in order to evaluate, more precisely, treatment efficacy and determine prognosis in patients following CeVD. The Leap Motion Controller (LMC) (Ultraleap Inc., Bristol, England), which has three IR light emitters and two IR cameras, can be used to detect hand movements with a temporal resolution of up to 120 Hz and a spatial resolution of 1/100 mm, without anatomical markers, and can also recognize hand gestures. As stated by the manufacturer, the accuracy of the detection of each fingertip position is approximately 0.01 mm, with a frame rate of up to 300 fps [9]. The LMC can reliably assess static objects with a 0.2 mm accuracy [10] and can obtain clinically meaningful data for measuring wrist flexion and extension [11]. In this study, we introduce our newly developed model, Fahrenheit, that uses the LMC as a sensor, given its demonstrated superiority, to measure hand movements during recovery from post-CeVD motor paralysis in accordance with the BRS criteria. In addition, we investigate whether Fahrenheit is able to predict the outcome of the BRS assessment of hand motor paralysis in patients with post-CeVD.
2. Materials and Methods
2.1. Participant Selection
Patients with post-CeVD who participated in the Fahrenheit Development Project at Saitama Prefectural University were recruited in the present study. The eligibility criterion for this study was participants aged 20 or above who were recruited among all inpatients admitted at the Tokyo Dental College Ichikawa General Hospital between 1 June 2016 and 31 March 2021 who had suffered a stroke. The exclusion criteria were as follows: (1) patients with a history of previous CeVD or transient ischemic attack, (2) patients who were missing one or more fingers or who had severely limited range of motion (ROM), (3) patients with difficulty understanding verbal instructions due to impaired consciousness, dementia, or aphasia, (4) patients who required bedrest, (5) patients who found it difficult to remain seated for at least 30 min, and (6) patients who experienced difficulty maintaining the required limb positions during measurement, even with assistance. The following baseline characteristics were determined for each participant: age, sex, hand dominance (right/bilateral/left), time since CeVD onset, CeVD lesion side(right/left), type of CeVD (ischemic/hemorrhagic), and BRS (Table 1).

Table 1.
Participants’ baseline characteristics.
2.2. Fahrenheit Procedures
We developed Fahrenheit using the LMC as an IR sensor with Unity game software [12]. The characteristics of each proximal, middle, and distal phalanx were derived from each set of measured joint coordinates: metacarpophalangeal (MCP), proximal interphalangeal (PIP), distal interphalangeal (DIP), and wrist. A software program was created to calculate the joint angles and distances from the vectors representing each phalanx, using the law of cosines. Fahrenheit was operated on a laptop computer with a Windows 8 64-bit operating system (Microsoft, Tokyo, Japan), and the data generated were stored on the hard drive of the same computer.
The nine tasks, according to the BRS assessments, were as follows: (A) finger mass flexion, the flexion of all fingers from maximum extension; (B) finger mass extension, the extension all fingers from maximum flexion; (C) radial thumb abduction, the abduction of the thumb towards the radius from maximum flexion; (D) thumb-index finger pinch, the opposition of the thumb and index finger from maximum extension of both fingers; (E) cylindrical grasp, the adduction and flexion of the palmar surface of all fingers against one other, from maximum extension, as if grasping a cylinder of approximately 40 mm in diameter; (F) spherical grasp, the adduction and flexion of the palmar surface of all fingers against one other, from maximum extension, as if grasping a baseball; (G) index-middle finger flexion, the flexion of the index and middle fingers from maximum extension of all fingers; (H) thumb-index finger extension, the radial abduction of the thumb and the full extension of the index finger from maximum flexion of all fingers; and (I) finger mass abduction/adduction, the adduction and extension of all fingers from maximum abduction and extension (Figure 1).
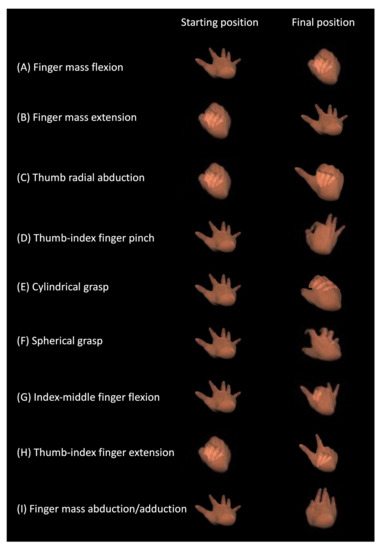
Figure 1.
The nine tasks of the BRS assessment.
Data recorded from the nine tasks were analyzed from the LMC coordinates using an algorithm. The algorithm was implemented using output measurement values ranging from 0 (the minimal value of movement; 14 finger joints per hand—metacarpophalangeal [5], proximal interphalangeal [4], distal interphalangeal [4], and interphalangeal [1]—in maximal extension in a normal adult; 0° converted to 0) to 1 (the maximum value of movement converted to 1; maximal flexion computed as 90° × 14 joints = 1260°). These measurements were obtained during voluntary ROM at a resolution of 0.001 mm and a sampling rate of 60 frames per second (fps).
Patients were placed in a sitting position during the recordings, on a chair or wheelchair, with the shoulder joint in a resting position [13], the elbow joint at approximately 90°, the forearm pronated, and the wrist joint in the neutral position (Figure 2A). We instructed each participant to assume the starting position for each task at a distance of approximately 20 cm above the LMC, and to maintain the starting position for 5 s from the start signal while looking at the laptop monitor. After 5 s, a verbal signal directed the participant to move their hand to the best of their ability to achieve the hand position for each task, and then to maintain their maximum attained position for 15 s from the start signal. The participant was provided with virtual hand images, obtained from the Fahrenheit recording, to demonstrate their hand movements in real time (Figure 2B).
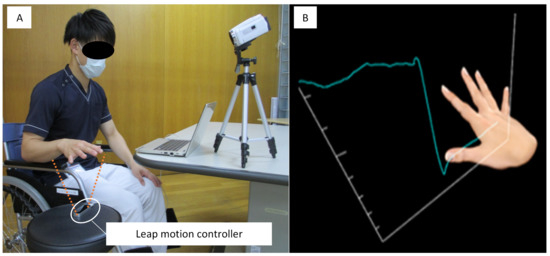
Figure 2.
The Fahrenheit System. (A) The position of the patient during measurement. (B) Virtual hand movements displayed in real time. The vertical axis represents the numeric value of finger movements, and the horizontal axis represents the measurement time-point.
For each participant, we assigned a maximum value for each task using a 5% trimmed mean, obtained from analyzing the hand position attained between 11 s and 14 s (661–840 fps) from the start of each measurement. In cases where we could not measure a minimum increase of 0.2 from the time of initiating hand movement, using a 5% trimmed mean obtained between 2 s and 4 s (61–240 fps) from the start of the measurement, the movement was deemed insufficient and assigned a value of 0 in the analysis.
2.3. Therapist’s Assessment of Hand Function
We recorded the hand movements of each subject with a video camera (Everio GZ-E9; JVC, Kanagawa, Japan) at 30 Hz temporal resolution (Figure 2A). Each therapist, either a licensed occupational or physical therapist (n = 3; mean age = 28 ± 3 years; mean duration of post-qualification experience = 6 ± 3 years), assessed the hand movements of each subject by audiovisual observation of the video recording. The therapists did not meet any of the subjects in person. The therapists assigned each movement a score of 0 (insufficient movement) or 1 (well-accomplished movement) in accordance with the BRS criteria. The therapists performed their assessments independently and were blinded to the results obtained by the other therapists and by Fahrenheit. If the three therapists assigned discrepant scores, we used the score obtained by the majority.
2.4. Statistical Analysis
Continuous data are presented as mean values ± SD. The mean values ± SD recorded with Fahrenheit (BRS score was assigned as 0/1) were compared between participants who obtained a BRS score of 1 (well-accomplished movement) and those who obtained a BRS score of 0 (insufficient movement) using an unpaired t-test to determine if they were significantly different.
To examine Fahrenheit’s predictive ability in terms of hand function, we constructed a receiver operating characteristic (ROC) curve for each participant’s performance of the nine tasks. The Fahrenheit and therapist’s assessments were evaluated against the BRS score as a dependent variable, and we computed the area under the curve (AUC) for each ROC curve. We interpreted the AUC values as follows: 0.5 < AUC < 0.7: low accuracy; 0.7 ≤ AUC < 0.9: moderate accuracy; and AUC ≥ 0.9: high accuracy. The Youden index was used to determine the ideal cutoff points (the higher the index, the better the prediction at the cutoff point) [14]. We compared the accuracy of the results obtained for each task during the Fahrenheit recording and the results of the therapists’ assessments, using the AUC and DeLong’s test [15] for two ROC curves. Statistical analysis was performed using the software Jamovi version 1.8 [16], with significance set at 5%.
3. Results
3.1. BRS Assessment
Thirty-two patients with post-CeVD who participated in the Fahrenheit Development Project at Saitama Prefectural University were included in the present study (Figure 3). All participants were categorized into the following six groups according to the BRS criteria: stage I (one participant), stage II (three participants), stage III (five participants), stage IV (seven participants), stage V (seven participants), and stage VI (nine participants). Table 2 shows the BRS score assigned to each task performed by each participant (0 = insufficient movement; 1 = well-accomplished movement).
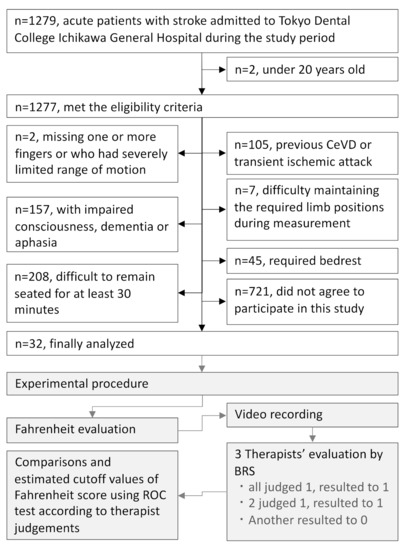
Figure 3.
Patient recruitment and experimental procedures.

Table 2.
The comparison of measurements in each task recorded with Fahrenheit.
3.2. Analysis Using Fahrenheit
We obtained Fahrenheit recordings for all movement parameters in all participants, with no omissions. The mean ± SD values of the nine tasks recorded with Fahrenheit among all participants were as follows: (A) finger mass flexion: 0.58 ± 0.18, (B) finger mass extension: 0.74 ± 0.17, (C) thumb radial abduction: 0.81 ± 0.32, (D) thumb-index finger pinch: 0.77 ± 0.22, (E) cylindrical grasp: 0.87 ± 0.31, (F) spherical grasp: 0.83 ± 0.38, (G) index-middle finger flexion: 0.80 ± 0.31, (H) thumb-index finger extension: 0.85 ± 0.26, and (I) finger mass abduction/adduction: 0.84 ± 0.30. The measurements obtained in each task recorded with Fahrenheit were compared to participants’ BRS scores of 0 (insufficient movement) and 1 (well-accomplished movement), and showed significantly different values (Table 2).
3.3. Therapists’ Assessment of Hand Functions
The therapists’ assessment of each task performed by each participant, assigned scores of 0 or 1, were as follows: (A) finger mass flexion, 4/28; (B) finger mass extension, 5/27; (C) thumb radial abduction, 7/25; (D) thumb-index finger pinch, 8/24; (E) cylindrical grasp, 6/26; (F) spherical grasp, 8/24; (G) index-middle finger flexion, 22/10; (H) thumb-index finger extension, 11/21; and (I) finger mass abduction/adduction: 12/20. The rate of agreement among the therapists’ assessments for each task were as follows: (A) finger mass flexion, 99.0%; (B) finger mass extension, 99.0%; (C) thumb radial abduction, 98.0%; (D) thumb-index finger pinch, 94.8%; (E) cylindrical grasp, 91.7%; (F) spherical grasp, 92.7%; (G) index-middle finger flexion, 90.6%; (H) thumb-index finger extension, 92.7%; and (I) finger mass abduction/adduction: 92.7%.
3.4. ROC Curves
The ROC curves were constructed for each task, using the measured values obtained from the Fahrenheit recordings as the dependent variable, and the BRS assessment as the independent variable. AUC analysis indicated that all tasks had significant AUC values (Figure 4 and Table 3). The cutoff value for each measured value of the tasks recorded with Fahrenheit were calculated from the coordinate points on the ROC curve using the Youden index. These results were highly accurate for the following three tasks: (A) finger mass flexion, (B) finger mass extension, and (C) thumb radial abduction, and moderately accurate for the following six tasks: (D) thumb-index finger pinch, (E) cylindrical grasp, (F) spherical grasp, (G) index-middle finger flexion, (H) thumb-index finger extension, and (I) finger mass abduction/adduction (Figure 4). The comparison of the ROC curves of the Fahrenheit and the therapists’ BRS assessments, using DeLong’s test, only showed statistically significant differences in (F) spherical grasp (Z = 1.9739, p = 0.0484), but not in the other tasks (Z = −1.4434–1.946; all p-values > 0.05).
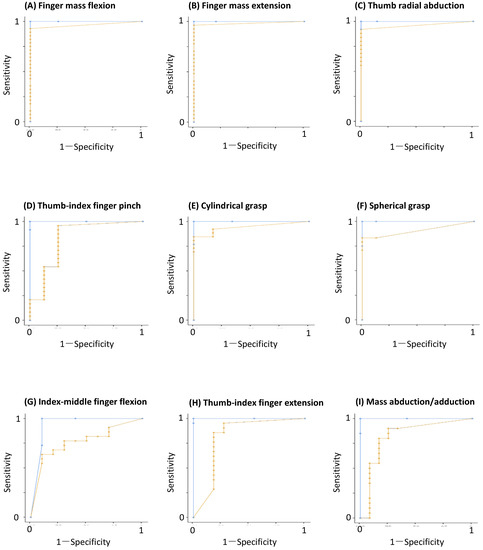
Figure 4.
Receiver operating characteristic (ROC) curves for each of the nine tasks. The light blue curve was estimated by three therapists (insufficient movement = 0, well-accomplished movement = 1). The orange curve was determined using the values determined with Fahrenheit, and the therapist’s clinical judgment.

Table 3.
Accuracy for cut-off values of nine tasks recorded with Fahrenheit.
4. Discussion
In this study, we investigated the predictive ability of the newly developed Fahrenheit in accordance with the BRS criteria and the derived cutoff values. The AUC analyses showed high predictability for the nine tasks recorded with Fahrenheit (all AUC values ≥ 0.7), which is comparable to the therapists’ assessments. These results indicate that all tasks recorded by Fahrenheit can be used to evaluate hand impairment after CeVD, similar to the BRS assessment.
Instruments using Microsoft Kinect version 2 as infrared cameras, which assess the synergistic patterns and muscle contractions of the upper limbs of CeVD patients, have been reported in previous studies [17,18]. One study, which developed the automated FMA tests, showed that both “finger mass flexion” and “finger mass extension” were measured by infrared cameras, but the other hand function items, such as “cylindrical grasp” or “spherical grasp” were measured by pressor sensors [18]. The agreement rate of each “finger mass flexion” and “finger mass extension” between the automated and original FMA tests were 66.7% and 88.9%, respectively [18]. In the present study, the agreement rate of each “finger mass flexion” (93.3%) and “finger mass extension” (93.3%) between the Fahrenheit and BRS were higher than those between the automated and original FMA tests. Other studies did not measure all hand function items using infrared cameras [17,19]. The virtual reality and hand tracking system, using the LMC, have been able to evaluate and classify tremors in patients with Parkinson’s disease in two recent studies [20,21]. No instrument has used infrared cameras to assess the synergistic patterns and muscle contractions in the hand function of patients with CeVD, other than Fahrenheit, described in the present study.
A systematic review of movement quality in robot-assisted upper limb rehabilitation reported the various parameters of kinematic assessments with statistically significant outcomes, but there was a controversial correlation between the various parameters of kinematic and clinical assessments for hand impairment in patients with CeVD [22]. Since Fahrenheit was developed based on the clinical assessment of hand motor function after CeVD, it may have clinical application as a clinical instrument to assess hand impairment in CeVD patients. The clinical utility of Fahrenheit is demonstrated by the significance of the differences in numeric values derived by Fahrenheit versus the therapists, according to the BRS assessment. The BRS assessment is not conducive to identifying subtle differences in hand motor function within each stage, although the BRS assessment is widely used in clinical rehabilitation. Fahrenheit enables more finely graded measurement by recording movements in the range of 0 to 1 with a temporal resolution of 60 Hz, from movement initiation to maximum attainment, and can potentially obtain clinically useful information for a therapist about hand motor recovery in patients post-CeVD. As a result, it may serve as a measurement instrument that will lead to the development of new methods of intervention for rehabilitation.
The Fahrenheit prediction accuracy as inferred from the AUC was low (0.77) for the index-middle finger flexion, similar to the agreement rate among the three therapists’ assessments. This movement is used to assess whether, starting from a position in which all fingers are extended, the radial index and middle fingers can move separately from the ulnar ring and little fingers. This would influence the functional restriction of the anatomy of the flexor digitorum profondus muscle (FDP) and common digital extensor muscle (CDE). The FDP, which is a protagonist muscle during flexing of the DIP joints, originates radially from the palmar side of the interosseous membrane. The ulnar portion of FDP originates from the ulnar and palmar sides. The radial portion forms the FDP tendon of the index finger, whereas the ulnar portion first forms one tendon and then divides into three tendons after reaching the distal end of the forearm. Then, each forms the FDP tendons of the middle, ring, and little fingers, which are jointed into the distal phalanges. Thus, in the middle, ring, and little fingers, if a DIP joint of the adjacent fingers is extended, the contraction of the muscle originating from the ulnar portion is restricted; thus, it is difficult or impossible to achieve the DIP flexion angle of the middle finger during the index-middle finger flexion task.
The CDE is an extrinsic finger extensor muscle, which is located in the posterior forearm, and gradually transitions into four tendons attached to the dorsal side of the middle and distal phalanges of the four fingers (from index to little fingers). These CDE tendons do not remain fully separated from one another and have an intertendinous connection. As a result, the extension of one finger can be influenced by the position of the other fingers. Thus, in this position, it is difficult or impossible to extend the ring finger at the MCP joints during this task. Therefore, it is necessary to develop a system in the future that can be used in accordance with individual differences in the FDP and CDE.
This study has several limitations. First, Fahrenheit cannot measure muscle contractions that occur in the absence of joint articulation, as it can only detect changes in the joint angles of the virtual hand derived from the recordings of the two IR cameras. Thus, Fahrenheit cannot detect changes during a transitional period, such as the transition between flaccid paralysis in BRS stage I to voluntary slight mass grasp in BRS stage II. Second, a selection bias could have limited the results of this study because the subjects of this study were patients with CeVD in the acute phase. It is unclear whether Fahrenheit can be used to evaluate hand motor function in patients with hemiparesis following CeVD in the chronic phase. In this study, we could not collect data on the muscle strength, tone, and somatosensory function that affect finger paralysis. Since the patients in this study were recruited in the acute phase, the experimental period was limited, and it was not possible to measure the muscle strength. With these detailed data, the prediction of finger paralysis would have been more accurate. Third, the study was likely underpowered based on the sample size calculation, which could contribute to an increased likelihood of type 2 errors. It is also difficult to predict whether these results are reproducible. To comprehensively evaluate Fahrenheit and reduce the likelihood of type 2 errors in future studies, it is necessary to repeat this study with a larger sample size, a homogeneous group of participants, where data from mild to severe will be obtained, and in which researchers ensure that patients comply with the described process and methods.
Regarding the hand movements of patients with stroke, the movements of the shoulders and elbow joints are small, the displacement of the trunk is large, and the exercise time is long [23]. In this study, since the hand movement involves multiple joints including the joints in the trunk, patients could maintain the sitting position, and their hand function was evaluated with the forearm fixed with the arm rest. This position was adopted because the patient’s hand movement had to be detected from beneath the position with an infrared camera. Therefore, the patients were required to maintain the wrist joint in the air, and it cannot not be ruled out in this experiment that this continuous movement induced spastic contraction. If the movement of the patient’s hand was affected by the position of the forearm and muscle tones of the other body, it is necessary to keep the wrist joint fixed and analyze the movement of fingers in a subsequent experiment.
5. Conclusions
The results of this pilot study suggest that Fahrenheit, a new method for assessing finger motor function, may be a valid method for evaluating prognosis and treatment efficacy in patients with hemiplegia following CeVD. In addition, we were able to find a cut-off value derived from the movements recorded by Fahrenheit to determine whether patients were able to perform the required hand movement in each task. Fahrenheit may be useful from two clinical perspectives. First, it enables clinicians to assign a numeric value to a specific finger movement, and thus, help quantify motor function. This makes pre- and post-treatment comparisons more detailed and easier than previous assessments. It also enables clinicians to detect subtle signs of recovery or deterioration and evaluate treatment efficacy. Second, Fahrenheit can aid the visualization and recording of results as electronic data. This can be used to motivate patients and set treatment goals, and may enable the provision of remote rehabilitation services in future. These findings are preliminary, and further research is required to validate these findings and determine whether the results obtained here are reproducible. Further development of Fahrenheit is needed before it may be suitable for clinical application.
Author Contributions
Conceptualization, T.H. and T.S.; methodology, T.H.; software, T.H.; validation, T.H.; formal analysis, T.S. and S.Y.; investigation, S.Y.; resources, T.I.; data curation, T.S. and S.Y.; writing—original draft preparation, T.S. and T.I.; writing—review and editing, T.H.; visualization, T.S. and S.Y.; supervision, T.H.; project administration, T.H.; funding acquisition, T.S. and T.I. All authors have read and agreed to the published version of the manuscript.
Funding
The project described was supported by the Saitama Prefectural University (SPU) Grant (15001) and JSPS KAKENHI (C) (21K11220).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Saitama Prefectural University (approval No. 27083) and the Independent Ethics Committee of Tokyo Dental College Ichikawa General Hospital (I15-71).
Informed Consent Statement
All participants received written and verbal explanations of the project, including the details of this study, and provided written consent to participate. Written informed consent has been obtained from patients to publish this paper.
Data Availability Statement
Study data will be provided on request from the corresponding author.
Acknowledgments
The authors would like to thank Masateru Katayama, Hiraku Hotta, Shin Doumae, Sayumi Shibui, and Ayumi Enami, the Department of Neurosurgery and Rehabilitation at Tokyo Dental College Ichikawa General Hospital for their contributions and the operational approval to conduct this study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CeVD | cerebrovascular disease |
| BRS | Brunnstrom recovery stage |
| ROC | receiver operating characteristic |
| UE | upper extremity |
| FMA | Fugl-Meyer assessment |
| 3D | three-dimensional |
| IR | infrared |
| LMC | leap motion controller |
| MCP | metacarpophalangeal |
| PIP | proximal interphalangeal |
| DIP | distal interphalangeal |
| Fps | frames per second |
| FDP | flexor digitorum profondus muscle |
| CDE | common digital extensor muscle |
References
- Mackay, J.; Mensah, G.A. The Atlas of Heart Disease and Stroke; World Health Organization: Geneva, Switzerland, 2004; pp. 50–51. [Google Scholar]
- Feydy, A.; Carlier, R.; Roby-Brami, A.; Bussel, B.; Cazalis, F.; Pierot, L.; Burnod, Y.; Maier, M.A. Longitudinal study of motor recovery after stroke: Recruitment and focusing of brain activation. Stroke 2002, 33, 1610–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawner, K.A.; LaVigne, J.M. Brunnstrom’s Movement Therapy in Hemiplegia, 2nd ed.; J. B. Lippincott Company: Philadelphia, PA, USA, 1992. [Google Scholar]
- Naghdi, S.; Ansari, N.N.; Mansouri, K.; Hasson, S. A neurophysiological and clinical study of Brunnstrom recovery stages in the upper limb following stroke. Brain Inj. 2010, 24, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [PubMed]
- Woytowicz, E.J.; Rietschel, J.C.; Goodman, R.N.; Conroy, S.S.; Sorkin, J.D.; Whitall, J.; McCombe-Waller, S. Determining levels of upper extremity movement impairment by applying a cluster analysis to the fugl-meyer assessment of the upper extremity in chronic stroke. Arch. Phys. Med. Rehabil. 2016, 98, 456–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabilit. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Hondori, H.M.; Khademi, M. A review on technical and clinical impact of microsoft Kinect on physical therapy and rehabilitation. J. Med. Eng. 2014, 2014, 846514. [Google Scholar] [CrossRef] [Green Version]
- Ultraleap. Available online: http://www.ultraleap.com (accessed on 30 May 2021).
- Weichert, F.; Bachmann, D.; Rudak, B.; Fisseler, D. Analysis of the accuracy and robustness of the leap motion controller. Sensors 2013, 13, 6380–6393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeragliuolo, A.H.; Hill, N.J.; Disla, L.; Putrino, D. Validation of the leap motion controller using markered motion capture technology. J. Biomech. 2016, 49, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Unity. Available online: http://japan.unity3d.com (accessed on 1 April 2019).
- Hsu, A.T.; Chang, J.H.; Chang, C.H. Determining the resting position of the glenohumeral joint: A cadaver study. J. Orthop. Sports Phys. Ther. 2002, 32, 605–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Jamovi. Available online: https://www.jamovi.org (accessed on 10 October 2020).
- Del Din, S.; Patel, S.; Cobelli, C.; Bonato, P. Estimating fugl-meyer clinical scores in stroke survivors using wearable sensors. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011. [Google Scholar]
- Lee, S.; Lee, Y.S.; Kim, J. Automated evaluation of upper-limb motor function impairment using fugl-meyer assessment. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Cho, S.; Baek, D.; Bang, H.; Paik, N.J. Upper extremity functional evaluation by Fugl-Meyer assessment scoring using depth-sensing camera in hemiplegic stroke patients. PLoS ONE 2016, 11, e0158640. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.H.; Rovini, E.; Dolciotti, C.; Bongioanni, P.; De Petris, G.; Cavallo, F. Leap motion evaluation for assessment of upper limb motor skills in Parkinson’s disease. In Proceedings of the 2017 IEEE International Conference on Rehabilitation Robotics, London, UK, 17–20 July 2017; pp. 116–121. [Google Scholar] [CrossRef]
- Lugo, G.; Ibarra-Manzano, M.; Ba, F.; Cheng, I. Virtual reality and hand tracking system as a medical tool to evaluate patients with Parkinson’s. In Virtual Reality and Hand Tracking System as a Medical Tool to Evaluate Patients with Parkinson’s, Proceedings of the 11th EAI International Conference on Pervasive Computing Technologies for Healthcare, Barcelona, Spain, 23–26 May 2017; Association for Computing Machinery: New York, NY, USA, 2017; pp. 405–408. [Google Scholar] [CrossRef]
- Nordin, N.; Xie, S.Q.; Wünsche, B. Assessment of movement quality in robot-Assisted upper limb rehabilitation after stroke: A review. J. Neuroeng. Rehabil. 2014, 11, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-W.; Liao, W.-W.; Chen, C.-L.; Wu, C.-Y. Kinematic descriptions of upper limb function using simulated tasks in activities of daily living after stroke. Hum. Mov. Sci. 2021, 79, 102834. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).